Abstract
MRI potentially offers an alternative to CT, and thus is central to an ALARA strategy. However, long exam times, limited magnet availability, and motion artifacts are barriers to expanded use of MRI. This article reviews developments in pediatric body MRI that may reduce these barriers: high field systems, acceleration, navigation, and newer contrast agents.
The Paradox
In the setting of pediatric body applications, MRI is both scarce and underutilized. This is particularly true for younger patients who conventionally require anesthesia. The task of obtaining an anesthesia slot (a scarce resource as well) that coincides with free magnet time can be quite daunting. As a result, although MRI can offer excellent spatial resolution and contrast, even an MRI enthusiast often shunts patients to CT, despite its ionizing radiation.
Ironically, MRI scanners often sit idle for significant lengths of time between cases. This is a result of the inherent turn-around time for anesthesia. In addition, the length of time the scanners sit idle is somewhat unpredictable. Utilization of this down-time is challenging because often sequences in MRI have to be repeated due to artifacts. Thus, pediatric MRI is paradoxically both an underutilized and scarce resource. However, a number of recent developments offer hope that these challenges can be addressed. This article highlights some of these developments.
Hardware
Higher field strength often yields higher signal to noise-ratio. Thus 3T potentially offers two-fold SNR increase over 1.5T, [1] though this is somewhat reduced by longer T1 relaxation times. [2, 3] Although this SNR gain is valuable in adult imaging, the impact is stronger on pediatrics. For a child being imaged without anesthesia, the signal gain may be expended for faster imaging: 4-fold faster imaging may be obtained with the same SNR. Thus, a 28 second breath-hold for abdominal imaging becomes 7 seconds, which is much more reliably obtained in a child. Alternatively, for the sedated child, who is often smaller, the signal can be used to obtain diagnostic images at higher resolution. The conventional wisdom has been that 3T will play a limited role in abdominal imaging. This largely stems from the adult imaging community, where the larger size of patients results in significant dielectric shading problems. However, for most children, dielectric shading issues are reduced.
Along with higher field strength, a new generation of phased-array receive coils has entered the market. Whereas prior generations of coils for body imaging typically had 8 elements, 32-channel systems are now available. [4–6] With a few exceptions [7], the current geometries of these coils are not optimal for children, as the individual coil elements are too large to provide adequate coil sensitivity variation over the smaller patient. Nonetheless, the relatively reduced element sizes of high channel coils provide a further enhancement of SNR. In fact, this SNR enhancement can equal or exceed that of transitioning to 3T [8, 9]. Alternatively, as seen below, the increased number of coils permits a degree of accelerated imaging.
Parallel Imaging and Beyond
Parallel imaging has become a mainstream component of pediatric body MRI exams and has been covered extensively in other articles[10]. Further, recent efforts have been devoted to combining parallel imaging with other undersampling strategies for even higher accelerations. These strategies include radial undersampling and model-based iterative image reconstruction, such as compressed sensing [11, 12]. Here, we will focus on three applications that have the strongest potential to reduce anesthesia need, depth, and duration.
Contrast-enhanced dynamic imaging
Cardiovascular, abdominal, and pelvic imaging protocols routinely incorporate dynamic contrast enhancement sequences. These sequences must contend with fast circulatory dynamics in children and limited breathholding capability; thus imaging speed is vital. [10, 13] Parallel imaging can largely address the encoding limitation of MRI, i.e. adequate sampling of k-space in a short time. However, parallel imaging does not address the compromised SNR of a short scan; this is best addressed by combining it with higher field strength and high-density receive coils. For the unsedated child, a volumetric acquisition using this approach can be obtained at high resolution in under 10 seconds.
Volumetric (3D) T2-weighted imaging
Conventional T2-weighted imaging employs fast spin echo techniques and requires a lengthy repetition interval for T1 relaxation, so a 3D T2-weighted acquisition is impractically long. However, a very long echo train can be obtained by slowly increasing the refocusing flip angle to counters the effects of T2 relaxation. [14–18] At the same time, the scan time of such a technique, despite the longer echo train, is still prohibitive. With parallel imaging however, scan times for the abdomen and pelvis can be under 5 minutes. Additionally, images can be obtained with near isotropic voxel size, permitting reformatting of image data set in arbitrary planes and 3D reconstructions. This approach works particularly well for the sedated child and may reduce the number of sequences in a protocol.
