Abstract
Sleep apnea among commercial drivers may increase the risk of fall-asleep crashes, which incur large expenses. Drivers of passenger cars whose apnea is treated experience lower crash risk. Among community-based holders of commercial driver’s licenses, we considered three methods for identifying sleep apnea syndrome: 1) in-lab polysomnography; 2) selective in-lab polysomnography for high-risk drivers, where high risk is first identified by body mass index, age and gender, followed by oximetry in a subset of drivers; and 3) not screening. The costs for each of these three programs equaled the sum of the costs of testing, treatment of identified cases, and crashes. Assuming that treatment prevents apnea-related crashes, polysomnography is not cost-effective, because it was more expensive than the cost of crashes when no screening is done. Screening with BMI, age and gender, however, with confirmatory in-lab polysomnography only on high-risk drivers was cost-effective, as long as a high proportion (73.8%) of screened drivers accepts treatment. These findings indicate that strategies that reduce reliance on in-laboratory polysomnography may be more cost-effective than not screening, and that treatment acceptance may need to be a condition of employment for affected drivers.
Keywords: Polysomnography, nocturnal pulse oximetry, questionnaire, economics, cost analysis
1. Introduction
An appropriate target population for screening for obstructive sleep apnea (OSA)(1) is that of commercial drivers. Risk factors for OSA are enriched in this usually male, middle-aged and obese group(2). Indeed, OSA is highly prevalent among commercial drivers(1, 3, 4). OSA is associated with daytime sleepiness and, as data from Wisconsin state employees show, impaired psychomotor performance(5). This association should be highlighted, as it may predispose affected drivers to experiencing occupational crashes. Data collected from passenger car drivers show that OSA increases vehicular crash rates (6–8) and off-road deviations in a driving simulator (9). Driving performance (9) and crash risk (10–12) may improve if affected drivers are identified and treated. These data signal a need for developing screening strategies that find OSA among commercial drivers and assessments to determine whether such strategies are cost-effective.
1.1 Diagnosing apnea
The standard diagnostic test for OSA is in-laboratory polysomnography, despite its high expense and relative inaccessibility (13, 14). We proposed using the alternative of “selective” polysomnography in the highest-risk group of commercial drivers(1) by first identifying drivers most likely to have apnea with questionnaire and oximetry(15). The questionnaire we used, the multivariable apnea prediction(16, 17), predicts likelihood of apnea by combining three symptom-frequency questions with body mass index (BMI), age and gender. BMI is a proxy variable for obesity. Oximetry evaluates desaturations during sleep, and can be a sensitive measure of sleep-disordered breathing(18–20).
In our “two-stage” strategy(1, 21), the multivariable apnea score classified subjects’ risk for OSA as high, low or intermediate. Oximetry was a second-stage test for the subgroup predicted by the questionnaire to be at intermediate risk. Confirmatory sleep studies would be administered selectively to those with high multivariable scores or those with positive oximetry studies. We simulated use of this two-stage strategy in a sleep-disorders clinic with a high prevalence of OSA(21), and a community-based sample of commercial drivers(1), who may experience increased risk of vehicular accidents due to untreated OSA.
1.2 Apnea screening, treatment and crashes: economic implications
Treatment of OSA may lower crash risk(12), and thereby lower the cost of crashes. However, even screened drivers may experience “residual” crashes if the program misses cases, or if identified cases do not accept treatment. Employers may bear high costs associated with such crashes(22). A decision to screen based on economics should thus balance expenditures incurred by screening, treatment and residual crashes against the cost of crashes when screening is not done. Such an analysis can help specify the proportion of drivers who must accept treatment in order to offset the costs of the program. If this number is high, then treatment acceptance may need to be a condition for employment of affected drivers.
1.3 Focus of the current study
We now focus on two questions: 1) Is screening cost-effective if we use a) in-laboratory polysomnography on all drivers, or b) selective polysomnography? 2) What is the minimum rate of treatment acceptance needed for each screening program to be cost-effective? For selective polysomnography, we used a modified two-stage strategy without apnea symptom-reporting, since such reporting may be inaccurate in the occupational setting (N. Hartenbaum, personal communication), and also evaluated an alternative, “one-stage” strategy which did not require oximetry. We chose the cost perspective of the employer, who would bear expenses related to screening, treatment or crashes.
2. Methods
All participants provided signed informed consent. The study was approved by the University of Pennsylvania’s Institutional Review Board.
2.1 Subject selection
During 1996–1998 we mailed the multivariable prediction questionnaire to commercial driver’s license-holders in Philadelphia. From 1,392/4,410 (32%) respondents, we recruited 247 drivers at higher-risk (defined by multivariable prediction≥0.436), then 159 in random order from among those at lower-risk for OSA. We have described this recruitment strategy previously (1, 23). This two-tiered sample (24, 25) allowed estimation of the proportion with sleep-disordered breathing of various severities(1) in our large community-based study of OSA in commercial drivers.
2.2 Definition of OSA syndrome
An apnea-hypopnea index (AHI) ≥5/h with sleepiness (Epworth Sleepiness Scale score>10) defined OSA syndrome (OSAS)(24, 26).
2.3 Diagnostic testing
2.3.1 Modified multivariable apnea prediction without symptoms
We used laboratory-assessed BMI, age and gender to compute the multivariable prediction, which ranges between 0–1 and quantifies relative risk for OSAS. Symptom information, traditionally part of this calculation(16), was excluded here because of possible under-reporting in the occupational setting. The formula we used was as follows. The probability that a driver will have AHI≥10 events/h is:
Probability = ex/(1 + ex), where x = −10.784 + (0.203 * BMI) + (0.043 * AGE) + (1.004 * GENDER), where GENDER = 1 if male and 0 if female, AGE is age in years and BMI is body mass index in kg/m2. We called this new tool the “modified multivariable apnea prediction,” and it predicts a high probability of OSA when it is close to 1, and low probability when it is close to 0.
2.3.2 Polysomnography and Oximetry
We monitored electro-encephalograms, eye, chin and pre-tibial myography, electrocardiography, oximetry, respiratory effort and airflow by thermistor. A trained technician scored data using standard methods (27, 28), while blinded to questionnaire data. The AHI was the number of apneas plus hypopneas divided by hours of sleep time(28). The Ohmeda-3700 (Ohmeda Inc., Louisville, CO) or N-200 oximeter (Nellcor Inc., Pleasanton, CA) recorded continuous finger oximetry during polysomnography. The oximetry desaturation index (ODI) was the number of desaturations ≥3% divided by hours of test time(1). Scorers were blinded to questionnaire and polysomnography data.
2.3.3 Selective polysomnography
2.3.3.1 Two-stage (Figure 1)
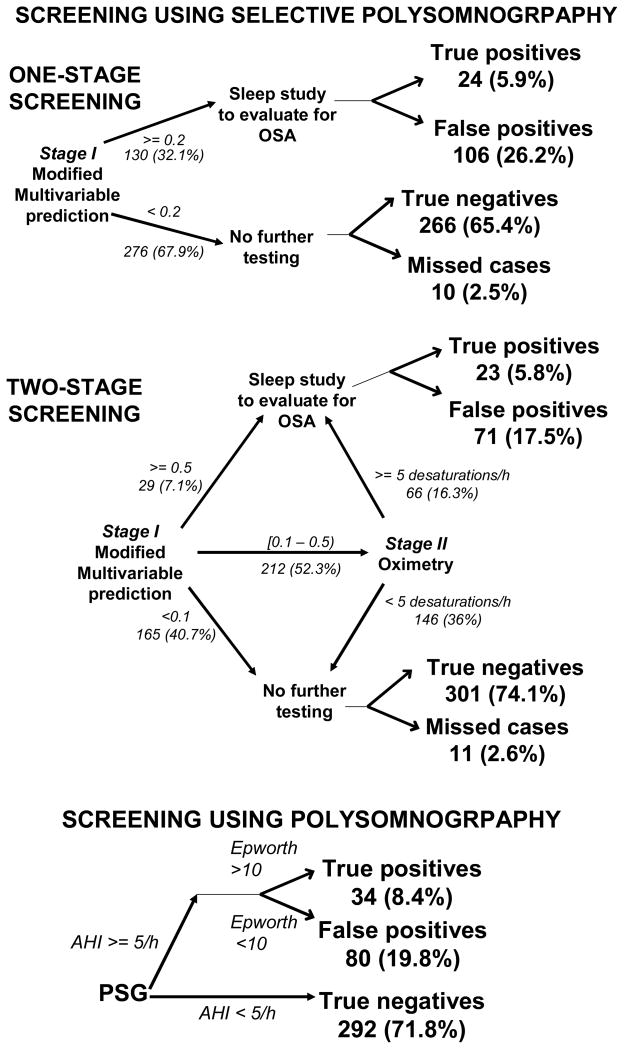
Figure 1.
