Abstract
Aim
To compare risk-adjusted outcomes at 18–22 months corrected age for extremely low birth weight (ELBW) infants who never received phototherapy (NoPTx) to those who received any phototherapy (PTx) in the NICHD Neonatal Research Network randomized trial of Aggressive vs. Conservative Phototherapy.
Methods
Outcomes at 18–22 months corrected age included death, neurodevelopmental impairment (NDI), and Bayley Scales Mental Developmental Index (MDI). Regression models evaluated the independent association of PTx with adverse outcomes controlling for center and other potentially confounding variables.
Results
Of 1972 infants, 216 were NoPTx and 1756 were PTx. For the entire 501–1000 g BW cohort, PTx was not independently associated with death or NDI (OR 0.85, 95% CI 0.60 –1.20), death, or adverse neurodevelopmental endpoints. However, among infants 501–750 g BW, the rate of significant developmental impairment with MDI<50 was significantly higher for NoPTx (29%) than PTx (12%) (p=0.004).
Conclusions
Phototherapy did not appear to be independently associated with death or NDI for the overall ELBW group. Whether PTx increases mortality could not be excluded due to bias from deaths before reaching conservative treatment threshold. The higher rate of MDI<50 in the 501–750g BW NoPTx group is concerning, and consistent with NRN Trial results.
INTRODUCTION
Phototherapy is one of the most frequently used treatments in the neonatal intensive care unit (NICU). It is generally considered to be a safe and effective therapy for neonatal hyperbilirubinemia, but these assumptions have been primarily extrapolated from observations in larger or late preterm and term infants. Phototherapy is potentially injurious, particularly in the smaller more translucent extremely low birth weight (ELBW) infants. In the only large trial of neonatal phototherapy prior to 2008, fewer than 80 ELBW infants were included; although the risk of death was not significantly increased with phototherapy in that subgroup, the odds ratio (OR) was consistent with an increased mortality 1.49 (95%CI: 0.93–2.40) (1–3). Phototherapy has also been implicated as a contributing factor in a number of neonatal morbidities including prolonged ductal patency, hemolysis, and platelet destruction via a range of proposed mechanisms including oxidative injury (4–12). However, the accumulation of bilirubin may itself be directly deleterious to preterm infants. Higher total serum bilirubin (TSB) levels and unbound bilirubin or “free” bilirubin (Bf) levels have been shown to be associated with higher risk of death or adverse neurodevelopmental outcomes among very low birth weight (VLBW) and ELBW infants (13–16), but the relationship is likely to be complex.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) Randomized Trial of Aggressive or Conservative Phototherapy for 501–1000 gram BW infants (the Phototherapy Trial) compared 18–22 month corrected age outcomes of infants randomized to two different phototherapy strategies based on bilirubin level thresholds (17). Aggressive phototherapy did not significantly reduce the rate of death or neurodevelopmental impairment (NDI) for the overall cohort. In post hoc analyses, a significant reduction in profound impairment among survivors was observed, but this reduction was offset by an increase in death among the 501–750 gram BW subgroup. However, the NRN trial was not designed to evaluate whether the lack of phototherapy exposure altogether was associated with better or worse outcomes; rather, the trial sought to evaluate two different phototherapy strategies.
There are no prospective studies of recent ELBW cohorts to determine whether exposure to phototherapy at all is associated with better or worse outcome, and a randomized trial of phototherapy vs. no phototherapy in this population could not be reasonably undertaken without an effective alternative treatment for jaundice. Nevertheless, the NICHD NRN Phototherapy Trial dataset provides an opportunity to explore this question in a clinical context. Therefore, we compared risk-adjusted rates of death or NDI at 18–22 months corrected age (primary outcome) among infants who received any phototherapy (PTx) to those who never received phototherapy (NoPTx) during the NICHD NRN Phototherapy Trial. We also compared NoPTx and PTx groups with respect to the secondary outcomes of death before hospital discharge, death by 18–22 months corrected age, and adverse neurodevelopmental outcomes among survivors. Despite the possibility of residual confounding, these observational analyses may serve to generate evidence-based hypotheses in future trials comparing phototherapy against new therapies to prevent bilirubin neurotoxicity.
SUBJECTS and METHODS
The Eunice Kennedy Shriver NICHD NRN conducted a multicenter trial to compare the outcomes of infants 501–1000 g BW randomized to aggressive or conservative phototherapy (17). Subjects were enrolled September 2002 through April 2005, and randomization was stratified on the basis of BW (501–750 g, or 751–1000 g) and NRN center. The protocol stipulated the use of phototherapy during the first 14 days after birth. Aggressive phototherapy was initiated at enrollment. For 501–750 g infants, phototherapy was continued or restarted whenever a value ≥5 mg/dL was identified. For 751–1000 g infants, aggressive phototherapy was continued or restarted whenever a value ≥5 mg/dL was identified during the first 7 days and ≥7 mg/dL in the next 7 days. Conservative phototherapy was initiated, continued, or restarted whenever the bilirubin was ≥ 8 mg/dL for 501–750g infants and ≥10 mg/dL for 751–1000 g infants. The trial did not require infants to receive phototherapy unless they reached bilirubin treatment thresholds.