Single shot imaging
The applicability of single shot imaging with T2-weighting (SSFSE, HASTE) to various abdominal and pelvic applications has been debated. For the unsedated child, the speed of these sequences and their robustness to motion are appealing. On the side of clear value is cholangiography and bowel imaging. The more debatable applications include oncologic and gynecologic. The questionable wisdom of substituting a single shot approach to T2 weighted imaging for conventional FSE sequences stems from several factors: (1) reduced SNR, (2) blurring due to T2 decay over the long echo train, and (3) image contrast that may be different from conventional T2. For cholangiography and bowel imaging, the long T2 of bile/bowel lumen mitigates these issues.
For evaluation of solid organs in the abdomen and pelvis, all of these issues may be addressed by the combination of 3T, a good coil, and parallel imaging. In my personal experience, adequate SNR can certainly be obtained at 3T with a 32 channel coil. Further, these coils typically have two plates, anterior and posterior, and each plate consists of roughly a 4x4 array. Thus, for coronal imaging, four-fold acceleration can be obtained and the resulting echo train length is comparable to conventional FSE T2 imaging.
Navigation
Besides shortening breath-holds with sheer imaging speed, body MRI exams may be enabled in partially cooperative patients by using navigation. Navigators are additional echoes that measure displacements and either reject or correct data based on those displacements, retrospectively or prospectively. [19, 20] These approaches are most widespread for handling respiration in cardiovascular MRI. [21] In the abdomen, navigators are most used with hepatobiliary examinations, again for mitigating respiratory motion. [22–25] While mostly used for T2-weighted imaging, T1-weighted imaging can be navigated so long as the navigator flip angle is kept to a minimum to avoid saturation artifacts. [26
CONTRAST AGENTS
Recently, two classes of MRI contrast agents have become available that potentially offer some unique advantages for pediatric body imaging, though they are approved by the FDA for adult imaging indications. One is gadoxetate disodium (Eovist), which has approximately 50% biliary excretion and has a hepatocyte parenchymal phase of contrast enhancement that begins about 15 minutes after administration and lasts for approximately an hour [27, 28] The other agent is gadofosveset trisodium (Ablavar), which lasts in the blood pool for considerably longer than conventional extracellular agents. [29–35] By offering a longer window of imaging time, these agents enable two approaches of particular value in pediatrics: (1) repeat scanning in the event of lack of cooperation with a breath-hold, and/or (2) respiratory triggered or navigated free-breathing imaging.
Summary and Synergies
The developments described here synergize to broaden the applicability of MRI to pediatric body imaging. Examples are shown in Figures 1–4. A combination of high field strength, high-density receive coils, advanced image reconstruction methods, and new intravenous contrast agents will permit more children to be scanned faster and with reduced use and depth of anesthesia. This will hopefully result in diminished radiation burden from CT.
Figure 1.