Figure 1 displays three screening options. The first two rely on selective polysomnography: one-stage screening, which uses the modified multivariable apnea prediction or two-stage screening, which uses both modified multivariable prediction and oximetry. The third is routine polysomnography on everyone. Selective polysomnography results in true or false positives, and true negatives or missed cases, while routine polysomnography misses no cases. The total numbers of drivers that are in each arm of the program are given, with percentages in parentheses. The branch points are defined by using the optimal parameters, which were determined as described in the Methods section 2.4.2. Cases can be missed in two situations: when the modified multivariable prediction is low and no further testing is done, or if an intermediate modified multivariable prediction results in an oximetry score below the threshold. In either situation, a missed case occurs if the AHI is ≥5 events/h and Epworth score is >10.
We sorted drivers into three risk groups based on scores from the modified multivariable prediction(1, 21). The “upper bound” score separated the high from the intermediate risk group. “Lower bound” separated intermediate from low risk. Individuals in intermediate-risk group had oximetry evaluation; if ODI ≥another variable (“ODIthreshold”), they were predicted to have OSAS. Those with low modified multivariable prediction, or with intermediate modified multivariable prediction and ODI<ODIthreshold were predicted to have no OSAS. All subjects had review of their polysomnogram and Epworth score to ascertain the presence or absence of OSAS. In order to improve the accuracy of our prediction rules for future application in occupational settings, we did not use self-reported sleepiness (Epworth score>10) in our prediction rules for OSAS. This is because accuracy of reporting might be incomplete in an occupational setting. Sleepiness, however, was common in our study population (32.6% had Epworth score>10), where information was kept confidential and therefore sleepiness was part of our case definition.
2.3.3.2 One-stage (Figure 1)
Subjects with modified multivariable predictions exceeding a predefined cut-point had review of polysomnography and Epworth score to ascertain the diagnosis of OSAS. The remainder had no further testing.
2.4 Testing-related expenses
2.4.1 Polysomnography
We reviewed expenses from 3,500 hospital-based, 16-channel sleep studies performed in the Delaware Valley. The average cost of polysomnography, $768/study, included payroll (technical, administrative and physician), supplies, equipment, services and facility rental.
2.4.2 Selective polysomnography (one- or two-stage)
Custom programming was developed using SAS (SAS Systems, Cary, NC) to implement the various algorithms. We considered 125 combinations (“parameter sets”) of upper bound, lower bound and ODIthreshold: we varied upper bound from 0.5–0.9 and lower bound from 0.1–0.5 both in 0.1-unit increments, and ODIthreshold from 5–25/h, in 5/h-increments. For each parameter set, we computed the 125 costs of two-stage screening (SAS Systems, Cary, NC). Each was a sum of the costs of modified multivariable prediction, oximetry (for those with intermediate predictions during two-stage testing), polysomnography (for those predicted to have OSAS) and the cost of missed OSAS, which we treated as a variable from $0–$200,000/case. We estimated oximetry-related expenses at $160/study using the same data we used for estimating the cost of polysomnography. Given the ease of administering the modified multivariable instrument, interpreting results and relaying them to the employer, the estimated expense was only $5.
The cost of two-stage screening was selected from these 125 costs. It was associated with the parameter set that minimized misclassification rate (sum of false positive plus false negative predictions). Because missing a case could lead to a crash, we counted each false negative prediction at 10 times the value of a false positive prediction (i.e., we weighted the ratio of FP: FN at 1:10). We have published this method and its rationale previously (1). We computed the cost of one-stage screening similarly, by selecting the cost associated with one of 9 modified multivariable prediction cut-points that minimized the sum of false positive plus 10 times false negative diagnoses. These cut-points ranged from 0.1–0.9, in 0.1-unit increments.
2.5 Expenses of OSAS-attributable crashes
In one analysis we treated the cost of a missed case as a variable. In a second analysis, we fixed the value at the cost of a crash attributable to OSAS at $8,200/driver/crash based on computations using federal data for large trucks(29), while assuming the odds ratio for a crash given OSAS equaled 2.5(26).
To ground our analyses in actual cost of crashes, we determined the cost of a crash attributable to OSAS based on current data. Of 11 million commercial drivers’ license holders(31), 3 million are employed as drivers(32). In our cohort, 8.7% had OSAS (AHI≥5/h plus Epworth score>10). Thus, (0.087)*(3 million) =~261,000 drivers with OSAS are at risk for fall-asleep crashes. Large trucks are involved in approximately 415,000 accidents yearly. Thus, average annual risk of accidents per truck driver is approximately 13.8% (=415,000/3,000,000). Each accident costs $59,000(22). This value includes the costs of medical treatment, emergency services, property damage, lost productivity, and a monetary value of the pain, suffering, and reduced quality of life experienced by the victim and his/her family. A recent meta-analysis using passenger car data indicates that OSAS is associated with an odds ratio (OR) for traffic accidents of 2.5 (26). We used this estimate in our base calculations.
For calculation of the cost of a crash attributable to OSA, we used the following approach. The overall risk for accidents (13.8%) is a weighted average of the elevated risk for traffic accidents among those with OSA and a background risk for traffic accidents in those without OSA. We do not know this background risk. We can, however, apply the average risk (13.8%) and the fractions of the driver population with (8.7%) and without (91.3%) OSA to derive this risk. The cost is a function of the odds ratio for a crash as a result of OSAS. Our computations show that if the odds for a crash equals 2.5, a crash attributable to OSAS costs $8,200/driver/year, but is as high as $22,400 if the odds ratio=10 and as low as $3,200 if the odds ratio is 1.5.
2.6 Treatment costs
We estimated the average annual cost of CPAP treatment for the supplier for the first year to be $1,806/case. This includes the costs of titration polysomnography ($768); the CPAP unit at [$708, broken down as CPAP machine equipped with compliance monitoring ($449), heated humidification ($135), mask ($25), headgear ($24) and labor for initial setup ($75)]; three office visits for the first year ($170 for the initial visit, plus two follow-up evaluations totaling $100); replacement masks ($60). We multiplied this total cost ($708+$768+170+100+60=$1806/identified case) by the number of cases identified by each program. We added this treatment cost to the cost of screening, and averaged the total amount to obtain the per-driver cost.
2.7 Total cost of a screening program
The total cost of a screening program equaled the sum costs of testing, treatment plus crashes. Crashes could result either from a missed diagnosis of OSAS or, among identified cases, due to non-acceptance of treatment, so total cost was a function of treatment acceptance. The overall cost of missed cases was the number of missed cases (which was all cases of OSAS in our sample, for the “no screening” strategy) multiplied by the cost of crashes attributable to OSAS (8,200/driver). We divided each overall cost of a program by the number of drivers in the sample to derive the cost per driver screened.
2.8 Determination of cost-effectiveness
A program was cost-effective if its total cost (diagnosis, treatment plus residual crashes) was lower than the cost of not screening. We determined the minimum percentage of drivers who must accept treatment in the first year in order for each screening strategy to be cost-effective. We assumed that 100% treatment acceptance prevents all apnea-related crashes in the first year, while 0% acceptance prevents no crashes.
2.9 Sensitivity analyses
While we assigned the attributable-odds for a crash at 2.5(26), in sensitivity analyses, we considered a range from 1.5–10, in 0.5-unit increments. We also evaluated the effects of doubling and halving the assumed costs of the modified multivariable prediction, oximetry, polysomnography and treatment. Finally, we evaluated the effect of changing the weighting of false positive:false negative predictions, from 1:10 to 1:20 and 1:5.