Research nurses collected demographic, perinatal, and infant data at each center using common definitions, as described previously (17, 18). At 18–22 months corrected age, a comprehensive neurodevelopmental assessment was performed on surviving infants. The follow-up visit as previously described (19, 20) consisted of a battery of developmental, neurologic, and behavioral assessments. Neurologic examinations were performed by annually certified examiners who had been trained to reliability in a 2-day workshop on the neurologic assessment. During the study period, the Bayley Scales of Infant Development-II (BSID-II) (21) were administered, which included determination of the Mental Developmental Index (MDI) and Psychomotor Developmental Index (PDI). MDI and PDI scores of 100±15 represent the normative mean ± 1 standard deviation. The BSID-II was administered by experienced examiners, who were certified annually by gold standard psychologists.
Cerebral palsy (CP) was defined as a non-progressive central nervous system disorder characterized by abnormal muscle tone in at least one extremity and abnormal control of movement and posture that interfered with or prevented age-appropriate motor activity. Children with moderate or severe CP were non-ambulatory or required an assistive device for ambulation. Neurodevelopmental impairment (NDI) was defined as any of the following: moderate/severe CP, MDI or PDI<70, bilateral permanent hearing loss, or bilateral blindness. Children with MDI<50 (scoring more than 3 standard deviations below the normative mean) were considered to have significant developmental impairment.
Statistical Analyses
Unadjusted comparisons between PTx and NoPTx groups were made using Χ2 or Fisher’s exact test for categorical data, and t-test for continuous data. Adjusted odds ratio (OR) with 95% confidence interval (CI) were obtained using multiple logistic regression analyses evaluating the independent association of PTx vs. NoPTx with the primary outcome of death or NDI at 18–22 months, and secondary outcomes of death before discharge, death by 18–22 months, and adverse neurodevelopmental outcomes among survivors (NDI, moderate-severe CP, MDI<70, and MDI<50). These analyses adjusted for the following potential confounders that could affect outcome and potentially obscure the independent effect of PTx vs. NoPTx: NRN center, antenatal steroids, inborn vs. outborn, sex, race, C-section delivery, BW, estimated gestational age (EGA), multiple gestation, and maternal education less than high school (HS). Because cranial ultrasonography was not performed before enrollment, it was not possible to control for intracranial hemorrhage at baseline. Separate regression analyses were conducted for the overall cohort (501–1000 g BW), as well as for each BW strata (501–750 g, and 751–1000 g). Modeling was not possible for some outcomes in each BW strata due to small numbers. All analyses were performed using SAS software (SAS Institute, Cary, NC) at RTI International (Research Triangle Park, NC), the Data Coordinating Center for the NICHD NRN.
RESULTS
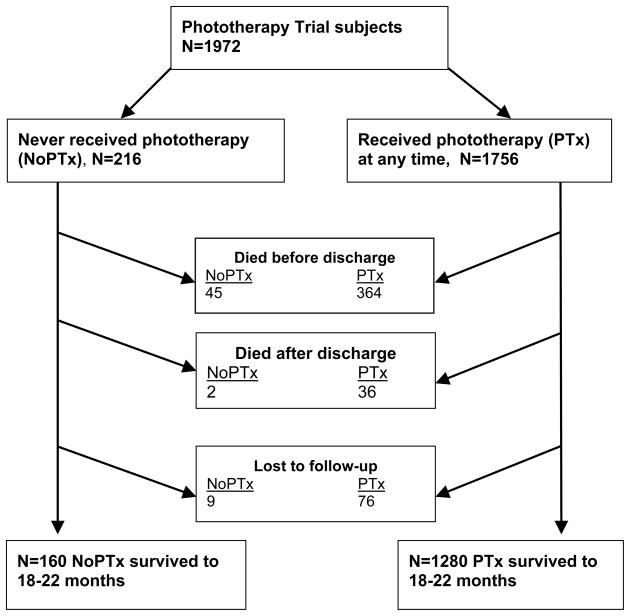
Of the 1974 infants enrolled in the NRN Phototherapy Trial, 2 were excluded from analysis due to missing data regarding phototherapy. Of the remaining 1972, 216 (11%) never received phototherapy (NoPTx) (70 infants 501–750 g BW, and 146 infants 751–1000 g BW) whereas 1756 (89%) received phototherapy at some time (PTx) per Phototherapy Trial protocol (790 infants 501–750 g BW, and 966 infants 751–1000 g BW). Survival at 18–22 months (mos) corrected age could be determined for 207 patients in NoPTx and 1680 patients in PTx (Figure 1). Nine patients in the NoPTx group (4.2%) and 76 in the PTx group (4.3%) were lost to follow-up. The outcome of death or NDI at 18–22 mos could be determined for 195 patients in NoPTx and 1607 patients in PTx (91.4% follow-up rate).
Figure 1.
Progression of NoPTx and PTx patients from NRN Phototherapy Trial to 18–22 mos corrected age.
