SUMMARY
Mitochondria are functionally and physically associated with heterotypic membranes, yet little is known about how these interactions impact mitochondrial outer-membrane permeabilization (MOMP) and apoptosis. We observed that dissociation of heterotypic membranes from mitochondria inhibited BAK/BAX-dependent cytochrome c (cyto c) release. Biochemical purification of neutral sphingomyelinases that correlated with MOMP sensitization suggested that sphingolipid metabolism coordinates BAK/BAX activation. Using purified lipids and enzymes, sensitivity to MOMP was achieved by in vitro reconstitution of the sphingolipid metabolic pathway. Sphingolipid metabolism inhibitors blocked MOMP from heavy membrane preparations but failed to influence MOMP in the presence of sphingolipid-reconstituted, purified mitochondria. Furthermore, the sphingolipid products, sphingosine-1-PO4 and hexadecenal, cooperated specifically with BAK and BAX, respectively. Sphingolipid metabolism was also required for cellular responses to apoptosis. Our studies suggest that BAK/BAX activation and apoptosis are coordinated through BH3-only proteins and a specific lipid milieu that is maintained by heterotypic membrane-mitochondrial interactions
INTRODUCTION
Mitochondria are dynamic organelles that interact with each other and heterotypic intracellular membrane compartments, such as the endoplasmic reticulum (ER) (Csordas et al., 2006; de Brito and Scorrano, 2008). It is proposed that mitochondria are structurally and functionally tethered to organelles via proteins that reside in the outer mitochondrial membrane (OMM), and that these organelle-organelle interactions form unique biochemical and physical loci that regulate lipid biosynthesis, lipid transport, and intra-organellar Ca2+ signaling (Hayashi et al., 2009; Scorrano et al., 2003; Walter and Hajnoczky, 2005). An area of particular interest is the impact of organelle communication on apoptosis.
The mitochondrial pathway of apoptosis is dependent upon the BCL-2 family for the release of proapoptotic factors (e.g., cyto c) from the mitochondrial intermembrane space (IMS), via the process of mitochondrial outer-membrane permeabilization (MOMP) (Chipuk et al., 2010). The BCL-2 family is divided into three functional groups based on their composition of up to four BCL-2 homology domains (BH1-4 domains) (Chipuk et al., 2010). Anti-apoptotic BCL-2 proteins (e.g., BCL-xL) function to directly bind and inhibit the proapoptotic BCL-2 proteins. The proapoptotic members are divided into two classes. The effector molecules (e.g., BAK, BAX) directly engage MOMP by creating proteolipid pores responsible for cyto c release (Kuwana et al., 2002; Lindsten et al., 2000; Wei et al., 2000; Wei et al., 2001). The BH3-only proteins function in distinct cellular stress pathways to either directly activate BAK/BAX-dependent MOMP (e.g., BID), or by inhibiting the anti-apoptotic repertoire (e.g., BAD), which alters sensitivity to the direct activators and effectors (Chipuk et al., 2008; Kuwana et al., 2005; Letai et al., 2002).
Sphingolipid metabolism also regulates apoptosis, but how sphingolipids mechanistically intersect with BCL-2 family function remains obscure (Hannun and Obeid, 2008). Apoptotic inducers promote alterations in sphingolipid profiles, in particular ceramide profiles (Taha et al., 2006b). Exogenous ceramides applied to cells promotes apoptosis (Obeid et al., 1993); and genetic or pharmacological inhibition of ceramide production can result in apoptotic resistance (Alphonse et al., 2004; Liu et al., 2004; Pchejetski et al., 2005; Rodriguez-Lafrasse et al., 2002; Taha et al., 2006a)(Dai et al., 2004; Deng et al., 2008; Mesicek et al., 2010). A challenge remains to determine if sphingolipids and the BCL-2 family cooperate to regulate MOMP. Here we show that mitochondria actively maintain a sphingolipid milieu that promotes BAK/BAX function and apoptosis.
RESULTS
Microsomes Restore Mitochondrial Sensitivity to Direct Activator Stimulation
The isolation of heavy membrane (HM) fractions usually results in mitochondria that are contaminated with ER markers. Using a refinement of a published protocol (Csordas et al., 2006), we eliminated the majority of ER contamination and were able to obtain a highly pure mitochondrial population (Figures S1A-B). This purified fraction was designated mitochondria, whereas the subsequent light membrane, heterotypic fraction was termed microsomes (μS). We then examined the influence of these heterotypic species on mitochondrial sensitivity to recombinant caspase-8 cleaved BID (C8-BID)-induced BAK activation and cyto c release. BAK is the effector molecule under investigation in these experiments because C57Bl/6 liver HM fractions only contain BAK due to its integration within the OMM. BAX is a cytosolic effector protein and does not copurify using standard HM isolation techniques. In Figure 1A, HM fractions were treated with C8-BID and this resulted in a dose-dependent release of cyto c as measured by a change in localization from the pellet (p) to the supernatant (s). In comparison, mitochondria purified from the HM fraction were treated with the same doses of C8-BID and demonstrated reduced cyto c release, requiring 10-20 fold higher C8-BID concentrations to achieve minimal release.
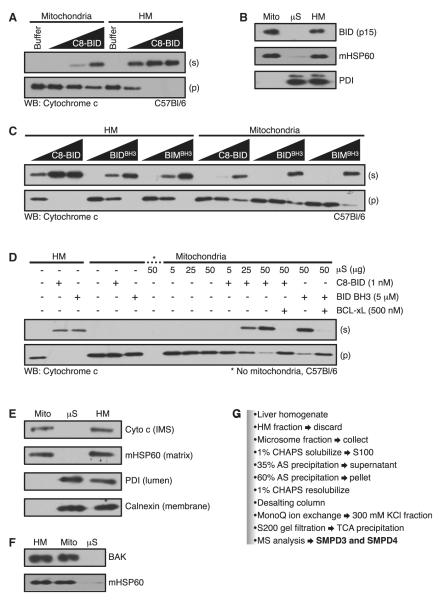
Figure 1. Microsomes Restore Mitochondrial Sensitivity to Direct Activator Stimulation.