2.10 Testing accuracy
Using the parameter set associated with the cost of each program, we computed true positive, true negative, false positive and false negative prediction rates and sensitivity, specificity, negative predictive value and negative likelihood ratio.
3. Results
3.1 Demographics and proportion of drivers with obstructive sleep apnea syndrome
The stratified sampling design requires us to weight sample data from each of the tiers based on the estimated proportion of drivers within each stratum in the population. We computed the weighted mean as (0.415*higher risk mean) + (0.585*lower risk mean), and the weighted standard error (SE) as the square root of [(0.415)2(higher risk SE)2]+[(0.585)2 (lower risk SE)2]. We have published details regarding this weighting method previously(1).
The average±SD age of the weighted sample was 45.4±11.0 years, BMI 29.9±5.2 kg/m2, and modified multivariable prediction 0.44±0.21(23). A total of 28.1% had AHI≥5/hour, and 32.6% had Epworth score >10. A total of 8.7% of the sample had OSAS, with both AHI≥5/hour and sleepiness (Epworth score >10). The average±SE AHI and Epworth score were 6.0±11.5 events/hour and 8.8±4.4, respectively.
3.2 Accuracy of screening when using parameters that minimize cost
For our assumption that the odds of a crash equals 2.5, the two-stage strategy required upper and lower bound values of the modified multivariable apnea prediction index of 0.5 and 0.1, respectively, and ODIthreshold ≥5 hourly desaturations of ≥3% to minimize cost (see Figure 1). For the one-stage strategy, the modified multivariable apnea prediction cutpoint value of 0.2 minimized cost of one-stage screening. Using these parameters, 276 drivers (67.9%) were excluded from any additional testing by the one-stage strategy, and 165 (40.7%) were excluded by the two-stage strategy. The two-stage strategy required that 212 (52.3%) drivers undergo oximetry and 95 (23.4%) undergo sleep studies, while the one-stage strategy required 130 (32.1%) to have sleep studies. In the two-stage strategy, 66 (16.3%) underwent both oximetry and polysomnography. Thus, a relatively large number of drivers (146, or 36%) had negative oximetry.
For the two-stage strategy, sensitivity, specificity and negative predictive values were 69%, 81% and 97%, respectively, with a misclassification rate of 20.0% and negative likelihood ratio of 0.385 (Table 1). We applied this value to a Bayesian nomogram(30), and determined that when two-stage screening predicts the absence of OSAS, the likelihood of having OSAS was 3.5%. For the one-stage strategy, the optimum questionnaire cutpoint value of 0.2 was associated with sensitivity, specificity, negative predictive value and negative likelihood ratio of 70.5%, 71.4%, 96.4% and 0.413, respectively (Table 1). This means that a modified multivariable score<0.2 using the one-stage strategy, which was seen in 276 (67.8%) drivers, is associated with 3.8%(30) likelihood of having OSAS.
TABLE 1.
ACCURACY OF THREE SCREENING STRATEGIES
| One-stage | Two-stage | Polysomnography | |
|---|---|---|---|
| Sensitivity | 70.5% | 68.8% | 100% |
| Specificity | 71.4% | 80.9% | 78.4% |
| Negative Predictive Value | 96.4% | 96.6% | 100% |
| Negative Likelihood Ratio | 0.413 | 0.385 | 0.000 |
3.3 Comparison of costs of administering each of the four screening strategies in relation to the cost of a missed case, when this cost is treated as a variable
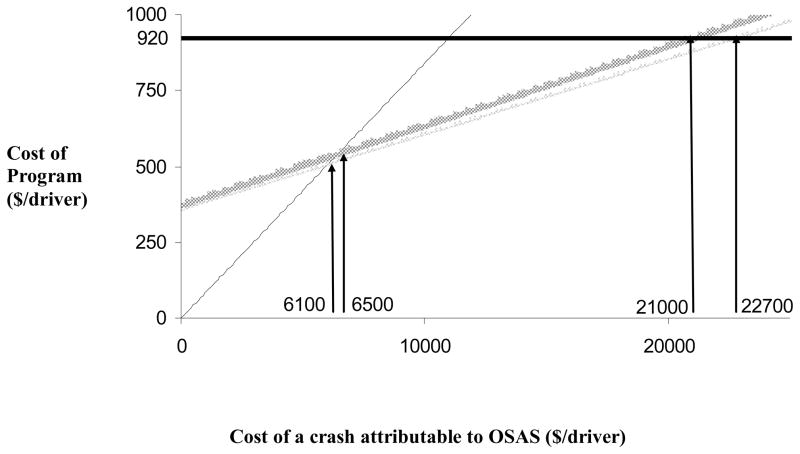
Figure 2 shows the costs of administering each program, which includes costs of screening and treating identified cases, and the cost of a missed case. (We consider crashes and incomplete treatment acceptance later, in Figure 3). The cost of each program is represented as a function of the cost of a missed case, which is treated in this analysis as an independent variable. Included are the costs for doing nothing (thin black line), one-stage screening (thin gray line), two-stage screening (heavy gray line) and polysomnography (heavy black horizontal line). The cost of performing polysomnography on all drivers remains fixed at $920/driver ($920=$768 for polysomnography + $152 for treatment, averaged over all drivers screened, see Table 3, “PSG” column). The line is flat because it is independent of the cost of a missed case, since polysomnography is assumed to miss no cases, but some false positives may still occur since not all individuals identified will be excessively sleepy. Doing nothing misses all cases, and so the cost of doing nothing is a linear function of the cost of a missed case (long dashed line). Since selective polysomnography misses some cases, these lines increase as the cost of a missed case increases.
Figure 2.
Total cost of screening strategy as a function of the cost of missing a case of sleep apnea syndrome (defined by AHI≥5 events/h with sleepiness defined by Epworth score >10). The cost of the polysomnography program (heavy black line) is is fixed at $920=$768 for testing each driver, plus $152/driver screened for the cost of treatment. Cost of oximetry and modified multivariable prediction were assumed to be $160 and $5, respectively. The figure shows costs (on y-axis) for full polysomnography (heavy black line) and doing nothing (thin black line) compared against one-stage screening (thin gray line) and two-stage screening (heavy gray line). The horizontal axis shows the variable cost of a missed case (bottom labels). Not screening is least expensive when a missed case costs less than $6,100. When missing a case costs between $6,100 and $22,700, one-stage screening is the least expensive strategy. When a missed case costs more than $6,500, two-stage screening is also less expensive than not screening. Polysomnography is less expensive than two-stage screening when a missed case costs more than $21,000, and less expensive than one-stage screening when the cost of a missed case is more than $22,700.
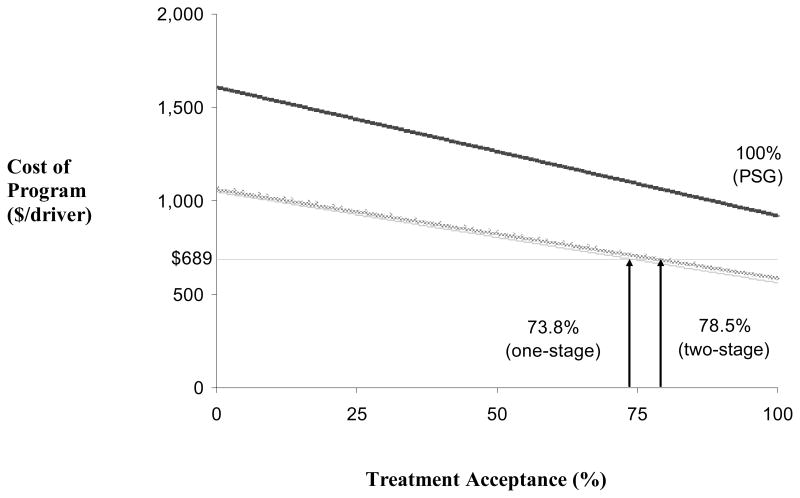
Figure 3.