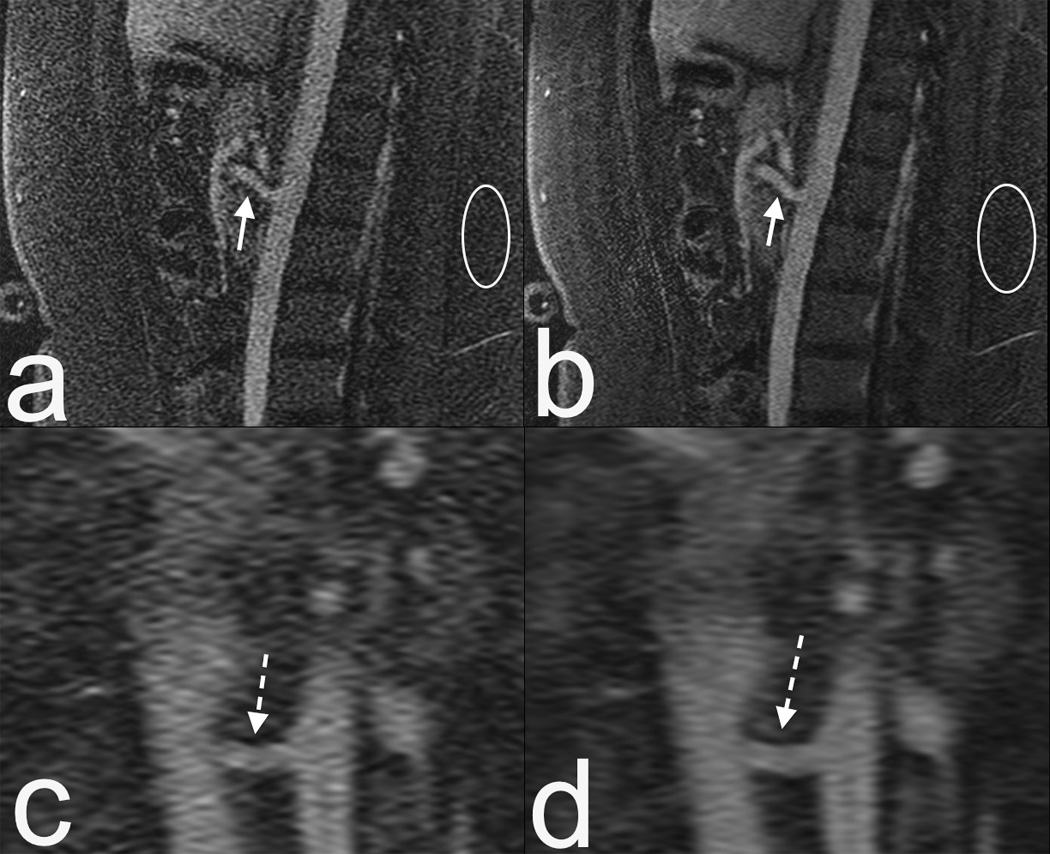
16 year old female MRI at 1.5T with an 8 channel coil. (a) Sagittal contrast enhanced MRA. Note the limited SNR, particularly at the superior mesenteric artery (SMA, arrow) and noise in the subcutaneous fat (circle). (b) Compressed sensing reconstruction of same data as in (a) results in enhanced delineation of the SMA. Noise is reduced, but to a lesser degree in regions that are at the boundaries of coil sensitivities (circle). (c and d) Zoomed coronal reformats of of (a) and (b) respectively, showing enhanced delineation of the left renal artery (dashed arrow). This highlights the potential of advanced reconstruction methods.
Figure 4.
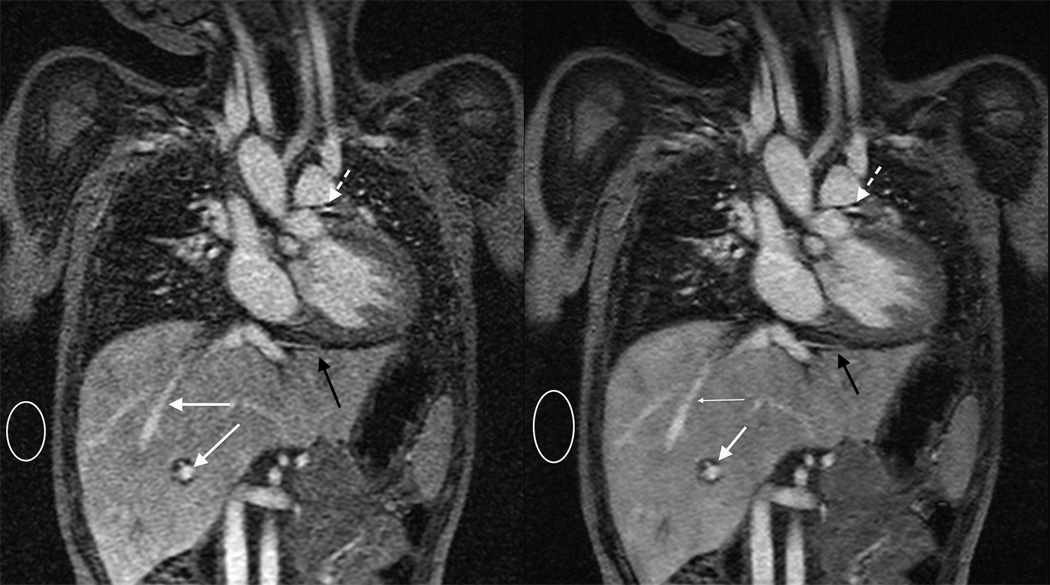
Blood pool agent free-breathing MRA in a 4 year old. The scan is obtained with a combined respiratory and cardiac triggering technique. Because of the combined triggering the scan takes five minutes, and thus requires a blood pool agent for optimal enhancement. (a) Traditional parallel imaging reconstruction highlights lack of respiratory motion at the diaphragm (black arrow) and cardiac motion at the left coronary (dashed arrow). Note enhancement of the entire blood pool, including portal and hepatic veins (white arrows). (b) Iterative reconstruction reduces the noise in the image, best seen by comparing the region in the white circles.