(A) HM and mitochondria were treated with C8-BID (0.5, 1, and 5 nM).
(B) The HM fraction was isolated, and the μS and mitochondrial (Mito) fractions were purified. All three fractions were subjected to western blot. In parallel, each fraction was incubated with 10 nM C8-BID for 1 hr at 37°C, washed, and subjected to western blot.
(C) Same as in (A), but treatments included BID BH3 and BIM BH3 (1, 5, and 20 μM).
(D) HM (50 μg) and mitochondria (50 μg) were treated as indicated (C8-BID, 1 nM; BID BH3, 5 μM; BCL-xL, 500 nM; μS, 5, 25, and 50 μg).
(E) HM, mitochondrial, and μS fractions were subjected to western blot.
(F) HM, mitochondrial, and μS fractions were treated with limited proteolysis, lysed, and subjected to western blot.
(G) Purification scheme to identify SMPD3 and SMPD4.
See also Figure S1.
To determine if resistance to C8-BID induced cyto c release was due to decreased binding of C8-BID to mitochondria, we incubated the HM and mitochondrial fractions with C8-BID, washed the fractions, and subjected them to western blot for associated BID. Both fractions bound equal amounts of the BID p15 fragment suggesting the decreased sensitivity of mitochondria was independent of BID association (Figure 1B). Direct activator BH3-only protein function can be mimicked by short peptides derived from the BH3 domains of BID and BIM (referred to as BID BH3 and BIM BH3) (Kuwana et al., 2005; Letai et al., 2002). We compared the responses of the HM fraction versus mitochondria to BID BH3 and BIM BH3 induced cyto c release. The HM fraction treated with BID BH3 or BIM BH3 demonstrated greater sensitivity to cyto c release compared to mitochondria (Figure 1C), suggesting the effect was not specific to BID. To ensure that the purification of mitochondria did not alter their integrity, we measured their ability to respire and compared their rates of O2 consumption to the original HM fraction. The HM fraction and mitochondria exhibited similar rates of O2 consumption (Figure S1A, available online), and mitochondria appeared normal by electron microscopy (Figure S1B).
When mitochondria were purified from the HM fraction, there was a loss of 30%–40% of protein, which was recovered in the resulting μS fraction. We hypothesized that the μS fraction contained an activity required for maintaining sensitivity to C8-BID. Mitochondria alone incubated with C8-BID did not release cyto c, but the addition of μS promoted dose-dependent release of cyto c (Figure 1D). The combination of μS and BID BH3 also promoted cyto c release (Figure 1D). For both C8-BID and BID BH3 cooperation with μS, BCL-xL blocked cyto c release suggesting that microsome-regulated MOMP proceeded through BAK (Figure 1D). Cyto c and BAK levels did not change after addition of μS (Figures 1E and 1F).
As low concentrations of C8-BID (0.1 nM) did not induce MOMP in HM fractions, but the addition of solubilized mS synergized with C8-BID to produce robust MOMP (Figures 1A and 1D and Figure S1C), we subjected μS proteins to the enrichment protocol shown in Figure 1G and monitored synergy with C8-BID (Figures S1C-E, left). Peptide analysis of the final, enriched preparation revealed the presence two neutral sphingomyelinases (N-SMase; also referred to as SM phosphodiesterases), SMPD3 and SMPD4. The MOMP-promoting fractions were then examined for N-SMase activity by 14C-SM hydrolysis (Figures S1C-E, right). N-SMase activity correlated with fractions that synergized with C8-BID to promote MOMP. We therefore directly examined the effects of N-SMases on MOMP.
A Neutral Sphingomyelinase Activity Cooperates with the BCL-2 Family to Promote MOMP
To determine if SMPD3 or SMPD4 uniquely promoted MOMP, we compared SMPD2, 3, and 4 for their ability to sensitize WT HM fractions for C8-BID induced cyto c release. SMPD2, SMPD3, and SMPD4 were in vitro transcribed and translated (IVTT), and each was tested for 14C-SM hydrolysis (Figures 2A and 2B). We next analyzed the IVTT proteins for their abilities to promote C8-BID induced cyto c release. SMPD2-4 cooperated with C8-BID to promote MOMP, which was blocked by either the N-SMase inhibitor GW4869 or BCL-xL (Figure 2C). These data suggest that mitochondria and C8-BID cooperate with a general N-SMase activity. Furthermore, inhibition by GW4869 and BCL-xL confirmed that MOMP was promoted via SMase activity and through the activation of BAK, respectively (Figures 2B and 2C).
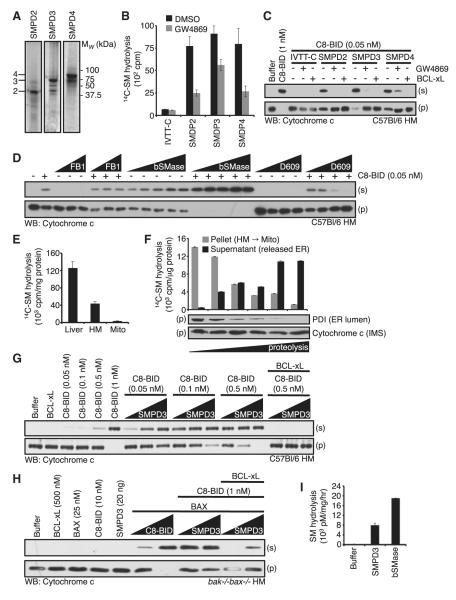
Figure 2. A Neutral SMase Activity Cooperates within the BCL-2 Family to Promote MOMP.
(A) SMPD2-4 IVTT with 35S-Met were subjected to SDS-PAGE. Lines denoting 2, 3, and 4 indicate full-length products.
(B) Nonradioactive IVTT reactions were performed and subjected to 14C-SM hydrolysis ± 10 μM GW4869.
(C) Nonradioactive IVTT reactions were performed and combined with WT HM fractions. C8-BID (1 nM for control; 0.05 nM for IVTT combinations); GW4869 (25 μM) and BCL-xL (50 nM).
(D) WT HM fractions were pretreated with FB1 (1, 5, 10, and 25 μM), bSMase (0.0001, 0.0005, 0.0010, and 0.0025U) or D609 (1, 5, and 25 μM) for 1 hr at 37°C before the addition of 0.05 nM C8-BID.