Cost per driver screened of screening, treatment, and crashes for each of four programs, as a function of the rate of treatment acceptance. The cost of doing nothing (horizontal line) is fixed at $689, due to the fixed cost of crashes due to missing all cases. Full polysomnography (heavy black line) derives its cost from testing, treatment, and from crashes among those who do not accept treatment. The cost declines with increased treatment acceptance but is always greater than doing nothing, even at 100% treatment acceptance. Screening with the one-stage (thin gray line) or the two-stage (wide solid gray line) methods incurs costs from testing, treatment, and crashes not only due to incomplete adherence, but also from variable numbers of missed cases. As long as treatment acceptance is at least some minimum value, a given screening program is less expensive than not screening. For one- and two-stage screening to be cost-effective, these rates are 73.8% and 78.5%, respectively. Full polysomnography is never cost-effective, since it never crosses the “do nothing” line, even when treatment acceptance is 100%.
Table 3.
Costs of crashes ($) for each of three programs due to undiagnosed OSAS or failure of identified cases to accept treatment*
| Cost of Crashes | One-Stage | Two-Stage | Polysomnography |
|---|---|---|---|
| DUE TO MISSED CASES (false negative) | 203 | 215 | 0 |
| DUE TO NON-ACCEPTANCE OF TREATMENT | |||
| Treatment acceptance (%) | |||
| 100 | 0 | 0 | 0 |
| 78.5 | 104 | 102 | 148 |
| 73.8 | 127 | 124 | 180 |
| 50 | 243 | 237 | 344 |
| 25 | 364 | 356 | 517 |
| 0 | 485 | 474 | 689 |
Data are based on the estimate that the cost of a single crash equals $8,200, which corresponds to an odds ratio for a crash of 2.5(26).
When a sleep study costs $768, oximetry $160 and questionnaire $5, compared to the one-stage strategy, not screening is the strategy of choice only if the cost of a missed case attributable to OSAS is less than $6,100. One-stage screening remains least expensive until the cost of a missed case is at least $22,700, when using a full sleep study on everyone could be justified (see Figure 2). Two-stage screening is less expensive than doing nothing or doing polysomnography, if the cost of a missed case is between $6,500 and $21,000. However, even within this range, it is marginally more expensive than one-stage screening.
3.4 Selection of a screening strategy based on a fixed cost of traffic accidents
Data provided by Sassani et al(26) assigned a value of 2.5 to the odds ratio for crashes related to OSAS. For this value of the odds ratio, we found that the cost of a missed case attributable to OSAS was $8,200 per driver. When we fixed the cost of a missed case at this value of the OSAS-attributable cost of a crash, we found that program costs per driver screened were as follows: doing nothing=$689/driver, one-stage screening=$562/driver, two-stage screening=$587/driver and uniform polysomnography=$920/driver. This program cost equaled the sum costs of administering the screening tests, plus the cost of treatment of identified cases, and the cost of crashes that result from missed cases. These costs also pertain to the condition in which treatment acceptance is a condition of employment, so that we assume there are no costs associated with crashes due to failed treatment. The impact of varying treatment acceptance is considered below. The cost of such crashes attributable to OSAS due to missed cases is $203/driver, $215/driver, and $0, for the one-stage, two-stage and polysomnography programs, respectively.
The basis for these total costs for the three strategies is shown in Table 2, which lists: 1) costs of screening, itemized by the outcome of the screening program prediction (whether true or false positive, or true or false negative); and 2) costs of treating identified cases, i.e., true positives.
Table 2.
Costs per driver screened ($) of screening and treatment for three* screening programs, based on correctness of prediction
| COST COMPONENT | One-Stage | Two-Stage | Polysomnography |
|---|---|---|---|
| 1.Cost of Screening, based on type of prediction) | 252 | 268 | 768 |
| TRUE POSITIVE PREDICTIONS | 45.76 | 51.26 | 64.51 |
| Modified Multivariable prediction | 0.30 | 0.29 | 0.00 |
| Oximetry | 0.00 | 6.56 | 0.00 |
| PSG | 45.47 | 44.41 | 64.51 |
| FALSE POSITIVE PREDICTIONS | 202.35 | 154.98 | 151.70 |
| Modified Multivariable prediction | 1.31 | 0.88 | 0.00 |
| Oximetry | 0.00 | 19.44 | 0.00 |
| PSG | 201.04 | 134.67 | 151.70 |
| FALSE NEGATIVE PREDICTIONS (missed cases) | 0.12 | 2.02 | 0.00 |
| Modified Multivariable prediction | 0.12 | 0.13 | 0.00 |
| Oximetry | 0.00 | 1.88 | 0.00 |
| PSG | 0.00 | 0.00 | 0.00 |
| TRUE NEGATIVE PREDICTIONS | 3.27 | 59.44 | 551.79 |
| Modified Multivariable prediction | 3.27 | 3.70 | 0.00 |
| Oximetry | 0.00 | 55.74 | 0.00 |
| PSG | 0.00 | 0.00 | 551.79 |
| 2. Cost of Treating Cases (true positives) | 107 | 104 | 152 |
| TOTAL COST of screening plus treating identified cases | 358 | 372 | 920 |
A fourth alternative program we considered, not screening, does not incur screening or treatment costs, but by comparison incurs a total cost of $689/driver. This cost is due to the cost of missed cases.
Performing polysomnography on all drivers incurs the greatest screening cost, at $768/driver. However, because <10% of drivers in our sample had OSAS, most of this cost, $552/driver, was expended for sleep studies done in drivers who were eventually found not to have OSAS (true negatives). Selective polysomnography eliminates the cost of these excess sleep studies completely. On the other hand, a number of false positive predictions resulted in excess cost, which was highest for one-stage screening ($202/driver). Because the background proportion with OSAS was relatively low, $152/driver screened was also expended with the routine polysomnography program among drivers who were eventually not labeled with OSAS.
In contrast, the option of not screening incurs no screening or treatment costs, but $689/driver due to the cost of crashes among cases that are not identified.
3.5 Effect of treatment acceptance on costs of screening programs
We next consider the case where treatment acceptance is not a condition of continued employment, and that the odds ratio for a crash is 2.5(26) so that the cost of a crash is fixed at $8,200. There is a rate of treatment acceptance at which cost of diagnosis and treatment as well as residual crashes (from missed cases) equals the cost of all crashes ($689/driver) in the absence of screening and therefore missing all cases. This relationship is shown in Figure 3. The rate of treatment acceptance affects costs since drivers not using therapy are still at risk for crashes. Even if treatment acceptance in the first year is mandatory (100%), routine polysomnography on all drivers was still not cost-effective and was more expensive than not screening. Selective polysomnography, however, requires lower rates of treatment acceptance in order to be justifiable based on cost, compared to doing nothing. Screening all drivers and treating identified cases is less expensive than not screening as long as treatment acceptance is ≥73.8% or ≥78.5% for one-stage and two-stage screening, respectively. Thus, the more expensive the screening program, the higher the treatment acceptance rate needed for the program to be cost-effective. With all strategies, however, high treatment acceptance was necessary for the total program to be cost-effective.
The graphs in Figure 3 are based on calculated cost of crashes (Table 3), calculated costs of screening (see Table 2) and of the total cost (see Table 4) as a function of treatment acceptance. Table 3 shows the costs related to crashes, which come from either failure to identify cases (false negative predictions) in the case of selective polysomnography or from cases that were identified but subsequently did not accept therapy for the first year. While the former cost is fixed, the latter cost is an inverse function of the proportion that accepts treatment. Crashes due to missed cases are $0 for polysomnography (since this strategy misses no cases), $203/driver for one-stage screening and $215/driver for two-stage screening, if everyone accepts treatment. As treatment acceptance falls, however, the total cost of crashes (the sum of costs of crashes from missed cases plus non-acceptance of treatment) rises despite screening, up to $689/driver when no driver accepts treatment. This value is identical to the cost of crashes when screening is not done, $689/driver.
Table 4.