Figure 2.
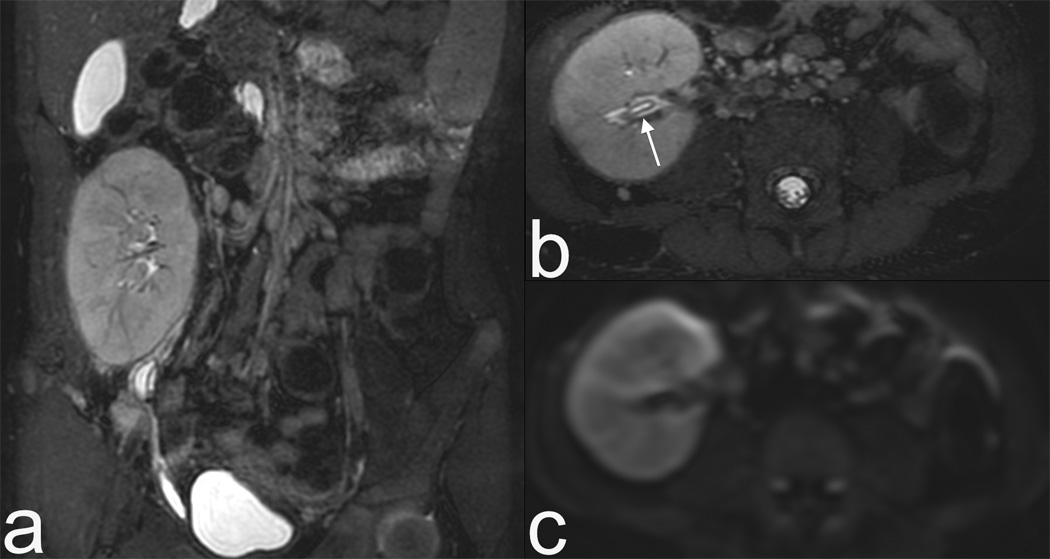
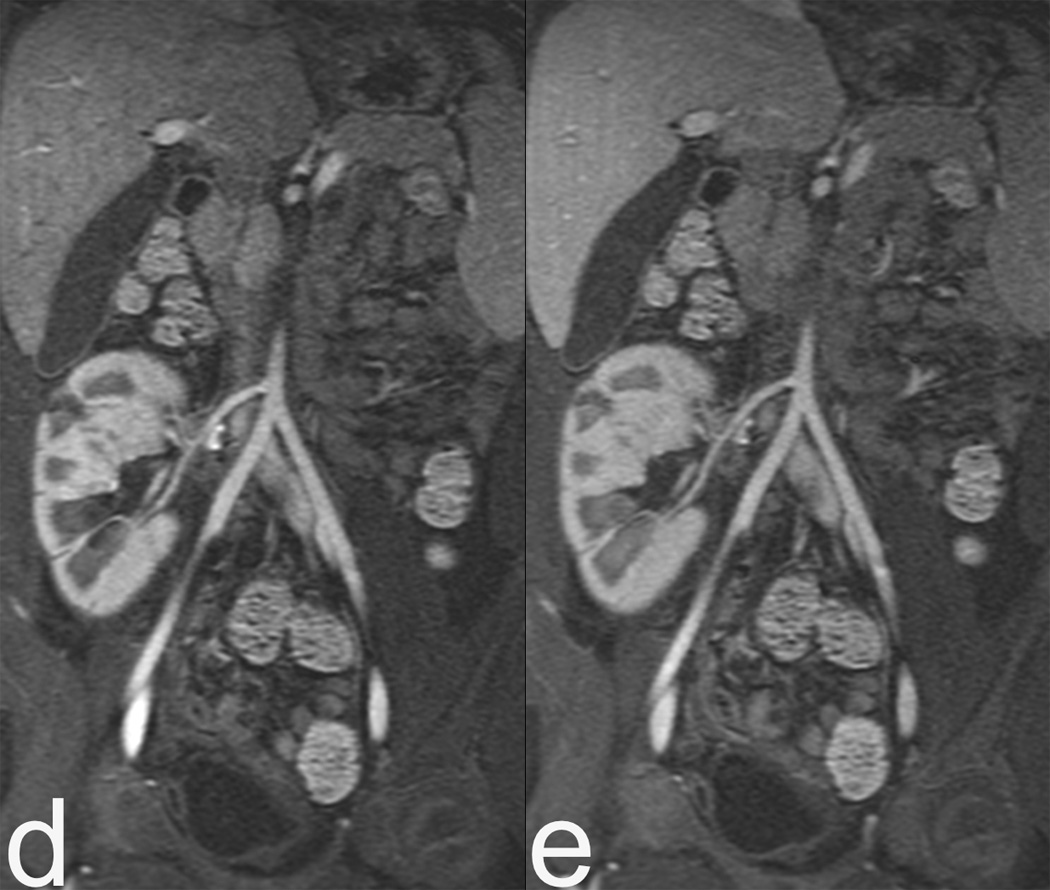
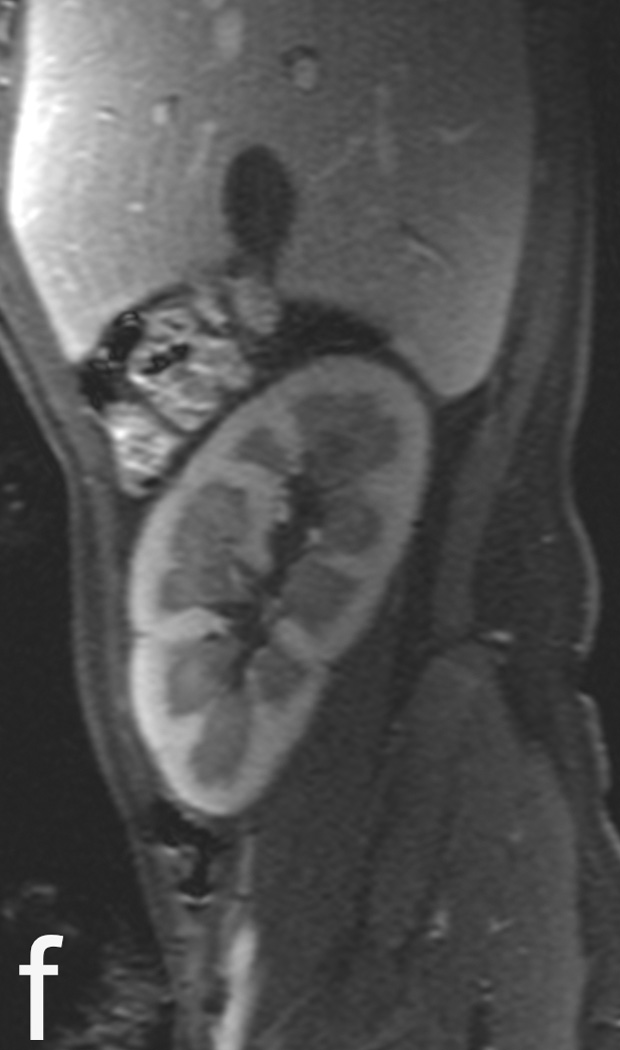
3T images with a 32 channel coil on a 5 year old female with a renal transplant. Rapid MRI protocol consisting of volumetric T2 (a) and its axial reformat (b). Note urothelial thickening (arrow), which can be seen in rejection, is well demonstrated even on reformats. (c) Diffusion weighted imaging is fast (1 minute) and may be relatively motion robust as it is a single shot EPI technique. Finally contrast enhanced images in early venous (d) and late venous (e) phase show high resolution despite 13 second scan time. The resolution rivals CT, as seen by zoomed sagittal reformat (f).