(E) Liver homogenate, solubilized HM fraction, or solubilized mitochondria (1 μg) were subjected to 14C-SM hydrolysis.
(F) HM fraction was subjected to limited proteolysis (0, 1, 2, 5, 10, and 20 min) on ice, and fractionated into pellet (mitochondria) and supernatant (contains released μS) before 14C-SM hydrolysis and western blot.
(G) WT HM fractions were combined with C8-BID (0.05, 0.1, 0.5, and 1 nM) or as indicated in the presence of SMPD3 (1, 5, and 20 ng) and BCL-xL (500 nM).
(H) bak-/-bax-/- HM fractions treated with SMPD3 (20 ng; or 1, 20 ng), BAX (25 nM), C8-BID (10 nM; or 1, 10 nM; or as indicated), and BCL-xL (500 nM).
(I) SMPD3 was tested for 14C-SM hydrolysis and compared to bSMase. All data are reported as ± SD.
See also Figure S2.
To further examine the cooperation between C8-BID and sphingolipid metabolism, we added a suboptimal concentration of C8-BID to WT HM fractions in the presence of either a ceramide synthase inhibitor in the de novo synthetic pathway (FB1), which does not rely on a SMase; or an inhibitor of SM synthase, D609, which can demonstrate coupling between SMases and SM synthases. A suboptimal concentration of C8-BID promoted a small amount of cyto c release that was inhibited by D609, but not FB1, further suggesting a potential role for a SMase in MOMP (Figure 2D). As an additional control, we compared WT and acid SMase knockout (asm-/-) HM fractions for their ability to sensitize WT HM fractions for MOMP as in figure S1E, and observed equivalent MOMP-promoting activities (Figures S2A–S2C). We next tested an N-SMase from B. cereus (bSMase) for its ability to cooperate with C8-BID. As shown in Figure 2D, bSMase promoted a small but detectible amount of cyto c release in WT HM; but when bSMase was titrated in the presence of C8-BID, the release of cyto c was robust and complete.
The above results suggest that removal of contaminating membranes from mitochondria reduced sensitivity to C8-BID induced MOMP. We hypothesized that mitochondrial purification from the HM fraction removed a SMase activity that was necessary for normal sensitivity to C8-BID. To address this, total liver homogenate, the HM fraction, and mitochondria were compared for 14C-SM hydrolysis. Approximately two-thirds of cellular N-SMase activity was lost during HM purification, yet the remaining N-SMase activity was present in the HM fraction. Limited proteolysis and further purification of mitochondria reduced the activity to below 5% (Figure 2E). This suggests that the N-SMase activity that cooperates with C8-BID is not mitochondrial in origin, but is present in the heterotypic membranes. To ensure that the purification protocol did not degrade or inhibit the released N-SMase activity, we analyzed the HM fraction, released heterotypic membranes, and mitochondria during a time course of proteolysis for 14C-SM hydrolysis. N-SMase activity in the HM fraction was released in a time-dependent manner, remained active, and was recoverable (Figure 2F). As controls, we monitored an ER luminal marker (PDI) and cyto c to ensure appropriate loss of heterotypic membrane species and equal analysis of mitochondria, respectively (Figure 2F).
We chose to focus on SMPD3 as the significant MOMP-promoting activity because it was consistently identified in our assays (Figure 1G) and was amenable to recombinant protein production (Figure 2I). We detected very few SMPD4 peptides in our mass spectroscopy analyses and could not produce recombinant SMPD4 after numerous attempts. Recombinant mouse SMPD3 was tested for 14C-SM hydrolysis and demonstrated significant activity (Figure 2I). SMPD3 was then analyzed for its ability to cooperate with C8-BID to promote MOMP. Using a WT HM fraction, we titrated suboptimal concentrations of C8-BID in the presence of increasing SMPD3. C8-BID at 0.05-0.5 nM promoted minimal cyto c release, but this was enhanced by the additional of SMPD3. For example, 0.5 nM C8-BID treatment of the HM fraction released only small amounts of cyto c; this was enhanced to nearly 100% release in the presence of all SMPD3 concentrations tested (Figure 2G). As WT HM fractions contain only BAK, we sought to examine the regulation of BAX-dependent MOMP by using a purified bak-/-bax-/- HM fraction. The addition of C8-BID and BAX reconstituted BAX-dependent MOMP in the bak-/-bax-/- HM fraction (Figure 2H). A concentration of C8-BID (1 nM) that was alone insufficient to promote MOMP was then added to BAX in the presence of increasing SMPD3. The addition of SMPD3 cooperated with 1 nM C8-BID and BAX to promote substantial MOMP (Figure 2H). For both BAK and BAX-dependent MOMP, the addition of BCL-xL blocked C8-BID induced cyto c release (Figures 2G and 2H).
Pharmacological Inhibition of Sphingolipid Metabolism Blocks MOMP
We next investigated a panel of SM pathway inhibitors to examine their effects on C8-BID induced BAK activation. The SM pathway products, enzymes, and inhibitors relevant to this study are summarized in Figure 3A. WT HM fractions were preincubated with increasing doses of GW4869, NOE (ceramidase inhibitor), MAPP (ceramidase inhibitor), SKI (sphingosine ‘SPH’ kinase inhibitor), DMS (SPH kinase inhibitor), DOP (sphingosine-1-PO4 ‘S1P’ lyase inhibitor), or THI (S1P lyase inhibitor) prior to the addition of C8-BID and analyzed for cyto c release (Figures 3B–3D). While none of the drugs alone promoted MOMP; GW4869, NOE, MAPP (Figure 3B), SKI, and DMS (Figure 3C) completely blocked C8-BID induced cyto c release. In the case of BAK-dependent MOMP, DOP and THI did not influence C8-BID induced cyto c release (Figure 3D).