Total costs ($) per driver of three programs arising from comprehensive screening, treatment of identified cases of OSAS, and crashes*
| Treatment acceptance (%) | One-Stage | Two-Stage | Polysomnography |
|---|---|---|---|
| 100 | 562 | 587 | 920 |
| 78.5 | 666 | 689 | 1,068 |
| 73.8 | 689 | 711 | 1,100 |
| 50 | 804 | 824 | 1,264 |
| 25 | 926 | 942 | 1,436 |
| 0 | 1,047 | 1,061 | 1,608 |
Because crashes may be due to missed cases of OSAS or failure of identified cases to accept treatment, data are shown as a function of treatment acceptance rates
Table 4 shows the total cost of the three programs, which includes screening and treatment (Table 2) plus crashes (Table 3), as a function of treatment acceptance. All three programs that involve active screening become less cost-effective as treatment acceptance drops. However, regardless of how many drivers accept treatment, doing full sleep studies on everyone is consistently the most expensive of the four choices. At a minimum, 73.8% of drivers must accept treatment in order to offset the costs of crashes and make screening less expensive than doing nothing. The program of choice is then one-stage screening. In conditions with acceptance rates below 73.8%, none of the screening programs are cost-effective.
3.6 Sensitivity analyses
3.6.1 Effect of variation in the cost of polysomnography, oximetry and treatment
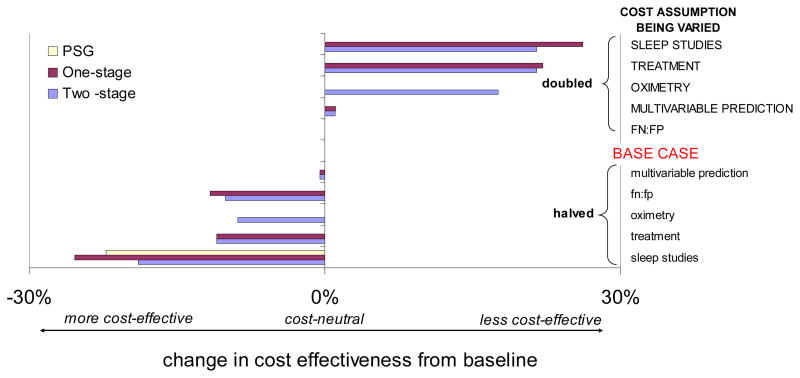
The greatest impact on cost-effectiveness occurred under the following conditions: 1) doubling the costs of either sleep studies or treatment, or 2) halving the cost of sleep studies (see Figure 4 uppermost and lowest bars, respectively). This figure shows the magnitude of change in the required treatment acceptance (%) compared to the baseline condition described above. A negative value implies that less acceptance is needed, so that the program is more cost-effective. Halving the cost of sleep studies made all programs more cost-effective, especially one-stage screening, which required only 48% treatment acceptance, followed by two-stage screening, which required 59.6% treatment acceptance. The lack of cost-effectiveness of full polysomnography was a robust finding: for all conditions, change in minimum treatment acceptance was 0%, with the exception of halving the cost of polysomnography. For this case, the program became more cost-effective, with a change in minimum rate of treatment acceptance equaling 22.2%, and a drop in the required minimum treatment acceptance rate dropped from 100% to 77.8%. Doubling either the costs of sleep studies or of treatment made all programs cost-ineffective, requiring ~100% treatment acceptance.
Figure 4.
Sensitivity analysis of the effect of varying costs of testing (modified multivariable prediction, oximetry or sleep study) or of treatment, and misclassification ratios, for one-stage screening strategy. The change in minimum treatment acceptance needed for cost-effectiveness compared to the baseline case is shown on the x-axis. A large positive value denotes an increase in treatment acceptance needed from baseline (and hence lower cost-effectiveness), and a negative value denotes improvement in cost-effectiveness. Doubling of the cost of treatment or of polysomnography makes one- and two-stage screening less cost-effective, while halving each, or changing the misclassification rate to 5:1 (rather than 10:1) makes one-stage screening more cost-effective. Doubling the cost of oximetry makes the two-stage program less cost-effective.
Appropriately, only two-stage screening was affected by cost of oximetry: doubling the cost of oximetry made two-stage screening much less cost-effective, increasing minimum treatment acceptance rate required to 96% (Figure 4). Halving the cost of oximetry was the only condition in which two-stage screening became more cost-effective than one-stage (Figure 4, third bar from the bottom).
The cost-effectiveness of each program did not differ from the base case significantly when we altered the expense of modified multivariable prediction (bars stayed close to the cost neutral range).
3.6.2 Effect of variation in the weighting of missed cases to false positive predictions (FN:FP)
Doubling the weight ratio of FN:FP from 10:1 (base case) to 20:1 did not change the cost-effectiveness of the three programs. Halving this ratio to 5:1 made one- and two-stage screening slightly more cost-effective. (See Figure 4, FN:FP conditions).
3.6.3 Effect of variation in the odds ratio for a crash
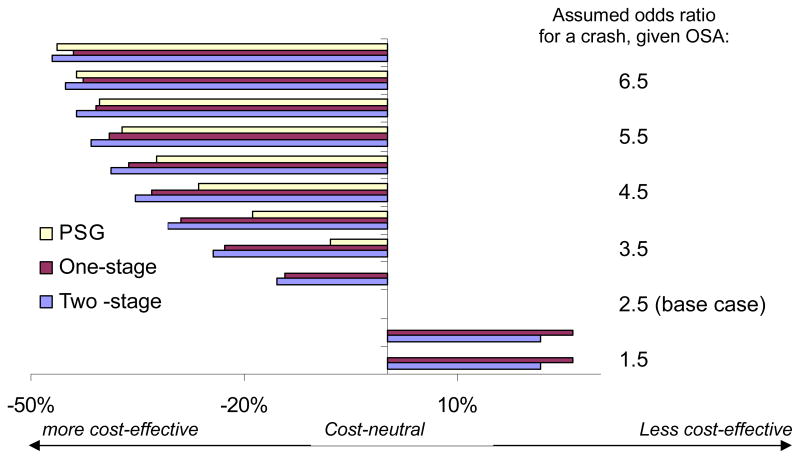
We assessed the effect of varying the odds ratio for crash risk attributable to OSAS on the acceptance rate that resulted in a given strategy being cost-effective. We increased the odds ratio in 0.5 intervals (Figure 5), beginning at 1.5. Lower odds for an OSAS-attributable crash (<=3.0 for full polysomnography, or <=2.0 for selective polysomnography) meant that 100% treatment acceptance was required to achieve cost-effectiveness. When the odds increased to 7, all programs became more cost-effective, as the required treatment acceptance rates were much lower: 53.7% for full polysomnography, 29.7% for one-stage screening and 31.5% for two-stage screening.
Figure 5.
Sensitivity analysis of the effect of changing the odds for a crash, given OSAS. As in Figures 4, the change in minimum treatment acceptance needed for cost-effectiveness compared to the baseline case is shown on the x-axis. A large positive value denotes an increase in treatment acceptance needed from baseline (and hence lower cost-effectiveness), and a negative value denotes improvement in cost-effectiveness. All programs become more cost-effective as the odds for a crash increases. At low odds for a crash, (≤2 for one- and two-stage screening, or ≤3 for polysomnography), the programs become cost-ineffective. When the odds for a crash is ≥3.5, however, both one-stage (red) or two-stage (blue) screening, both of which are comparable in cost-effectiveness, become more cost-effective than polysomnography (yellow). (Compare leftward magnitude of bars for one- and two-stage against that for polysomnography).
4. Discussion
Our results indicate that an untreated driver may be expected to incur $8,200/year if the odds of a crash given OSAS is 2.5, an odds estimate offered by Sassani et al(26) in a recent meta-analysis. Given this high cost, and given the proportion of drivers expected to have sleep apnea syndrome, we estimated that an employer’s decision not to screen commercial drivers costs the industry ~$2.4 billion/year, or $689/driver due to vehicular accidents alone.
We compared this value of $689/driver against the costs of systematic screening and treatment. Screening using full sleep studies and treating identified cases is not cost-effective: at $920/driver, it is one third more expensive than not screening at all. Selective polysomnography, however, is cost-effective. Using a combination of BMI, age and gender to determine who should undergo polysomnography and then treating identified cases is considerably less expensive, at $358/driver, or $372/driver if combined with oximetry. These approaches are nearly 50% less expensive than not screening.
Oximetry used in conjunction with BMI, age and gender is only marginally more expensive ($14/driver more) but offers one advantage: it requires fewer in-laboratory studies. Only 95 (23%) sleep studies were done under two-stage screening, instead of 130 (32%) for one-stage screening. This could prove valuable in settings of limited access to the sleep laboratory(13) and also be more acceptable to drivers.