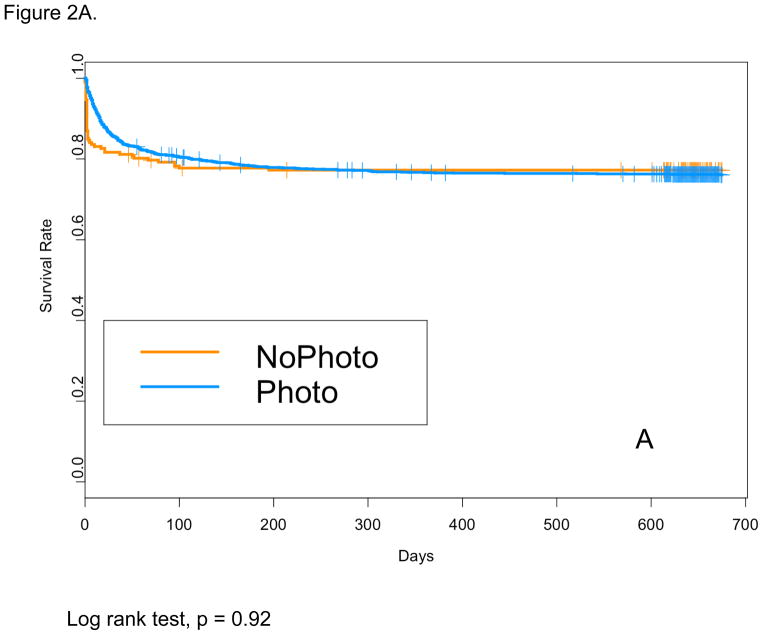
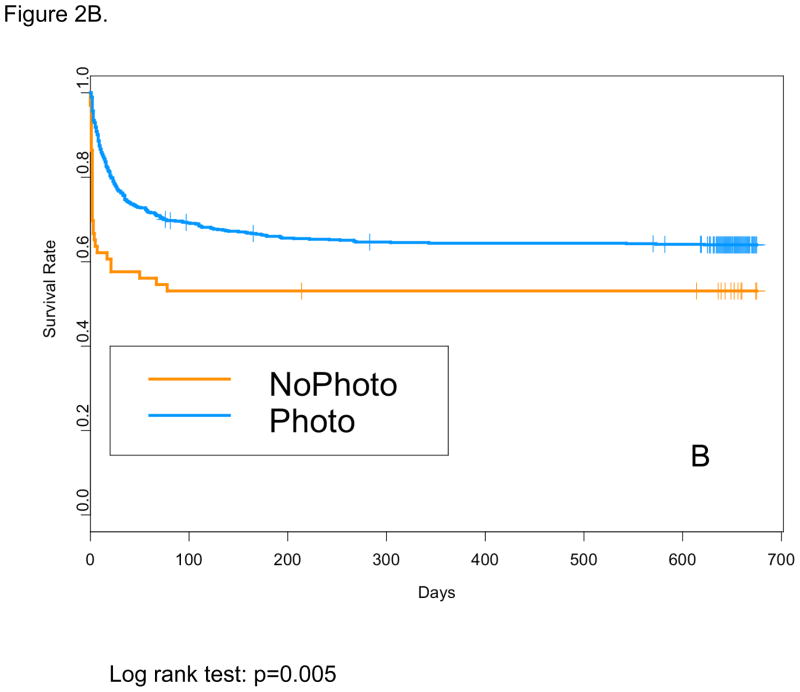
Baseline characteristics for the NoPTx and PTx groups are shown in Table 1. For the total cohort (501–1000 g BW), mean BW, mean EGA, and rate of cesarean section were higher, and rate of maternal education less than high school was lower in NoPTx on unadjusted comparison. The mean age at Phototherapy Trial enrollment was not different between the NoPTx and PTx groups (22+/−7.5 hours vs. 21+/−7.2 hours, p=0.29). For the total cohort (NoPTx 21% vs. PTx 21%, OR 0.84, 95%CI 0.56–1.27), and for the 751–1000 g subgroup (10% vs. 11%, OR 1.24, 95% CI 0.65–2.36) death before discharge and death at 18–22 mos were not different for NoPTx compared with PTx (Table 2). For the 501–750 g BW subgroup only, the odds of death before discharge were lower for PTx compared with NoPTx (33% vs. 44%, OR 0.54, 95%CI 0.30–0.96). This can be explained by early deaths prior to meeting the Phototherapy Trial treatment initiation criteria in the NoPTx group as shown by Kaplan-Meier survival curves (Figure 2A and 2B). Table 3 shows in-hospital morbidities among survivors to discharge. The rates of intraventricular hemorrhage (IVH) grade 1 or 2, IVH grade 3 or 4 or cystic periventricular leukomalacia (PVL), peak TSB, and length of stay were lower in NoPTx compared with PTx infants.
Table 1.
Baseline characteristics of NoPTx and PTx groups
| TOTAL COHORT (501–1000 g BW) | 501–750 g BW | 750–1000 g BW | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NoPTx | Photo | p value | NoPTx | Ptx | p value | NoPTx | PTx | p value | |
| N | 216 | 1756 | 70 | 790 | 146 | 966 | |||
| Birth weight (mean+/−SD), g | 801+/−128 | 774+/−135 | 0.006 | 645+/−69 | 646+/−66 | 0.947 | 875+/−70 | 878+/−71 | 0.58 |
| EGA (mean+/− SD), weeks | 26.2+/− 2 | 25.9+/−1.9 | 0.036 | 25.0+/−1.8 | 24.9+/−1.6 | 0.72 | 26.8+/−1.9 | 26.7+/−1.8 | 0.59 |
| Male | 111 (51%) | 900 (51%) | 0.97 | 36 (51%) | 382 (48%) | 0.622 | 75 (51%) | 518 (54%) | 0.611 |
| Race | 0.74 | 0.40 | 0.89 | ||||||
| Black | 85 (39%) | 735 (42%) | 27 (39%) | 369 (47%) | 58 (40%) | 366 (38%) | |||
| Other | 51 (24%) | 383 (22%) | 18 (26%) | 166 (21%) | 33 (23%) | 217 (22%) | |||
| White | 80 (37%) | 638 (36%) | 25 (36%) | 255 (32%) | 55 (38%) | 383 (40%) | |||
| Antenatal steroids | 176 (82%) | 1388 (79%) | 0.33 | 54 (78%) | 608 (77%) | 0.863 | 122 (84%) | 780 (81%) | 0.377 |
| Inborn | 189 (88%) | 1596 (91%) | 0.11 | 62 (89%) | 724 (92%) | 0.379 | 127 (87%) | 872 (90%) | 0.221 |
| Maternal education <HS* | 38 (18%) | 449 (26%) | 0.01 | 10 (15%) | 194 (25%) | 0.076 | 28 (19%) | 255 (27%) | 0.051 |
| ROM >24 hours | 44 (21%) | 325 (20%) | 0.64 | 15 (23%) | 157 (21%) | 0.787 | 29 (21%) | 168 (19%) | 0.601 |
| C-section** | 157 (73%) | 1125 (64%) | 0.01 | 52 (75%) | 512 (65%) | 0.079 | 105 (72%) | 613 (64%) | 0.048 |
| 5 minute Apgar score <5 | 25 (12%) | 238 (14%) | 0.43 | 9 (13%) | 144 (18%) | 0.253 | 16 (11%) | 94 (10%) | 0.623 |
| Coombs positive mother | 8 (5%) | 51 (4%) | 0.50 | 3 (6%) | 20 (4%) | 0.385 | 5 (5%) | 31 (5%) | 0.879 |
HS: High School
C-section: cesarean section delivery
Table 2.