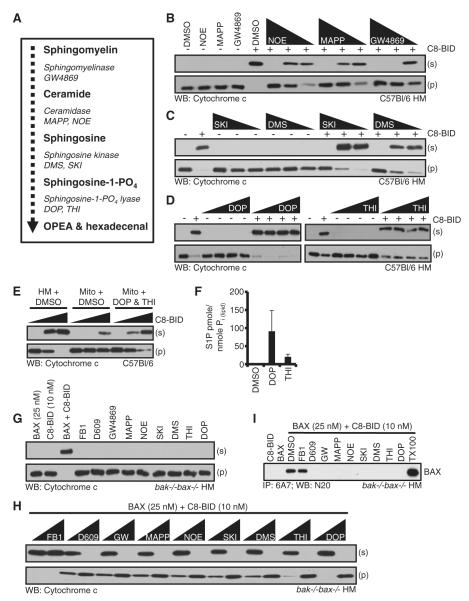
Figure 3. Inhibition of the Sphingolipid Pathway Blocks MOMP.
(A) Substrates, products, and enzymes, together with enzyme inhibitors tested.
(B–D) WT HM fractions were pretreated with the indicated drugs for 1 hr at 37°C prior to addition of C8-BID (1 nM). GW4869 (2, 10, and 20 μM), MAPP (2, 10, and 20 μM), NOE (0.1, 0.5, and 1 μM), DMS (2.5, 12.5, and 25 μM), SKI (5, 25, and 50 μM), DOP (1, 5, and 10 μM), THI (1, 5, and 10 μM).
(E) HM and subsequent mitochondria were purified in the presence of DOP/THI (10 μM each) before treatment with C8-BID (0, 0.5, and 1 nM).
(F) The levels of S1P were measured following isolation as in (E).
(G and H) bak-/-bax-/- HM fractions were pretreated with drugs (two higher doses from above) for 1 hr at 37°C prior to addition of BAX (25 nM) and C8-BID (10 nM).
(I) The HM fraction was pretreated with the highest dose of the inhibitors listed in (B)–(D) for 1 hr at 37°C, prior to addition of BAX (25 nM) and C8-BID (10 nM). Reactions were then incubated for 1 hr at 37°C before lysis in 1% CHAPS buffer and IP. A MOMP reaction lysed in 1% Triton X-100 is the positive control. All graphed data are reported as ± SD.
See also Figure S3.
These data suggest that S1P synthesis is essential for sensitivity of BAK activation in response to C8-BID. To test this hypothesis, a WT HM fraction and subsequent mitochondria were purified in the presence of DOP and THI to prevent S1P degradation. Mitochondria purified in the presence of DMSO were incubated with increasing doses of C8-BID and demonstrated resistance to C8-BID induced cyto c release (Figure 3E). However, mitochondria purified in the presence of DOP and THI demonstrated C8-BID sensitivity similar to the HM fraction (Figure 3E). Indeed, mitochondrial purification in the presence of either DOP or THI prevented the degradation of S1P and levels remained markedly higher compared with mitochondria purified in the presence of DMSO (Figure 3F and Figure S3A). The resulting concentrations of mitochondrial S1P following DOP or THI inhibition equaled approximately 1.8 μM and 0.42 μM, respectively. We based these calculations on 50 μg of mitochondrial protein per 50 μl MOMP reaction equals 10 nmole of total lipid Pi (Wagner and Rafael, 1977). As an example for DOP treatment, 91.118 pmoles of S1P/nmole lipid Pi was detected; therefore, 911.18 pmoles of S1P equals 1.8 μM S1P per 50 μl reaction.
As an additional control for the inhibitor studies, we analyzed GW4869, NOE, and MAPP for their capacity to regulate C8-BID induced MOMP in the absence of pretreatment (same treatments as in Figure 3B). This control was done to ensure that pretreatment was necessary as inhibition of MOMP following simultaneous drug and C8-BID treatment would likely suggest these drugs are not directly affecting sphingolipid levels (Figure S3B).
The same panel of SM pathway inhibitors was tested for their influence on C8-BID induced BAX activation using bak-/-bax-/- HM fractions. This fraction was preincubated with different doses of FB1, D609, GW4869, NOE, MAPP, SKI, DMS, DOP, or THI prior to the addition of BAX and C8-BID (Figures 3G and 3H). The inhibitors had no effect on the HM fraction in the absence of C8-BID and BAX (Figure 3G), but all the sphingolipid metabolism inhibitors blocked C8-BID plus BAX-induced cyto c release (Figure 3H). We performed the same assay as in Figures 3B–3D, but also probed for SMAC to ensure the effect is specific for MOMP and not cyto c mobilization (Figure S3C).
BAX-dependent cyto c release is associated with conformational changes at the amino terminus of BAX, which is recognized with the conformation-specific antibody, 6A7 (Hsu and Youle, 1997). To determine if the sphingolipid inhibitors blocked BAX conformational changes, we preincubated bak-/-bax-/- HM with the highest doses of the drugs used in Figure 3H for 1 hr, prior to the addition of BAX and C8-BID. The reactions were lysed and subjected to 6A7 immunoprecipitation (IP). The addition of C8-BID to the DMSO-treated HM fraction lead to 6A7-positive BAX. However, pretreatment with D609, GW4869, NOE, MAPP, SKI, DMS, DOP, or THI, but not FB1, led to no 6A7-positive BAX (Figure 3I). Furthermore, the same sphingolipid inhibitors that prevent BAX structural rearrangements also lead to the inhibition of BAX oligomerization (Figure S3D). These data suggest that a SM metabolic product beyond S1P is essential for sensitivity to C8-BID induced BAX activation (Figures 3H and 3I), which contrasts to the lipid requirement for BAK-dependent MOMP (Figure 3D).