While selective polysomnography is more cost-effective than full sleep studies or not screening, and lowers the numbers of sleep studies required, this strategy may still miss some cases, which can contribute to crashes. Both one-stage and two-stage strategies missed cases with similar frequency: 2.5% (10/406) of drivers screened were missed by one-stage screening and 2.6% (11/406) by two-stage screening. Additionally, selective polysomnography may lead to “excess” sleep studies in individuals without sleep apnea. Although the one-stage protocol avoids reliance on oximetry, this strategy required a larger number of sleep studies to be performed in individuals who did not have apnea. Consistent with this finding is the specificity of the two programs: the two-stage strategy had greater specificity (81%) compared to one-stage screening (71%).
We assumed a wide range of rates of treatment acceptance, and found that all programs require relatively high rates of treatment acceptance to be cost-effective: 73.8%, 78.5% or 100%, for one-stage, two-stage or full polysomnography, respectively. Rates this high are rarely seen in clinical practice. Up to 50% of patients refuse CPAP when it is first offered (33). Among regular users, about half use it less often than prescribed (34). Within three years, about 12 – 25% of patients abandon this therapy altogether (33).
Whether rates as low as these would be seen following screening of non-clinic populations such as commercial drivers is unknown. Data regarding treatment adherence among commercial drivers have not been reported. We may yet find that this group would be more willing to use therapy given the occupational implications of not being treated, and hence also experience a greater degree of utility with screening and treatment. Indeed, our analysis shows that cost-effectiveness is not achieved unless high numbers of drivers accept therapy. With this finding, a reasonable public health policy would be to mandate documentation of ongoing adherence to CPAP treatment as a condition for continued employment in this occupation. Otherwise, the risk of crashes will persist.
Other critical assumptions that we made pertain to the efficacy of CPAP therapy. We assumed that the rate of OSA-attributable crashes may be eliminated by full treatment acceptance, and reduced to the background risk seen in those without OSA. Whether this assumption is credible or not must be addressed by future prospective studies that quantify this efficacy rate; CPAP may not eliminate all apneas even if the driver adheres to therapy, and if it does, complete elimination of crash risk due to residual OSAS-related sleepiness may still not ensue. Additionally, we made no assumptions or conclusions regarding the cost of crashes that are not due to OSA in our analyses, regardless of whether the driver opts to be treated or refuses treatment.
Reporting of sleepiness by our subjects was common in this research setting, where we provided detailed confidentiality guarantees a priori. Over 95% of the group completed the Epworth questionnaire. Adjusting for stratified sampling by risk group, the estimated mean Epworth score was 8.8 (95% CI 8.4 to 9.2), with 32.6% (95% CI 28.2% to 36.9%) having scores in the pathological range, above 10. This is similar to the prevalence of 24% for elevated Epworth scores reported by Howard et al(3), who also studied a large sample of commercial drivers in Australia.
Our successful collection of sleepiness data allowed us to predict OSA syndrome rather than an isolated apnea-hypopnea index value. We chose to model on OSA syndrome rather than AHI alone because increasing levels of sleepiness were associated with increasing accident risk in the study by Howard et al, with a twofold increased risk of an accident in the sleepiest 5% of drivers based on Epworth scores(3). Additionally, in a case-control study of fatigue-related crashes among 1,403 drivers in North Carolina, individuals having such crashes were more than twice as likely to have elevated Epworth sleepiness scale scores (35).
Although sleepiness was therefore part of our case definition, we chose not to include self-reported sleepiness in our decision rule in making predictions about apnea risk, because such symptom-reporting is likely to be inaccurate in the occupational setting (personal communication, N. Hartenbaum, MD). Given these concerns about accuracy of symptom reporting, we also developed a version of the multivariable apnea prediction instrument that was independent of symptoms. This change did not prevent selective polysomnography from being more cost-effective than routine polysomnography, while maintaining a high negative predictive value of 97%. This is a reasonable rate for a program whose purpose is screening (i.e., low numbers of missed cases). In our prior analyses of commercial drivers, addition of symptom information to two-stage screening and use of different parameters (upper bound = 0.9, lower bound = 0.3, and ODIthreshold=10/h) was more accurate; the negative likelihood ratio was lower, 0.1(1), instead of 0.44 in this study.
Our economic analysis considers the cost of screening as a function of the cost of a missed case of apnea. In one analysis, we treated this cost of a missed case as a variable, since this cost is still not precisely known. This approach allows one the ability to use Figure 2 to assess the costs of screening when considering only the direct costs of untreated OSAS, indirect costs or both. For example, another indirect cost might be due to loss of productivity related to untreated sleep apnea: an affected driver may require longer hours to complete a haul if he has to stop en route due to sleepiness. Future studies need to focus on delineating these direct and indirect costs more clearly. These costs might include other outcomes of untreated apnea other than crashes, such as lost productivity and increased health care expenditures(36, 37).
Likewise, the role of apnea in crash risk in commercial vehicles(38) also warrants more investigation. Our study derives the attributable risk of crashes among commercial drivers from passenger car data (26) because these data are not available for commercial drivers. Since this odds ratio is not known for commercial drivers, we conducted a sensitivity analysis where we varied the odds ratio for a crash. These data show that selective polysomnography is the least expensive program to administer compared to full polysomnography, independent of the odds ratio, as long as the odds ratio is at least 2.0. Not screening is the least expensive option only if the risk of a crash attributable to OSAS in commercial drivers is <1.6. Indeed, crash risk may be mitigated among commercial drivers because of their greater driving experience, but they also drive continuously for long periods, and often at night (39), so their crash risk may indeed be higher than 1.6. An assessment of actual crash rates attributable to OSAS among commercial drivers is urgently needed. As we have shown, conclusions about the most cost-effective strategy and the degree of treatment acceptance that are needed for cost-effectiveness are dependent on the odds ratio for crashes attributed to OSAS. In addition, our odds ratio for crashes is based the number of crashes of large trucks divided by the number of commercial drivers. This denominator includes only drivers of large trucks, whose gross vehicle weight rating (GVWR) exceeds 10,000 pounds, so it excludes taxi drivers, light vehicle drivers (including couriers and motorcycle couriers) and chauffeurs. However, our sensitivity analysis around the odds ratio addresses the possibility that this may be an under- or over-estimate of the actual odds of a crash.
Another area of interest is that of fatal crashes; federal data report that a single fatal crash costs $3.4 million(22). We have opted not to confine our analysis to fatal crashes alone, since this value is orders of magnitude higher than $59,000 for all crashes, and may therefore favor screening and treatment. Furthermore, the role of OSAS specifically in sleepiness-related fatal accidents in commercial drivers is even less well defined.
We compare our results to those of Chervin et al(40), who conducted a cost-utility analysis of polysomnography, not testing at all, and home diagnostic testing using portable monitors in a hypothetical cohort of patients suspected of having OSAS, presumably such as those referred to a sleep center. Treatment was offered not only to the subset with confirmed OSAS on home study or polysomnography, but to everyone in the “no-testing” group. Measuring quality-of-life five years after diagnosis, they reported that polysomnography is cost-effective in comparison to home study or no testing. This conclusion is different from ours, and is likely related to the populations studied; the rates of sleep apnea in general populations such as commercial drivers are lower than those found in the specialized cohort of individuals being evaluated in a sleep center for suspected sleep apnea. Our results suggest that among commercial drivers, even when the odds of having a crash related to OSAS is ≥7, polysomnography would become more cost-effective than not screening, but remains less cost-effective than doing a pre-evaluation with BMI, age, and gender ±oximetry.
Given the paucity of data regarding longer-term health outcomes and costs, our analysis assessed costs of diagnosis and treatment of sleep apnea which occur within the first year, when these costs are presumably the highest (40). One study has addressed this issue in a more general population. Ronald et al(36) suggested that untreated OSA imposes significant economic burden on the Canadian health care system. Physician claims totaled an equivalent of $3,039/OSA patient/10 years in U.S. currency, compared to the cost of $1,506/subject without OSA/10 years, although confounding effects of obesity and tobacco use could not be excluded. Bahammam et al subsequently showed, however, that acceptance to treatment by OSA patients was associated with a reduction in physician claims in the two years after OSA diagnosis(37). The difference in physician claims (not actual claims) between OSA cases and controls during the year before diagnosis totaled $199/year, and dropped to $133/year in the 2 years after diagnosis. This comparison reached statistical significance in the group adhering with therapy.