Death at 18–22 months corrected age
| TOTAL COHORT 501–1000 g | 501–750 g | 751–1000 g | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NoPTx | PTx | OR (95%CI)* | NoPTx | PTx | OR (95%CI)* | NoPTx | PTx | OR (95%CI)* | |
| N | 216 | 1756 | 70 | 790 | 146 | 966 | |||
| Death at 18–22 months | 23% | 24% | 0.94 (0.63, 1.41) | 47% | 36% | 0.58 (0.32, 1.04) | 11% | 14% | 1.33 (0.73, 2.44) |
Adjusted odds ratios and 95% confidence intervals obtained by regression analyses using models that included the following independent variables: NRN site, antenatal steroids, inborn vs. outborn, gender, race, C-section delivery, BW, EGA, multiple gestation, and maternal education less than high school
Figure 2.
Kaplan-Meier survival curves for the: (A) total cohort (501–1000 g) and (B) lower birth weight (BW) subgroup (501–750 g)
Table 3.
Major morbidities and in-hospital findings among survivors to discharge
| Total cohort (501–1000 g) | 501–750 grams | 751–1000 grams | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NoPTx | PTx | P value | NoPTx | PTx | P value | NoPTx | PTx | P value | |
| N | 171 | 1392 | 59 | 531 | 132 | 861 | |||
| PDA | 71 (42%) | 656 (47%) | 0.17 | 15 (38%) | 293 (55%) | 0.043 | 56 (42%) | 363 (42%) | 0.95 |
| Grade 1 or 2 IVH | 35 (21%) | 416 (30%) | 0.011 | 4 (10%) | 173 (33%) | 0.003 | 31 (24%) | 243 (28%) | 0.28 |
| Grade 3 or 4 IVH or PVL | 18 (11%) | 246 (18%) | 0.019 | 3 (8%) | 100 (19%) | 0.077 | 15 (12%) | 146 (17%) | 0.11 |
| Proven NEC | 14 (8%) | 112 (8%) | 0.95 | 7 (18%) | 49 (9%) | 0.077 | 7 (5%) | 63 (7%) | 0.40 |
| BPD | 66 (40%) | 592 (43%) | 0.41 | 20 (51%) | 313 (60%) | 0.31 | 46 (36%) | 279 (33%) | 0.46 |
| Postnatal steroids | 17 (10%) | 199 (14%) | 0.12 | 8 (21%) | 119 (22%) | 0.78 | 9 (7%) | 80 (9%) | 0.35 |
| Late sepsis | 70 (41%) | 540 (39%) | 0.59 | 16 (41%) | 258 (49%) | 0.36 | 54 (41%) | 282 (33%) | 0.065 |
| Peak TSB (mean+/− SD, mg/dL) | 7.4+/−1.7 | 8.5+/−2.5 | <.001 | 6.1+/−1.6 | 8.0+/−2.2 | <.001 | 8.0+/−1.4 | 8.9+/−2.6 | <.001 |
| Length of stay (mean+/−SD, days) | 91+/−44 | 99+/−45 | 0.021 | 124+/−58 | 117+/−45 | 0.36 | 81+/−33 | 88+/−41 | 0.055 |
Table 4 shows the rates of 18–22 mos corrected age outcomes, including the primary outcome of death or NDI, and adjusted OR and 95% CI for PTx compared with NoPTx. For the total cohort, death or NDI occurred in 53% of both NoPTx and PTx groups. Phototherapy exposure was not independently associated with death or NDI (OR 0.85, 95% CI 0.6–1.2, p=0.35). Among survivors to 18–22 month neurodevelopmental follow-up, phototherapy exposure was also not independently associated with NDI (OR 0.81, 95% CI 0.55–1.2, p=0.30), components of NDI, or MDI<50. Similarly, for the 751–1000 g BW subgroup, phototherapy exposure was not independently associated with death or NDI, or any of the neurodevelopmental endpoints analyzed. Among 501–750 g BW survivors to 18–22 month follow-up, NDI was found in 47% of both NoPTx and PTx groups. Of concern however, MDI<70 was found in 47% of 501–750 g BW NoPTx, and 39% of PTx, although this difference did not reach statistical significance on unadjusted (p=0.36) or multivariable analysis (OR 0.5, 0.22–1.10; p=0.096). Furthermore, MDI<50 was found in 29% (n=10) of the 501–750 g BW NoPTx compared with 12%(n=57) of the PTx (unadjusted p= 0.004). An independent association of phototherapy exposure could not be definitively determined because regression modeling was not possible due to small numbers.