Figure 3.
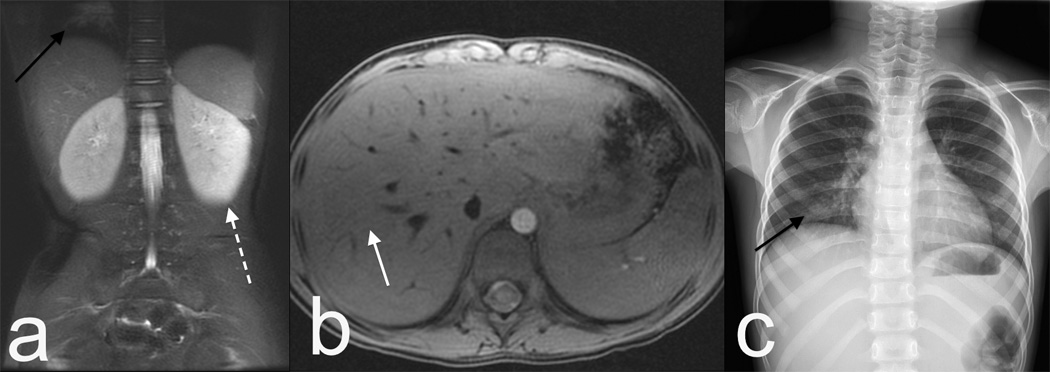
3T images with 32 channel custom coil sized to this 5 year old child with a fever and abdominal pain. (a) Single shot technique with acceleration of 4 shows little blurring due to the shortened echo train. Although fluid sensitive and with some T2 weighting, the contrast is not identical to conventional FSE, as seen by lack of corticomedullary differentiation in the kidneys (dashed arrow). Note pneumonia that was the source of fever (black arrow). (b) Axial T1 volumetric imaging with navigation and without intravenous contrast shows minimal respiratory artifact (white arrow) despite free breathing. Pneumonia was confirmed on a chest x-ray (c). The MRI protocol consisted of a localizer and two sequences, and was completed in under ten minutes without anesthesia or sedation. Traditionally fever and abdominal pain would trigger a CT scan.
REFERENCES
- 1.Edelstein WA, Glover GH, Hardy CJ, et al. The intrinsic signal-to-noise ratio in NMR imaging. Magn Reson Med. 1986;3:604–618. doi: 10.1002/mrm.1910030413. [DOI] [PubMed] [Google Scholar]
- 2.Gold GE, Han E, Stainsby J, et al. Musculoskeletal MRI at 3.0 T: relaxation times and image contrast. AJR Am J Roentgenol. 2004;183:343–351. doi: 10.2214/ajr.183.2.1830343. [DOI] [PubMed] [Google Scholar]
- 3.Schindera ST, Merkle EM, Dale BM, et al. Abdominal magnetic resonance imaging at 3.0 T what is the ultimate gain in signal-to-noise ratio? Acad Radiol. 2006;13:1236–1243. doi: 10.1016/j.acra.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Hardy CJ, Darrow RD, Saranathan M, et al. Large field-of-view real-time MRI with a 32-channel system. Magn Reson Med. 2004;52:878–884. doi: 10.1002/mrm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soher BJ, Dale BM, Merkle EM. A review of MR physics. 3T versus 1.5T. Magn Reson Imaging Clin N Am. 2007;15:277–290. doi: 10.1016/j.mric.2007.06.002. v. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y, Hardy CJ, Sodickson DK, et al. Highly parallel volumetric imaging with a 32-element RF coil array. Magn Reson Med. 2004;52:869–877. doi: 10.1002/mrm.20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erberich SG, Friedlich P, Seri I, et al. Functional MRI in neonates using neonatal head coil and MR compatible incubator. Neuroimage. 2003;20:683–692. doi: 10.1016/S1053-8119(03)00370-7. [DOI] [PubMed] [Google Scholar]
- 8.Bolog N, Nanz D, Weishaupt D. Muskuloskeletal MR imaging at 3.0 T: current status and future perspectives. Eur Radiol. 2006;16:1298–1307. doi: 10.1007/s00330-006-0184-7. [DOI] [PubMed] [Google Scholar]
- 9.O'Regan DP, Fitzgerald J, Allsop J, et al. A comparison of MR cholangiopancreatography at 1.5 and 3.0 Tesla. Br J Radiol. 2005;78:894–898. doi: 10.1259/bjr/28094700. [DOI] [PubMed] [Google Scholar]
- 10.Chung T, Muthupillai R. Application of SENSE in clinical pediatric body MR imaging. Top Magn Reson Imaging. 2004;15:187–196. doi: 10.1097/01.rmr.0000135285.76847.ab. [DOI] [PubMed] [Google Scholar]