Reconstitution of Sphingolipid Metabolism Replaces the Heterotypic Membrane Requirement for MOMP
To examine further if BAK and BAX-dependent cyto c release require different components of the SM pathway, we reconstituted the sphingolipid pathway with purified enzymes and lipids in the presence of WT or bak-/-bax-/- mitochondria. SM hydrolysis to ceramide was recapitulated by combining bSMase, SM, and WT mitochondria. After 1 hr incubation at 37°C, C8-BID was added. Reconstitution of SM hydrolysis alone did not promote MOMP, but the addition of C8-BID allowed for cyto c release, which was blocked by GW4869 (Figure 4A). The conversion of ceramide to SPH was recapitulated by combining recombinant N-acylsphingosine amidohydrolase 2 (ASAH2, neutral ceramidase), C16-Ceramide (C16), and WT mitochondria. After 1 hr incubation at 37°C, C8-BID was added. Reconstitution of ceramide hydrolysis alone did not promote MOMP, but the addition of C8-BID allowed for cyto c release, which was blocked by MAPP (Figure 4A). The conversion of ceramide to SPH was also recapitulated by combining SPH and WT mitochondria. SPH treatment alone did not promote MOMP, but the addition of C8-BID allowed for cyto c release, which was insensitive to both GW4869 and MAPP (Figure 4A). These data suggest that SPH is upstream of C8-BID induced BAK activation and MOMP.
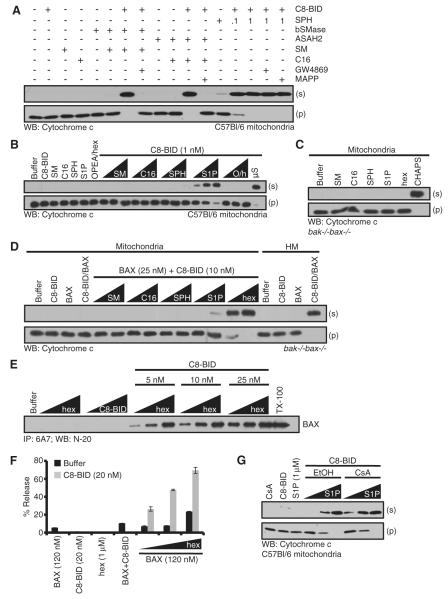
Figure 4. Reconstitution of the Sphingolipid Pathway Replaces the Heterotypic Membrane Requirement for MOMP.
(A) Purified mitochondria were preincubated for 1 hr with indicated sphingolipids (SM or C16, 1 μM), purified sphingolipid enzymes (bSMase or ASAH2, 0.001 U/μl) or drugs (GW4869 or MAPP, 25 μM) before the addition of C8-BID (1 nM). SPH was added at 0.1 or 1 μM.
(B) WT mitochondria were incubated with indicated sphingolipids (0.1, 0.5, and 1 μM) prior to the addition of C8-BID (1 nM). As a positive control, purified μS (50 μg) was also tested for sensitization.
(C) bak-/-bax-/- mitochondria were treated with indicated sphingolipids (1 μM).
(D) bak-/-bax-/- HM fractions and mitochondria were pretreated with BAX (25 nM), C8-BID (10 nM), and indicated sphingolipids (0.1 and 1 μM).
(E) BAX was incubated with hex (0.1, 0.5, and 1 μM) and C8-BID (5, 10, and 25 nM) for 1 hr at 37°C before IP with anti-BAX 6A7. Triton X-100 (0.5%) was added as a positive control for detergent-treatment activation.
(F) LUVs (PC, PE, PI, PS, no CL) were treated with indicated proteins and hex.
(G) Mitochondria were pretreated with CsA (100 nM) for 1 hr at 37°C prior to the addition of S1P (0.1, 0.5, and 1 μM) and C8-BID (1 nM). All data are reported as ± SD.
See also Figure S4.
Sphingolipid metabolites ranging from SM to ortho-phosphoethanolamine (OPEA) and hexadecenal (hex) were examined for their ability to sensitize WT mitochondria for C8-BID induced BAK activation. SM, C16, SPH, S1P, OPEA, and hex were titrated on WT mitochondria in the presence of C8-BID. The sphingolipids and C8-BID were added simultaneously to thwart the conversion of intermediates by mitochondrial sphingolipid enzymes. Based on our results in Figure 3F and the calculations detailed above, we used an average of the DOP and THI induced S1P concentrations (1.8 μM and 0.42 μM, respectively) as a benchmark for an approximate physiological concentration of sphingolipids to examine per assay (0.1, 0.5, and 1 μM). As shown in Figure 4B, the combination of S1P and C8-BID resulted in the complete release of cyto c. The same experiment was then conducted using bak-/-bax-/- mitochondria combined with BAX, and C8-BID induced BAX-dependent MOMP was reconstituted by the fatty aldehyde, hex (Figure 4D). None of the sphingolipid metabolites alone had an observable effect on bak-/-bax-/- mitochondria (Figure 4C); and the bak-/-bax-/- HM fraction responded to C8-BID and BAX as expected (Figure 4D). These results suggest that BAK and BAX-dependent MOMP require different components of the sphingolipid pathway: BAK cooperates with S1P, while BAX cooperates with hex.
To determine if hex promoted BAX activation and subsequent 6A7 positivity, we titrated C8-BID and hex with recombinant BAX (Figure 4E). Titration of hex or C8-BID with BAX did not promote 6A7 IP, but the combined treatment of C8-BID, hex, and BAX promoted dose-dependent 6A7 positivity (Figure 4E).
BAX, BID, and hex cooperation was examined using a large unilamellar vesicles (LUV) model system that faithfully measures BAX activation (Kuwana et al., 2002). LUVs containing 7% cardiolipin (CL) are generally used to promote BAX and C8-BID synergy and permeabilization, but CL is not highly represented at the OMM. Therefore, CL-free LUVs were examined for BAX function by combining them with C8-BID and titrating hex (Figure 4F). BAX, N/C-BID, and hex separately did not promote LUV permeabilization, and the combined treatment of BAX and N/C-BID resulted in only minimal release (Figure 4F). BAX and N/C-BID combined with hex caused dose-dependent permeabilization of the LUVs suggesting BAX, N/C-BID, and hex cooperation in BAX-mediated membrane permeabilization (Figure 4F). Importantly, the exogenous addition of ceramide, SPH, or S1P did not promote BAX activation to similar degrees as hex (Figures S4A and S4B). For additional control and comparison, we generated LUVs where the 7% CL was replaced with ceramide, SPH, or S1P, but none of these LUVs promoted efficient BAX activation compared to hex (Figure S4C). Together, these data suggest that hex can directly regulate BAX and BID function to promote MOMP.