Table 4.
Primary outcome (death or NDI) and neurodevelopmental endpoints at 18–22 months corrected age
| TOTAL COHORT 501–1000 g | 501–750 g | 751–1000 g | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NoPTx | PTx | OR (95%CI)* | NoPTx | PTx | OR (95%CI)* | NoPTx | PTx | OR | |
| N | 195 | 1607 | 65 | 743 | 130 | 864 | |||
| Death or NDI | 104 (53%) | 852 (53%) | 0.85 (0.60, 1.20) | 47 (72%) | 495 (67%) | 0.73 (0.38, 1.40) | 57 (44%) | 357 (41%) | 0.90 (0.59, 1.37) |
| Among survivors to follow-up | |||||||||
| NDI | 57/148 (39%) | 452/1207 (37%) | 0.81 (0.55, 1.20) | 16 (47%) | 221 (47%) | 0.80 (0.35, 1.79) | 41 (36%) | 231 (31%) | 0.84 (0.53, 1.34) |
| Mod-severe CP | 7 (5%) | 83 (7%) | ** | 3 (9%) | 42 (9%) | ** | 4 (3%) | 41 (5%) | ** |
| MDI <70 | 51 (34%) | 376 (31%) | 0.74 (0.49, 1.11) | 16 (47%) | 183 (39%) | 0.50 (0.22, 1.13) | 35 (30%) | 193 (26%) | 0.88 (0.54, 1.43) |
| MDI <50 | 19 (13%) | 123 (10%) | 0.64 (0.36, 1.15) | 10 (29%) | 57 (12%) | ** | 9 (8%) | 66 (9%) | ** |
Adjusted odds ratios and 95% confidence intervals obtained by regression analyses using models that included the following independent variables: NRN site, antenatal steroids, inborn vs. outborn, gender, race, C-section delivery, BW, EGA, multiple gestation, and maternal education less than high school
Full modeling was not possible due to small numbers.
DISCUSSION
The NRN Phototherapy Trial (17) randomized 501–1000 g BW infants to two different phototherapy strategies rather than investigating absolute phototherapy exposure. Our analysis therefore did not compare outcomes of randomly assigned phototherapy treatment groups; thus, interpretation is susceptible to bias due to unmeasured or unknown intrinsic differences in NoPTx vs. PTx, and residual confounders. However, a randomized trial of phototherapy vs. no phototherapy would not currently be possible or advisable for this extremely high-risk population without an effective alternative treatment. Nevertheless, given concerns regarding effects of possible phototoxicity in ELBW infants, the NRN Phototherapy Trial dataset provided an opportunity for post-hoc exploration of whether lack of phototherapy altogether was independently associated with better or worse outcomes. In the current analysis, we found that phototherapy exposure did not appear to be independently associated with death or NDI at 18–22 months corrected age for the overall ELBW cohort. However, among the smallest infants, those with 501–750 g BW, the rate of MDI<50 was higher among the NoPTx compared with PTx. Although patient numbers were small in this subgroup, this result is concerning. This finding is furthermore consistent with those of the main NRN Phototherapy Trial (17), which demonstrated that aggressive phototherapy significantly reduced the rate of NDI, attributable almost entirely to a reduction in the rate of profound developmental impairment in the aggressive phototherapy group.
We found that 11% of the total cohort (216/1972) was never exposed to phototherapy during the NRN Phototherapy trial (8.1% of 501–750 g BW infants, 13.1% of 750–1000 g BW infants). Clearly, some of these infants never received phototherapy because they died before reaching the defined phototherapy treatment threshold. Mortality before discharge was higher in NoPTx compared with PTx among 501–750 g BW infants, which would appear to be contradictory with results from the NRN Phototherapy Trial. These findings may be entirely due to early deaths before reaching treatment threshold, as shown by the Kaplan-Meier survival curve (Figure 2B), rather than by an effect of phototherapy itself. This underscores the limitations of a post-hoc observational analysis, in that the main trial was designed to compare outcomes of infants randomized to two different phototherapy strategies based on bilirubin level thresholds rather than phototherapy vs. no phototherapy. Indeed, analyses of infants as randomized suggests a high probability that phototherapy increases mortality of the smallest infants while reducing their rate of NDI, particularly profound NDI (17). Potential mechanisms to explain this finding include photo-oxidative injury to cell membranes or dermal biomolecules leading to cell death and mediated by endogenous photosensitizers (22). This could be particularly plausible among these most immature infants whose skin is highly penetrable to light and whose antioxidant defenses are extremely impaired (12, 23). Furthermore, neither the extent of possible toxicity of bilirubin photoisomers, nor their interference with Bf measurements, have yet to be fully elucidated (24).