- 11.Vasanawala SS, Alley MT, Hargreaves BA, et al. Improved pediatric MR imaging with compressed sensing. Radiology. 2010;256:607–616. doi: 10.1148/radiol.10091218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58:1182–1195. doi: 10.1002/mrm.21391. [DOI] [PubMed] [Google Scholar]
- 13.White LM, Buckwalter KA. Technical considerations: CT and MR imaging in the postoperative orthopedic patient. Semin Musculoskelet Radiol. 2002;6:5–17. doi: 10.1055/s-2002-23160. [DOI] [PubMed] [Google Scholar]
- 14.Busse RF, Hariharan H, Vu A, et al. Fast spin echo sequences with very long echo trains: design of variable refocusing flip angle schedules and generation of clinical T2 contrast. Magn Reson Med. 2006;55:1030–1037. doi: 10.1002/mrm.20863. [DOI] [PubMed] [Google Scholar]
- 15.Lebel RM, Wilman AH. Intuitive design guidelines for fast spin echo imaging with variable flip angle echo trains. Magn Reson Med. 2007;57:972–975. doi: 10.1002/mrm.21220. [DOI] [PubMed] [Google Scholar]
- 16.Hennig J, Weigel M, Scheffler K. Multiecho sequences with variable refocusing flip angles: optimization of signal behavior using smooth transitions between pseudo steady states (TRAPS) Magn Reson Med. 2003;49:527–535. doi: 10.1002/mrm.10391. [DOI] [PubMed] [Google Scholar]
- 17.Alsop DC. The sensitivity of low flip angle RARE imaging. Magn Reson Med. 1997;37:176–184. doi: 10.1002/mrm.1910370206. [DOI] [PubMed] [Google Scholar]
- 18.Mugler JP, Kiefer B, Brookeman JR. Three-Dimensional T2-Weighted IMaging of the Brain Using Very Long Spin-Echo Trains. International Society of Magnetic Resonance in Medicine 8th Meeting; Denver. 2000. p. 687. [Google Scholar]
- 19.Ehman RL, Felmlee JP. Adaptive technique for high-definition MR imaging of moving structures. Radiology. 1989;173:255–263. doi: 10.1148/radiology.173.1.2781017. [DOI] [PubMed] [Google Scholar]
- 20.Sachs TS, Meyer CH, Hu BS, et al. Real-time motion detection in spiral MRI using navigators. Magn Reson Med. 1994;32:639–645. doi: 10.1002/mrm.1910320513. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Rossman PJ, Grimm RC, et al. Navigator-echo-based real-time respiratory gating and triggering for reduction of respiration effects in three-dimensional coronary MR angiography. Radiology. 1996;198:55–60. doi: 10.1148/radiology.198.1.8539406. [DOI] [PubMed] [Google Scholar]
- 22.Huang J, Raman SS, Vuong N, et al. Utility of breath-hold fast-recovery fast spin-echo t2 versus respiratory-triggered fast spin-echo T2 in clinical hepatic imaging. AJR Am J Roentgenol. 2005;184:842–846. doi: 10.2214/ajr.184.3.01840842. [DOI] [PubMed] [Google Scholar]
- 23.Kim BS, Kim JH, Choi GM, et al. Comparison of three free-breathing T2-weighted MRI sequences in the evaluation of focal liver lesions. AJR Am J Roentgenol. 2008;190:W19–W27. doi: 10.2214/AJR.07.2043. [DOI] [PubMed] [Google Scholar]