Epstein et al conducted a cost-effectiveness analysis of nocturnal oximetry as a screening tool for OSA(41) in a sleep center population. However, their study did not include the major cost of a missed case. Using a pattern of brief, repetitive fluctuations in saturation to define an event, and using an ODIthreshold of 10/hour, they showed that oximetry had a sensitivity of only 61%(41). Perhaps because of this low diagnostic accuracy, as well as the lack of inclusion of the costs of missed cases, they concluded that using oximetry for screening is not cost-effective. Including the cost of missed cases in our analysis, we have shown that, contrary to the study by Epstein et al, screening is economically valuable, particularly in a more general population such as commercial drivers.
Future analyses in this area need to take into account patient adherence not only with treatment, but also with screening strategies. Adherence with polysomnography may be less than adherence with the modified multivariable prediction, which can be done within minutes, or oximetry, which can be done directly in the patient’s home. Additionally, future technologies for confirmatory testing will likely involve use of home-based diagnostic systems(42–46). If such systems become validated, they may eventually replace in-lab polysomnography. Our sensitivity analysis supports the use of such systems: halving the cost of full polysomnography decreases the rates of treatment adherence necessary for screening with full polysomnography to be cost-effective. In the future, an initial screen with BMI, age and gender followed by out-of-laboratory diagnostic options could result in substantial gains in cost-effectiveness.
In our exercise, we did not explore the impact of screening on intangible outcomes, such as drivers’ quality of life (47–49). Quality of life may improve with treating cases that are identified by screening (49, 50), provided that patients adhere to therapy. Utility of screening, however, depends not only on patient acceptance and adherence with therapy and on demographic variables, but also on patient-assessed ratings of satisfaction and importance of being treated. This can certainly be different among commercial drivers than in a more general population, given the importance of maintaining vigilance in order to meet occupational requirements safely and effectively.
In conclusion, our results suggest that from an economic perspective, a simple approach to screening using BMI, age and gender alone or with oximetry is a viable strategy to detect obstructive sleep apnea in commercial drivers, and lowers the need for in-laboratory polysomnography studies. Despite missing some cases, this strategy requires lower rates of treatment acceptance than routine polysomnography in order to be cost-effective, given the high cost of crashes in drivers with OSAS. Reasonable estimates of crash risk attributable to OSAS supports the use of screening strategies in commercial drivers from an economic perspective. Implementation of such strategies will require the policy that treatment adherence needs to be monitored once drivers with OSAS are identified.
Acknowledgments
This work was supported by Trucking Research Institute contract DTFH61-93-C-00088 funded by the Federal Highway Administration (FHA) (now Federal Motor Carriers Safety Administration). The Trucking Research Institute is part of the American Trucking Association. Also supported by NIH grants 3-M01-RR00040-39S2, P01-HL-60287, and K23 RR16068-03. As part of the contract, both the Trucking Research Institute and Federal Motor Carriers Safety Administration could comment on the manuscript but could not mandate change.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gurubhagavatula I, Maislin G, Nkwuo JE, Pack AI. Occupational screening for obstructive sleep apnea in commercial drivers. Am J Respir Crit Care Med. 2004;170:371–376. doi: 10.1164/rccm.200307-968OC. [DOI] [PubMed] [Google Scholar]
- 2.Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Int Med. 2005;142(3):187–97. doi: 10.7326/0003-4819-142-3-200502010-00010. [DOI] [PubMed] [Google Scholar]
- 3.Howard ME, Desai AV, Gurunstein RR, Chukins C, Armstrong JG, Joffe D, Swann P, Campbell DA, Pierce RJ. Sleepiness, sleep disordered breathing and accident risk factors in commercial vehicle drivers. Am J Respir Crit Care Med. 2004;170:1014–1021. doi: 10.1164/rccm.200312-1782OC. [DOI] [PubMed] [Google Scholar]
- 4.Stoohs R, Bingham L, Itoi A, Guilleminault C, Dement W. Sleep and sleep-disordered breathing in commercial long-haul truck drivers. Chest. 1995;107:1275–82. doi: 10.1378/chest.107.5.1275. [DOI] [PubMed] [Google Scholar]
- 5.Kim H, Young T, Matthews C, Weber S, Woodward A, Palta M. Sleep-disordered breathing and neuropsychological deficits. A population-based study. Am J Respir Crit Care Med. 1997;156:1813–9. doi: 10.1164/ajrccm.156.6.9610026. [DOI] [PubMed] [Google Scholar]
- 6.American Thoracic Society. Sleep apnea, sleepiness and driving risk. Am J Respir Crit Care Med. 1994;150:146373. doi: 10.1164/ajrccm.150.5.7952578. [DOI] [PubMed] [Google Scholar]
- 7.Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med. 1999;340(11):847–51. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 8.Young T, Blustein J, Finn L, Palta M. Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep. 1997;20:608–13. doi: 10.1093/sleep/20.8.608. [DOI] [PubMed] [Google Scholar]
- 9.Hack M, Davies RJ, Mullins R, Choi SJ, Ramdassingh-Dow S, Jenkinson C, Stradling JR. Randomised prospective parallel trial of therapeutic versus subtherapeutic nasal continuous positive airway pressure on simulated steering performance in patients with obstructive sleep apnoea. Thorax. 2000;55(3):224–31. doi: 10.1136/thorax.55.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassel W, Ploch T, Becker C, Dugnus D, Peter JH, von Wichert P. Risk of traffic accidents in patients with sleep-disordered breathing: reduction with nasal CPAP. Eur Resp J. 1996;9(12):2606–11. doi: 10.1183/09031936.96.09122606. [DOI] [PubMed] [Google Scholar]
- 11.Findley L, Smith C, Hooper J, Dineen M, Suratt PM. Treatment with nasal CPAP decreases automobile accidents in patients with sleep apnea. Am J Respir Crit Care Med. 2000;161(3 Pt 1):857–9. doi: 10.1164/ajrccm.161.3.9812154. [DOI] [PubMed] [Google Scholar]
- 12.George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax. 2001;56(7):508–12. doi: 10.1136/thorax.56.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pack AI. Sleep-disordered breathing: access is the issue.[comment] Am J Respir Crit Care Med. 2004;169(6):666–7. doi: 10.1164/rccm.2401008. [DOI] [PubMed] [Google Scholar]
- 14.Flemons WW, Douglas NJ, Kuna ST, Rodenstein DO, Wheatley J. Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med. 2004;169(6):668–72. doi: 10.1164/rccm.200308-1124PP. [DOI] [PubMed] [Google Scholar]
- 15.Vazquez JC, Tsai WH, Flemons WW, Masuda A, Brant R, Hajduk E, Whitelaw WA, Remmers JE. Automated analysis of digital oximetry in the diagnosis of obstructive sleep apnoea. Thorax. 2000;55(4):302–7. doi: 10.1136/thorax.55.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maislin G, Pack A, Kribbs N, Smith P, Schwartz A, Kline L, Schwab R, Dinges D. A survey screen for prediction of apnea. Sleep. 1995;18:158–166. doi: 10.1093/sleep/18.3.158. [DOI] [PubMed] [Google Scholar]
- 17.Maislin G, Gurubhagavatula I, Hachadoorian R, Pack F, O’Brien E, Bogage A, Staley B, Dinges D, Pack A. Operating Characteristics of the Multivariable Apnea Prediction Index in Non-Clinic Populations. Sleep. 2003;26(S):A247. [Google Scholar]