One alternative explanation for S1P regulation of C8-BID induced, BAK-dependent cyto c release could be that sphingolipid metabolism induces transient openings of the mitochondrial permeability transition pore (MPTP) impacting on sensitivity to MOMP (Scorrano et al., 2002). To test this, we determined if S1P regulation of C8-BID induced, BAK-dependent cyto c release was sensitive to cyclosporine A (CsA), an inhibitor of MPTP. As shown in Figure 4G, S1P and C8-BID promoted BAK-dependent cyto c release that was insensitive to CsA, suggesting the MPTP was not involved.
Hexadecenal Binds and Promotes BAX Activation
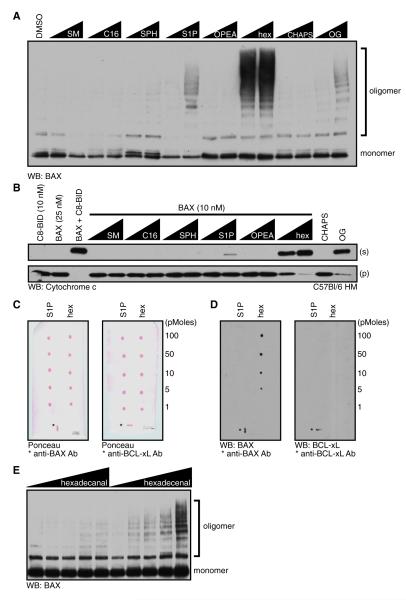
We noted that mid-range μM concentrations of hex directly promoted BAX activation akin to detergent treatment (Figures S4A and S4B). We took advantage of this observation to better understand the functional relationship between BAX and hex. Sphingolipid metabolites ranging from SM to hex were examined for their ability to directly induce BAX activation as measured by oligomerization (Figure 5A). BAX was treated with SM, C16, SPH, S1P, OPEA, or hex in the presence of BMH before analyzing oligomerization. All doses of hex promoted BAX oligomerization, whereas S1P promoted only weak BAX activation (Figure 5A). Octylglucoside (OG) and CHAPS were positive and negative controls in the assay, respectively. To determine if the BAX oligomers in Figure 5A could induce MOMP, the sphingolipid or detergent treated BAX samples were extensively dialyzed after treatment and added to bak-/-bax-/- mitochondria. Indeed, hex-activated BAX induced MOMP and cyto c release (Figure 5B).
Figure 5. Hexadecenal Binds and Promotes BAX Activation.
(A) BAX (10 nM) was incubated with indicated lipids or detergents (0.1 and 1 mM) in the presence of BMH (100 nM) for 1 hr at 37°C before western blot.
(B) As in (A), but instead of oligomerization analysis, samples were extensively dialyzed, combined with bak-/-bax-/- HM, and incubated for 1 hr at 37°C before fractionation, and western blot for cyto c.
(C and D) Nitrocellulose membranes spotted with indicated picomoles of S1P or hex (combined with BSA and ponceau red for visualization) were incubated with either 50 ng of BCL-xL or BAX and probed with anti-BCL-xL or anti-BAX (horizontal mark for anti-BCL-xL, vertical for anti-BAX). The positive controls (*) on the blots are spots containing an IP antibody for BCL-xL or BAX.
(E) BAX (10 nM) was incubated with hexadecanal or hex (0, 0.1, 1, 5, and 10 μM) in the presence of BMH (100 nM) for 1 hr at 37°C.
See also Figure S5.
The ability of hex to directly promote BAX oligomerization suggests that BAX and hex physically associate. To determine the specificity of this association, we analyzed the binding of BAX to commercially available sphingolipid arrays (Figures S5A and S5B). BAX was incubated with the sphingolipid arrays according to the manufacturer’s protocol, but we observed no detectable binding between BAX and the lipids tested. We therefore compared the binding of BAX to S1P and hex with a chloroform-spotted array using the same detection methods and observed a dose-dependent, BAX-hex association (Figure S5C and Figures 5C and 5D). The same assay was repeated comparing BAX and BCL-xL for hex binding, and while BAX bound to hex, a BCL-xL-hex interaction was not observed, suggesting that the binding of hex to the BAX protein is specific (Figure 5D). An immunoprecipitating antibody against BAX or BCL-xL was spotted onto the arrays as a positive control for the protein capture and detection conditions (Figure 5D). To probe the specificity of the hex-BAX association, hex and a closely related compound, hexadecanal, were titrated in the presence of BAX and BMH before analyzing BAX oligomerization (Figure 5E). Hex induced dose-dependent BAX oligomerization, whereas hexadecanal did not, suggesting that not all long chain aldehydes have the ability to induce BAX activation.
Sphingolipid Metabolism Regulates Sensitivity to Apoptosis and Clonogenic Survival
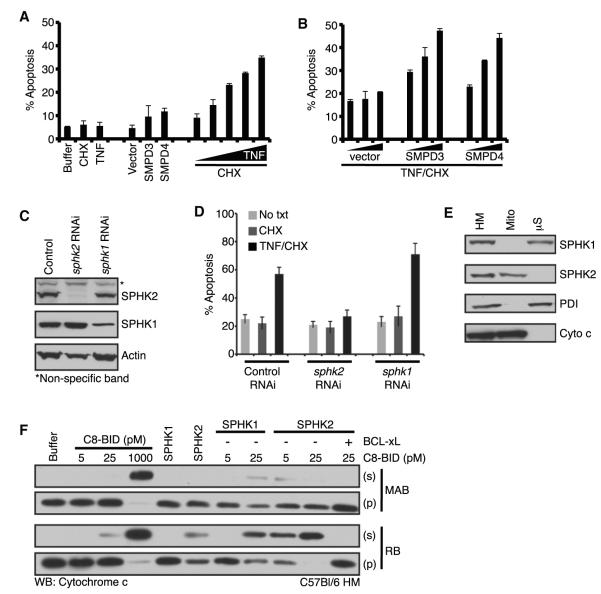
We tested whether the sphingolipid pathway regulates mitochondrial sensitivity to BAK and BAX activation and MOMP in a cellular context by observing the effect of enhancing or inhibiting sphingolipid metabolism. As a cellular model for death receptor-mediated, BID-dependent apoptosis, HeLa cells were titrated with different doses of TNF in the presence of cyclohexamide (CHX) and analyzed 8h later to determine an appropriate TNF concentration for further study. HeLa cells did not respond to TNF or CHX treatment alone; but they underwent dose-dependent apoptosis with TNF in the presence of CHX (Figure 6A). In a parallel experiment, we transiently transfected empty vector, SMPD3, or SMPD4. The exogenous expression of SMPD3 or SMPD4 enhanced the basal level of apoptosis, but not markedly, and the response 24 hr posttransfection did not increase when analyzed 48 and 72 hr posttransfection (Figure 6A and data not shown). We then examined the effect of exogenous SMPD3 or SMPD4 expression on TNF-induced apoptosis. We observed that the exogenous expression of SMPD3 or SMPD4 greatly enhanced sensitivity to TNF-induced apoptosis in a dose-dependent manner, whereas expression of the vector alone did not influence survival (Figure 6B).