Compared with NoPTx, we found that PTx was associated with a reduction in significant developmental impairment with MDI<50 among 501–750 g BW infants. The pathway by which phototherapy may protect the vulnerable, developing brain from the neurotoxic effects of bilirubin is likely to involve reduction of Bf levels specifically. The recently published results of a prospective secondary study of the NRN Phototherapy Trial by Oh, et. al. (16) showed that higher Bf levels in ELBW infants at 5 days of life, regardless of clinical status, was directly related to adverse outcomes including death, and death or adverse neurodevelopmental outcomes at 18–22 months. Higher TSB level in the first week of life was related to adverse outcomes only among clinically unstable ELBW infants; this relationship was not observed among clinically stable infants, although this finding may reflect residual confounding. A recent study by Ahlfors, et. al. also showed that elevated Bf, but not TSB concentration, was associated with abnormal automated auditory brainstem response (AABR) (25). In our current analysis, ELBW infants surviving to discharge who reached PTx threshold were more likely than NoPTx infants to have IVH, and had a longer hospital stay (Table 3). This may suggest that those infants had been clinically less stable. Yet those observed clinical morbidities, including IVH, may have led to the PTx group reaching phototherapy treatment threshold. Furthermore, although the peak TSB in the first two weeks for the PTx group was higher than for NoPTx, there was considerable overlap in the range of TSB values between the groups. Finally, the underlying clinical and genetic phenomena explaining why one premature infant progresses to a higher TSB, perhaps meeting a specific threshold for treatment, while another does not, remain obscure. Given that our clinical goal is to better predict outcomes, and to identify appropriate measures and cut points for therapeutic intervention, our findings and others suggest the need to aggressively evaluate more specific measures related to bilirubin neurotoxicity.
In summary, this post-hoc analysis demonstrates that MDI<50 was more common for NoPTx compared with PTx among the smallest infants (501–750 g BW). This finding is consistent with the NRN Phototherapy Trial results showing significant reduction in profound impairment with aggressive phototherapy, although this was offset by an increased rate of death in this subgroup. Nevertheless, our finding is of concern, and points to the need for further investigation into the effects of phototherapy on the most vulnerable and immature infants.
Acknowledgments
The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) provided grant support for the Neonatal Research Network’s Phototherapy Trial (ClinicalTrials.gov NCT00114543).
Data collected at participating sites of the NICHD Neonatal Research Network (NRN) were transmitted to RTI International, the data coordinating center (DCC) for the Network, which stored, managed and analyzed the data for this study. On behalf of the NRN, Dr. Abhik Das (DCC Principal Investigator) and Ms. Nellie Hansen (DCC Statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study:
NRN Steering Committee Chair: Alan H. Jobe, MD PhD, University of Cincinnati (2001–006); Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006-present).
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904) – Abbot R. Laptook, MD; Betty R. Vohr, MD; Angelita Hensman, BSN RNC; Theresa M. Leach, MEd CAES; Martha R. Leonard, BA BS; James R. Moore, MD; Lucy Noel RN; Bonnie E. Stephens, MD; Robert Burke, MD; Yvette Yatchmink, MD; Rachel V. Walden, MD; Victoria E. Watson, MS CAS.
Case Western Reserve University, Rainbow Babies & Children’s Hospital (U10 HD21364, M01 RR80) – Avroy A. Fanaroff, MD; Michele C. Walsh, MD MS; Deanne Wilson-Costello, MD; Nancy S. Newman, RN; Bonnie S. Siner, RN.
Cincinnati Children’s Hospital Medical Center, University Hospital, and Good Samaritan Hospital (U10 HD27853, M01 RR8084) – Kurt Schibler, MD; Edward F. Donovan, MD; Jean J. Steichen, MD; Kate Bridges, MD; Barbara Alexander, RN; Marcia Worley Mersmann, RN CCRC; Holly L. Mincey, RN BSN; Jody Hessling, RN; Teresa L. Gratton, PA.
Duke University School of Medicine, University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (U10 HD40492, M01 RR30) – Ronald N. Goldberg, MD; C. Michael Cotten, MD MHS; Ricki F. Goldstein, MD; Kathy J. Auten, MSHS; Kimberley A. Fisher, PhD FNP-BC IBCLC; Melody B. Lohmeyer, RN MSN; Kathryn E. Gustafson, PhD.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (U10 HD27851, M01 RR39) – Barbara J. Stoll, MD; Ira Adams-Chapman, MD; Ellen C. Hale, RN BS CCRC.
Eunice Kennedy Shriver National Institute of Child Health and Human Development – Linda L. Wright, MD; Elizabeth M. McClure, MEd.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (U10 HD27856, M01 RR750) – Brenda B. Poindexter, MD MS; James A. Lemons, MD; Anna M. Dusick, MD; Diana D. Appel, RN BSN; Dianne E. Herron, RN; Lucy C. Miller, RN BSN CCRC; Leslie Richard, RN.
RTI International (U10 HD36790) – Betty Hastings; Elizabeth N. McClure, MEd; Jamie E. Newman, PhD MPH; Rebecca L. Perritt, MS; Carolyn M. Petrie Huitema, MS; Kristin M. Zaterka-Baxter, RN BSN.
Stanford University, Lucile Packard Children’s Hospital (U10 HD27880, M01 RR70) – M. Bethany Ball, BS CCRC; Joan M. Baran, PhD; Barbara Bentley, PhD; Lori E. Bond, PhD; Ginger K. Brudos, PhD; Maria Elena DeAnda, PhD; Anne M. DeBattista, RN PNP; Jean G. Kohn, MD MPH; Julie C. Lee-Ancajas, PhD; Renee P. Pyle, PhD; Nicholas H. St. John, PhD.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32) – Waldemar A. Carlo, MD; Namasivayam Ambalavanan, MD; Myriam Peralta-Carcelen, MD MPH; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN; Vivien A. Phillips, RN BSN.