- 18.Series F, Marc I, Cormier Y, La Forge J. Utility of nocturnal home oximetry for case finding in patients with suspected sleep apnea hypopnea syndrome. Ann Int Med. 1993;119:449–453. doi: 10.7326/0003-4819-119-6-199309150-00001. [DOI] [PubMed] [Google Scholar]
- 19.Yamashiro Y, Kryger M. Nocturnal oximetry: is it a screening tool for sleep disorders? Sleep. 1995;18:167–171. doi: 10.1093/sleep/18.3.167. [DOI] [PubMed] [Google Scholar]
- 20.Levy P, Pepin J, Deschaux-Blanc C, Paramelle B, Brambilla C. Accuracy of oximetry for detection of respiratory disturbances in sleep apnea syndrome. Chest. 1996;109:395–399. doi: 10.1378/chest.109.2.395. [DOI] [PubMed] [Google Scholar]
- 21.Gurubhagavatula I, Maislin G, Pack AI. An algorithm to stratify sleep apnea risk in a sleep disorders clinic population. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1904–9. doi: 10.1164/ajrccm.164.10.2103039. [DOI] [PubMed] [Google Scholar]
- 22.Cost of large truck- and bus-involved crashes. Office of Research and Technology. Vol. 4 Federal Motor Carrier Safety Administration, U.S. Department of Transportation; Washington, D.C: 2001. [Google Scholar]
- 23.Pack AI, Maislin G, Staley B, Pack FM, Rogers WC, George CF, Dinges DF. Impaired Performance in Commercial Drivers: Role of Sleep Apnea and Short Sleep Duration. Am J Respir Crit Care Med. 2006;174:446–54. doi: 10.1164/rccm.200408-1146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 25.Gislason T, Almqvist M, Eriksson G, Taube A, Boman G. Prevalence of sleep apnea syndrome among Swedish men--an epidemiological study. J Clin Epi. 1988;41(6):571–6. doi: 10.1016/0895-4356(88)90061-3. [DOI] [PubMed] [Google Scholar]
- 26.Sassani A, Findley LJ, Kryger M, Goldlust E, George C, Davidson TM. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep. 2004;27(3):453–8. doi: 10.1093/sleep/27.3.453. [DOI] [PubMed] [Google Scholar]
- 27.Rechtschaffen A, Kales A. A manual of standardized terminology techniques and scoring system for sleep stages of human subjects. National Institute of Health, Washington, DC: US Government Printing Office; 1968. pp. 2–12. [Google Scholar]
- 28.Sleep-Related Breathing Disorders in Adults: Recommendations for Syndrome Definition and Measurement Techniques in Clinical Research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 29.National Center for Statistics and Analysis. U.S. Department of Transportation, National Highway Traffic Safety Administration; 2002. Traffic Safety Facts - Large Trucks. [Google Scholar]
- 30.Fagan T. Letter: Nomogram for Bayes Theorem. N Engl J Med. 1975;293:257. doi: 10.1056/NEJM197507312930513. [DOI] [PubMed] [Google Scholar]
- 31.2000. Employed Truck Drivers. U.S. Department of Transportation, Bureau of Transportation Statistics (BTS), based on U.S. Department of Labor, Bureau of Labor Statistics, 2000 National Occupational Employment and Wage Estimates, Transportation and Material Moving Occupations (occupational employment statistics); and 2000-10 National Employment Matrix (detailed occupation by industry, employment projections).
- 32.2000. CDL Records. FMCSA, Commercial Drivers License Information System (CDLIS).
- 33.Engleman H, Wild M. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS) Sleep Med Rev. 2003;7:81–99. doi: 10.1053/smrv.2001.0197. [DOI] [PubMed] [Google Scholar]
- 34.Weaver TE, Kribbs NB, Pack AI, Kline LR, Chugh DK, Maislin G, Smith PL, Schwartz AR, Schubert NM, Gillen KA, Dinges DF. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20(4):278–83. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 35.Stutts JC, Wilkins JW, Scott Osberg J, Vaughn BV. Driver risk factors for sleep-related crashes. Accid Anal Prev. 2003;35(3):321–31. doi: 10.1016/s0001-4575(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 36.Ronald J, Delaive K, Roos L, Manfreda J, Bahammam A, Kryger MH. Health care utilization in the 10 years prior to diagnosis in obstructive sleep apnea syndrome patients. Sleep. 1999;22(2):225–9. doi: 10.1093/sleep/22.2.225. [DOI] [PubMed] [Google Scholar]
- 37.Bahammam A, Delaive K, Ronald J, Manfreda J, Roos L, Kryger MH. Health care utilization in males with obstructive sleep apnea syndrome two years after diagnosis and treatment. Sleep. 1999;22(6):740–7. doi: 10.1093/sleep/22.6.740. [DOI] [PubMed] [Google Scholar]
- 38.Stoohs RA, Guilleminault C, Itoi A, Dement WC. Traffic accidents in commercial long-haul truck drivers: the influence of sleep-disordered breathing and obesity. Sleep. 1994;17(7):619–23. [PubMed] [Google Scholar]
- 39.FMCSA Annual Report. Share the Road Safely. Office of Research and Technology, Federal Motor Carrier Safety Administration, U.S. Department of Transportation; Washington, D.C: 2001. [Google Scholar]
- 40.Chervin RD, Murman DL, Malow BA, Totten V. Cost-utility of three approaches to the diagnosis of sleep apnea: polysomnography, home testing, and empirical therapy. Ann Int Med. 1999;130(6):496–505. doi: 10.7326/0003-4819-130-6-199903160-00006. [DOI] [PubMed] [Google Scholar]
- 41.Flemons W, Remmers J. The diagnosis of sleep apnea: questionnaires and home studies. Sleep. 1996;19:S243–7. [PubMed] [Google Scholar]
- 42.Parra O, Garcia-Esclasans N, Montserrat JM, Garcia Eroles L, Ruiz J, Lopez JA, Guerra JM, Sopena JJ. Should patients with sleep apnoea/hypopnoea syndrome be diagnosed and managed on the basis of home sleep studies? Eur Resp J. 1997;10(8):1720–4. doi: 10.1183/09031936.97.10081720. [DOI] [PubMed] [Google Scholar]
- 43.Reuven H, Schweitzer E, Tarasiuk A. A cost-effectiveness analysis of alternative at-home or in-laboratory technologies for the diagnosis of obstructive sleep apnea syndrome. Med Dec Making. 2001;21(6):451–8. doi: 10.1177/0272989X0102100603. [DOI] [PubMed] [Google Scholar]
- 44.Schafer H, Wig S, Hasper E, Luderitz B. Predictive diagnostic value of clinical assessment and nonlaboratory monitoring system recordings in patients with symptoms suggestive of obstructive sleep apnea syndrome. Respiration. 1997;64:194–199. doi: 10.1159/000196670. [DOI] [PubMed] [Google Scholar]
- 45.Whittle AT, Finch SP, Mortimore IL, MacKay TW, Douglas NJ. Use of home sleep studies for diagnosis of the sleep apnoea/hypopnoea syndrome. Thorax. 1997;52(12):1068–73. doi: 10.1136/thx.52.12.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fletcher EC, Stich J, Yang KL. Unattended home diagnosis and treatment of obstructive sleep apnea without polysomnography. Arch Fam Med. 2000;9(2):168–74. doi: 10.1001/archfami.9.2.168. [DOI] [PubMed] [Google Scholar]
- 47.Reimer MA, Flemons WW. Quality of life in sleep disorders. Sleep Med Rev. 2003;7(4):335–349. doi: 10.1053/smrv.2001.0220. [DOI] [PubMed] [Google Scholar]
- 48.Weaver TE. Outcome measurement in sleep medicine practice and research. Part 1: assessment of symptoms, subjective and objective daytime sleepiness, health-related quality of life and functional status. Sleep Med Rev. 2001;5(2):103–128. doi: 10.1053/smrv.2001.0152. [DOI] [PubMed] [Google Scholar]
- 49.Moyer CA, Sonnad SS, Garetz SL, Helman JI, Chervin RD. Quality of life in obstructive sleep apnea: a systematic review of the literature. Sleep Med. 2001;2(6):477–491. doi: 10.1016/s1389-9457(01)00072-7. [DOI] [PubMed] [Google Scholar]
- 50.Pichel F, Zamarron C, Magan F, del Campo F, Alvarez-Sala R, Suarez JR. Health-related quality of life in patients with obstructive sleep apnea: effects of long-term positive airway pressure treatment. Resp Med. 2004;98(10):968–76. doi: 10.1016/j.rmed.2004.03.009. [DOI] [PubMed] [Google Scholar]