Figure 6. SMPD3 and SMPD4 Regulate Sensitivity to Apoptosis, and a Mitochondrial Sphingosine Kinase Activity Sensitizes for MOMP.
(A) HeLa cells were transfected with vector, SMPD3, or SMPD4; or treated with CHX (10 μg/ml), TNF (25 ng/ml; or 5, 10, 15, 20, and 25 ng/ml); cultured for 24 hr and stained with Annexin V-PE.
(B) HeLa cells were transfected with 100, 500, 1000 ng of vector, SMPD3 or SMPD4, cultured for 24 hr, treated with CHX (10 μg/ml) and TNF (15 ng/ml), cultured for 6 hr and stained with Annexin V-PE.
(C) WT MEFs retrovirally infected to silence sphk1 or sphk2 were analyzed by western blot.
(D) Control, sphk1, and sphk2 RNAi cell lines were treated with CHX (5 μg/ml) and TNF (10 ng/ml), cultured for 6 hr, and stained with Annexin V-PE.
(E) HM fraction, mitochondria, and μS were subjected to western blot.
(F) WT HM fractions were treated with C8-BID (5, 25, 1000* pM, *positive control), SPHK1 (25 ng), SPHK2 (25 ng), BCL-xL (50 nM) in MAB or RB.
See also Figure S6.
As BH3-only proteins are essential to the induction of MOMP, we tested if SMPD3 cooperated with exogenous BH3-only protein expression to promote apoptosis. We chose to evaluate three BH3-only proteins: BIM-S and tBID, two well-defined direct activator BH3-only proteins and potent inducers of apoptosis; and BAD, a de-repressor BH3-only protein that is not a strong inducer of apoptosis (Kuwana et al., 2005; Letai et al., 2002). WT mouse embryonic fibroblasts (MEFs) were transiently transfected with either empty vector or SMPD3 in the presence of increasing masses of BIM-S, BAD, or tBID cDNA and analyzed 24 hr later. As shown in Figure S6A, BIM-S and tBID expression demonstrated dose-dependent induction of apoptosis, and this was enhanced by SMPD3 expression. BAD expression alone did not induce marked apoptosis, as expected, but there was an observable cooperation between BAD and SMPD3. To ensure that these observations were due to the enzymatic activity of SMPD3, we tested two SMPD3 catalytic mutants (SMPD3D428A and SMPD3H639A) for synergy with BIM-S (Milhas et al., 2010). While WT SMPD3 synergized with BIM-S in a dose-dependent manner, both SMPD3 mutants failed to synergize with BIM-S to promote apoptosis (Figure S6B). We probed BCL-2 family expression following SMPD3 and SMPD4 transient expression to ensure that the enhanced apoptosis was not due to changes in pro- or antiapoptotic protein expression (Figure S6C).
Our study suggests that a SPH kinase activity is required upstream of both BAK- and BAX-dependent MOMP. To examine this requirement in cells, we used WT MEFs in which sphk1 or sphk2 was silenced by RNAi and tested their sensitivity to TNF-induced apoptosis. Lysates from the sphk1 and sphk2 knockdown cells were confirmed for downregulation of SPHK1 and SPHK2 protein (Figure 6C). Control, sphk1, and sphk2 RNAi cell lines were then treated with CHX and TNF, cultured for 6 hr, and analyzed by flow cytometry (Figure 6D). Control and sphk1 RNAi cells were sensitive to TNF-induced apoptosis; however, the loss of sphk2 expression resulted in resistance to apoptosis (Figure 6D). Silencing of sphk2 in MEFs also resulted in marked resistance to the ActD induced apoptosis (Figure S6D). Reduction in apoptosis was not due to changes in BCL-2 family expression following sphk2 knockdown (Figure S6E).
As loss of sphk2 expression correlated with reduced apoptosis and our previous data demonstrate that changes in S1P regulate mitochondrial sensitivity to MOMP, we evaluated the subcellular localization of SPHK2. While the HM fraction contained both SPHK1 and SPHK2, the majority of SPHK1 and SPHK2 were localized to the mS and mitochondria, respectively. The purity of the fractions was confirmed by localization of PDI and cyto c (Figure 6E). SPHK2 activity was also analyzed by an enzymatic assay specific to SPHK2 comparing μS and mitochondria; and this confirmed that mitochondria contain functional SPHK2 (Figures S6F and S6G). We also compared the endogenous mitochondrial SPHK2 activity against recombinant SPHK2 to ensure specificity within our assay (Figures S6F and S6G).
To directly test the role of SPHK2 in regulating sensitivity to C8-BID, we analyzed the influence of SPHK2 (Figures S6F and S6G) on mediating C8-BID induced, BAK activation, and MOMP. WT HM fractions were treated with C8-BID in the presence or absence of SPHK2. Neither C8-BID at 5 or 25 pM, nor SPHK2 independently promoted MOMP, but when combined, C8-BID and SPHK2 induced complete cyto c release that was inhibited by BCL-xL (Figure 6F, lower panels). Using traditional MOMP conditions, our pharmacological inhibitor data in Figures 3C and 3H demonstrate that a SPH kinase is active, suggesting that this enzyme has access to a residual ATP pool within the HM fraction. As we are adding SPHK2 to the reactions and the accessibility of the ATP pool was unknown, we provided a means of ATP production. We compared MOMP reactions using mitochondrial assay buffer (MAB, typical buffer used in MOMP assays, no additional ATP source, upper panels), and respiration buffer (RB, supplemented with mitochondrial complex substrates and ADP to generate ATP, lower panels), and observed that the effect of SPHK2 indeed required an ATP source (Figure 6F, compare upper and lower panels). Additionally, we examined recombinant SPHK1 plus C8-BID and also observed a MOMP-promoting activity, albeit less than with SPHK2, suggesting that the mitochondrial localization of SPHK2 likely promotes MOMP (Figure 6F).