University of California – San Diego Medical Center and Sharp Mary Birch Hospital for Women and Newborns (U10 HD40461) – Neil N. Finer, MD; Yvonne E. Vaucher, MD MPH; Maynard R. Rasmussen MD; David Kaegi, MD; Kathy Arnell, RNC; Clarence Demetrio, RN; Martha G. Fuller, RN MSN; Chris Henderson, RCP CRTT; Wade Rich, BSHS RRT.
University of Miami Holtz Children’s Hospital (U10 HD21397, M01 RR16587) – Charles R. Bauer, MD; Shahnaz Duara, MD; Silvia Hiriart-Fajardo, MD; Ruth Everett-Thomas, RN BSN; MS; Mary Allison, RN; Alexis N. Diaz, BA; Silvia Frade Eguaras, Yamiley C. Gideon, BA; Alexandra Stoerger, BA; Andrea Garcia, MA; Kasey Hamlin-Smith, PhD.
University of Rochester Medical Center, Golisano Children’s Hospital (U10 HD40521, M01 RR44, UL1 RR24160) – Ronnie Guillet, MD, PhD; Gary J. Myers, MD; Linda J. Reubens, RN CCRC; Erica Burnell, RN; Mary Rowan, RN; Diane Hust, MS RN CS; Rosemary L. Jensen; Kelly Yost, PhD; Lauren Zwetsch, RN MS PNP; Julie Babish Johnson, MSW; Emily Kushner, MA; Joan Merzbach, LMSW.
University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children’s Medical Center Dallas (U10 HD40689, M01 RR633) – Walid A. Salhab, MD; Pablo J. Sánchez, MD; Charles R. Rosenfeld, MD; Abbot R. Laptook, MD; Roy J. Heyne, MD; Sally S. Adams, MS RN CPNP; Alicia Guzman; Gaynelle Hensley, RN; Jackie F. Hickman, RN; Linda A. Madden, RN CPNP; Susie Madison, RN; Nancy A. Miller, RN; Janet S. Morgan, RN; Catherine Twell Boatman, MS CIMI.
University of Texas Health Science Center at Houston Medical School, Children’s Memorial Hermann Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District (U10 HD21373, KL2 RR24149, UL1 RR24148) – Kathleen A. Kennedy, MD MPH; Pamela J. Bradt, MD MPH; Patricia Evans, MD; Terri Major-Kincade, MD MPH; Brenda H. Morris, MD; Laura L. Whitely, MD; Nora I. Alaniz, BS; Esther G. Akpa, RN BSN; Patty A. Cluff, RN; Susan Dieterich, PhD; Anna E. Lis, RN, BSN; Georgia E. McDavid, RN; Stacey Reddoch, BA; Maegan C. Simmons, RN; Patti L. Tate, RCP; Sharon L. Wright, MT(ASCP).
Wake Forest University, Baptist Medical Center, Brenner Children’s Hospital, and Forsyth Medical Center (U10 HD40498, M01 RR7122) – T. Michael O’Shea, MD MPH; Lisa K. Washburn, MD; Robert G. Dillard, MD; Nancy J. Peters, RN CCRP; Barbara G. Jackson, RN BSN; Korinne Chiu, MA; Deborah Evans Allred, MA LPA; Donald J. Goldstein, PhD; Raquel Halfond, MA; Carroll Peterson, MA; Ellen L. Waldrep, MS; Melissa Whalen Morris, MA; Gail Wiley Hounshell, PhD.
Wayne State University, Hutzel Women’s Hospital and Children’s Hospital of Michigan (U10 HD21385) – Seetha Shankaran, MD; Yvette R. Johnson, MD MPH; Athina Pappas, MD; Rebecca Bara, RN BSN; Geraldine Muran, RN BSN; Deborah Kennedy, RN BSN; Laura A. Goldston, MA.
Yale University, Yale-New Haven Children’s Hospital and Bridgeport Hospital (U10 HD27871, UL1 RR24139, MO1 RR125, M01 RR6022) – Richard A. Ehrenkranz, MD; Patricia Gettner, RN; Harris C. Jacobs, MD; Christine Butler, MD; Patricia Cervone, RN; Monica Konstantino, RN BSN; Elaine Romano, MSN; JoAnn Poulsen, RN; Joanne Williams, RN; Sheila Greisman, RN.