- 24.Klessen C, Asbach P, Kroencke TJ, et al. Magnetic resonance imaging of the upper abdomen using a free-breathing T2-weighted turbo spin echo sequence with navigator triggered prospective acquisition correction. J Magn Reson Imaging. 2005;21:576–582. doi: 10.1002/jmri.20293. [DOI] [PubMed] [Google Scholar]
- 25.Lee SS, Byun JH, Hong HS, et al. Image quality and focal lesion detection on T2-weighted MR imaging of the liver: comparison of two high-resolution free-breathing imaging techniques with two breath-hold imaging techniques. J Magn Reson Imaging. 2007;26:323–330. doi: 10.1002/jmri.21002. [DOI] [PubMed] [Google Scholar]
- 26.Vasanawala SS, Iwadate Y, Church DG, et al. Navigated abdominal T1-W MRI permits free-breathing image acquisition with less motion artifact. Pediatr Radiol. 2010;40:340–344. doi: 10.1007/s00247-009-1502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bluemke DA, Sahani D, Amendola M, et al. Efficacy and safety of MR imaging with liver-specific contrast agent: US multicenter phase III study. Radiology. 2005;237:89–98. doi: 10.1148/radiol.2371031842. [DOI] [PubMed] [Google Scholar]
- 28.Jung G, Breuer J, Poll LW, et al. Imaging characteristics of hepatocellular carcinoma using the hepatobiliary contrast agent Gd-EOB-DTPA. Acta Radiol. 2006;47:15–23. doi: 10.1080/02841850500406795. [DOI] [PubMed] [Google Scholar]
- 29.Grist TM, Korosec FR, Peters DC, et al. Steady-state and dynamic MR angiography with MS 325: initial experience in humans. Radiology. 1998;207:539–544. doi: 10.1148/radiology.207.2.9577507. [DOI] [PubMed] [Google Scholar]
- 30.Lauffer RB, Parmelee DJ, Ouellet HS, et al. MS-325: a small-molecule vascular imaging agent for magnetic resonance imaging. Acad Radiol. 1996;3(Suppl 2):S356–S358. doi: 10.1016/s1076-6332(96)80583-6. [DOI] [PubMed] [Google Scholar]
- 31.Lin W, Abendschein DR, Haacke EM. Contrast-enhanced magnetic resonance angiography of carotid arterial wall in pigs. J Magn Reson Imaging. 1997;7:183–190. doi: 10.1002/jmri.1880070128. [DOI] [PubMed] [Google Scholar]
- 32.Prompona M, Cyran C, Nikolaou K, et al. Contrast-enhanced whole-heart MR coronary angiography at 3.0 T using the intravascular contrast agent gadofosveset. Invest Radiol. 2009;44:369–374. doi: 10.1097/rli.0b013e3181a40d1d. [DOI] [PubMed] [Google Scholar]
- 33.Stuber M, Botnar RM, Danias PG, et al. Contrast agent-enhanced, free-breathing, three-dimensional coronary magnetic resonance angiography. J Magn Reson Imaging. 1999;10:790–799. doi: 10.1002/(sici)1522-2586(199911)10:5<790::aid-jmri25>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 34.Wagner M, Rief M, Asbach P, et al. Gadofosveset trisodium-enhanced magnetic resonance angiography of the left atrium--a feasibility study. Eur J Radiol. 2010;75:166–172. doi: 10.1016/j.ejrad.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 35.Gadofosveset: MS 325, MS 32520, Vasovist, ZK 236018. Drugs R D. 2004;5:339–342. doi: 10.2165/00126839-200405060-00005. [DOI] [PubMed] [Google Scholar]