References
- 1.Brown AK, Kim MH, Wu PYK, Bryla DA. Efficacy of phototherapy in prevention of neonatal hyperbilirubinemia. Pediatrics. 1985;75:393–400. [PubMed] [Google Scholar]
- 2.Lipsitz PJ, Gartner LM, Bryla DA. Neonatal and infant mortality in relation to phototherapy. Pediatrics. 1985;75(suppl):422–441. [PubMed] [Google Scholar]
- 3.Maisels MJ. In: Neonatal jaundice. Effective Care of the Newborn Infant. Sinclair JC, Bracken MB, editors. Oxford University Press; 1992. p. 532. [Google Scholar]
- 4.Benders MJNL, Van Bel F, Van de Bor M. Cardiac output and ductal reopening during phototherapy in preterm infants. Acta Paediatrica. 1999;88:1014–1019. doi: 10.1080/08035259950168540. [DOI] [PubMed] [Google Scholar]
- 5.Barefield ES, Dwyer MD, Cassady G. Associated of PDA and phototherapy in infants weighing less than 1000 grams. J Perinatol. 1993;XIII:376–380. [PubMed] [Google Scholar]
- 6.Scheidt PC, Bryla DA, Hoffman HJ. Phototherapy and PDA. Pediatrics. 1987;80:593–594. [PubMed] [Google Scholar]
- 7.Furchgott RF, Ehrreich SJ, Greenblatt E. The photoactivated relaxation of smooth muscle of rabbit aorta. J Gen Physiol. 1961;44:499–519. doi: 10.1085/jgp.44.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Travadi J, Simmer K, Ramsay J, Doherty D, Hagan R. Patent ductus arteriosus in extremely preterm infants receiving phototherapy: Does shielding the chest make a difference? A randomized controlled trial. Acta Paediatr. 2006;95:1418–1423. doi: 10.1080/08035250600771458. [DOI] [PubMed] [Google Scholar]
- 9.Tozzi E, Tozzi-Ciancarelli MG, DiGiula A, D’Alfonso A, Farello G, Sennati GF, et al. In vitro and in-vivo effects of erythrocyte phototherapy on newborns. Biol Neonate. 1989;56:204–209. doi: 10.1159/000243124. [DOI] [PubMed] [Google Scholar]
- 10.Tozzi-Ciancarelli MG, Amicosante G, Menichelli A, Di Giulio S, Del Principe D. Photodynamic damage induced by bilirubin on human platelets: possible relevance to newborn pathology. Biol Neonate. 1985;48:336–340. doi: 10.1159/000242190. [DOI] [PubMed] [Google Scholar]
- 11.Dani C, Cecchi A, Bertini G. Role of oxidative stress as physiopathologic factor in the preterm infant. Minerva Pediatr. 2004;56:381–94. [PubMed] [Google Scholar]
- 12.Saugstad OD. Bronchopulmonary dysplasia – oxidative stress and antioxidants. Semin Neonatol. 2003;8:39–49. doi: 10.1016/s1084-2756(02)00194-x. [DOI] [PubMed] [Google Scholar]
- 13.Van de Bor M, van Zeben-van der Aa TM, Verloove-Vanhorick SP, Brand R, Ruys JH. Hyperbilirubinemia in preterm infants and neurodevelopmental outcome at 2 years of age: results of a national collaborative survey. Pediatrics. 1989;83:915–920. [PubMed] [Google Scholar]
- 14.Hack M, Wilson-Costello D, Friedman H, Taylor GH, Schluchter M, Fanaroff AA. Neurodevelopment and predictors of outcomes of children with birth weights of less than 1000g. Arch Pediatr Adolesc Med. 2000;154:725–731. doi: 10.1001/archpedi.154.7.725. [DOI] [PubMed] [Google Scholar]
- 15.Oh W, Tyson JE, Fanaroff AA, Vohr BR, Perritt R, Stoll BJ, et al. Association between peak serum bilirubin and neurodevelopmental outcomes in extremely low birth weight infants. Pediatrics. 2003;112:773–779. doi: 10.1542/peds.112.4.773. [DOI] [PubMed] [Google Scholar]
- 16.Oh W, Stevenson SK, Tyson JE, Morris BH, Ahlfors CE, Bender GJ, et al. Influence of clinical status on the association between plasma total and unbound bilirubin and death or adverse neurodevelopmental outcomes in extremely low birth weight infants. Acta Paediatrica. 2010;99:673–678. doi: 10.1111/j.1651-2227.2010.01688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris BH, Oh W, Tyson TE, Stevenson DK, Phelps DL, O’Shea TM, et al. Aggressive vs. conservative phototherapy for infants with extremely low birth weight. N Engl J Med. 2008;359:1885–1896. doi: 10.1056/NEJMoa0803024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al. Trends in neonatal morbidity and mortality for very low birth weight infants. AJOG. 2007;196:147.e1–147.e8. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Hintz SR, Poole WK, Fanaroff AA, Kendrick DE, Laptook AR, Goldberg R, et al. Changes in mortality and morbidity among infants born at less than 25 weeks during the post-surfactant era. Arch Dis Child Fetal Neonatal Ed. 2005;90:F128–F133. doi: 10.1136/adc.2003.046268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vohr BR, Wright LL, Poole SK, McDonald SA for the NICHD Neonatal Research Network. Neurodevelopmental outcomes of extremely low birth weight infants <32 weeks’ gestational between 1993–1998. Pediatrics. 2005;116:635–643. doi: 10.1542/peds.2004-2247. [DOI] [PubMed] [Google Scholar]
- 21.Bayley N. Bayley Scales of Infant Development II. San Antonio, TX: Psychological Corp; 1993. [Google Scholar]
- 22.Vreman HJ, Knauer Y, Chan ML, Wong RJ, Stevenson DK. Dermal carbon monoxide (CO) excretion in neonatal rats during light exposure. Pediatr Res. 2009;66:66–69. doi: 10.1203/PDR.0b013e3181a7be77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vreman HJ, Wong RJ, Stevenson DK. Phototherapy: current methods and future directions. Semin Perinatol. 2004;28:326–333. doi: 10.1053/j.semperi.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 24.McDonagh AF, Vreman HJ, Wong RJ, Stevenson DK. Photoisomers: Obfuscating factors in clinical peroxidase measurements of unbound bilirubin? Pediatrics. 2009;123:67–76. doi: 10.1542/peds.2008-0492. [DOI] [PubMed] [Google Scholar]
- 25.Ahlfors CE, Amin SB, Parker AE. Unbound bilirubin predicts abnormal automated auditory brainstem response in a diverse newborn population. J Perinatol. 2009;29:305–309. doi: 10.1038/jp.2008.199. [DOI] [PMC free article] [PubMed] [Google Scholar]