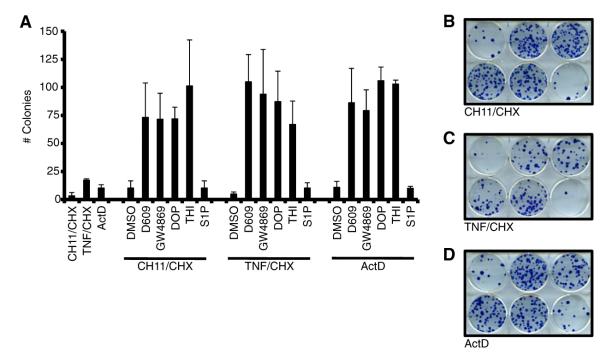
We next tested several pharmacological regulators of the sphingolipid pathway to determine their influence on cellular apoptosis and clonogenic survival. HeLa cells were treated with all the previous inhibitors of sphingolipid metabolism; however, only D609, GW4869, DMS, SKI, DOP, and THI permitted further analysis as the other drugs (FB1, MAPP, and NOE) were toxic at the doses and/or treatment times required for the survival assays (Figure S7A, and data not shown). HeLa cells were pretreated with D609, GW4869, DMS, SKI, DOP, and THI for 6 h; proapoptotic stimulation was added, cells were cultured for 24 hr, and analyzed. D609, GW4869, DMS, SKI, DOP, and THI each afforded protection from the three proapoptotic stimuli, suggesting that pharmacologically targeting the sphingolipid metabolic pathway at various stages can directly impact on cellular survival (Figures S7B-D). Moreover, the clonogenic potential of these cells was also assayed; however, DMS and SKI were toxic in this assay and could not be evaluated (data not shown). As in Figures S7B–S7D, HeLa cells were pretreated with D609, GW4869, DOP, and THI, proapoptotic stimulation was added and maintained for 12 hr, then cultured for two weeks prior to staining with methylene blue. Inhibition of N-SMase activity or the S1P lyase activity resulted in observable clonogenic survival despite apoptosis signaling through the extrinsic or intrinsic pathways (Figures 7A–7D). As a control for the loss of S1P lyase function, which could result in enhanced extracellular S1P signaling, a S1P treatment group was also included, but this did not result in enhanced clonogenic survival (Pyne et al., 2009).
Figure 7. The SMase Pathway Regulates Sensitivity to Apoptosis.
(A) HeLa cells were pretreated with indicated pharmacological inhibitors for 6 hr, apoptotic inducers (CH11/CHX, 0.4 μg/ml + 10 μg/ml CHX; 30 ng/ml TNF + 10 μg/ml CHX; or 50 nM ActD) added for 24 hr, and colonies were stained with methylene blue 12 days later. S1P (0.25 μM) was added at the same time as the inhibitors.
(B–D) Colonies from CH11/CHX, TNF/CHX, and ActD treatments are shown. Wells were treated as indicated (top to bottom, left to right): (1) DMSO, (2) 50 μM D609, (3) 20 μM GW4869, (4) 100 μM DOP, (5) 100 μM THI, and (6) 0.25 μM S1P.
All data are reported as ± SD. See also Figure S7.
DISCUSSION
Prior to the elucidation of caspase-activation mechanisms, a prominent notion held that SM hydrolysis and subsequent ceramide formation had a direct fundamental role in engaging cell death (Obeid et al., 1993); yet molecular mechanisms whereby ceramide initiates and executes apoptosis have been scant (Bartke and Hannun, 2009). A direct role for ceramide in mitochondrial integrity has been proposed (Siskind et al., 2002, 2006), but our studies suggest an alternative mechanistic view, such that ceramide is a precursor of two molecules, S1P and hex, which facilitate BAK/BAX activation leading to MOMP.
We propose that ceramide is produced by the constitutive hydrolysis of SM by a N-SMase in a mitochondria-associated compartment. Ceramide is then transferred to mitochondria, where enzymes further convert ceramide to produce S1P and hex. Indeed, evidence exists that N-SMases are associated with the heavy and light membrane fractions, and ceramide transport between organelles is described (Hanada et al., 2009; Sawai et al., 2000; Stiban et al., 2008; Wu et al., 2010; Yabu et al., 2009). Upon purification of mitochondria from the HM fraction, sensitivity to C8-BID induced BAK/BAX activation rapidly wanes unless components of the sphingolipid pathway are provided or metabolism is inhibited. We propose that mitochondria may regulate their sphingolipid composition through both forward (ceramide - SPH - S1P) and reverse pathways (S1P - SPH - ceramide).
SM hydrolysis and subsequent changes in sphingolipid profiles are associated with enhanced sensitivity to proapoptotic stimulation. In breast cancer cells, a bSMase targeted to mitochondria was shown to promote apoptosis, and it was suggested that mitochondrial SM hydrolysis enhanced BAX mitochondrial translocation (Birbes et al., 2001, 2005). Proapoptotic stimuli promote SM hydrolysis and marked alterations to the intracellular sphingolipid profile, perhaps to establish the appropriate milieu at the OMM to promote BAK/BAX function. For instance, TNF specifically induces SMPD3 function in MCF-7 cells to promote SM hydrolysis to ceramide (Clarke et al., 2011). Indeed, ceramide has been suggested to directly regulate the permeabilization function of BAX using isolated mitochondria and phospholipid membranes; and a recent report suggests that mitochondrial macrodomains rich in ceramide promote BAX activity (Ganesan et al., 2010; Lee et al., 2011). In support of this hypothesis, reversing sphingolipid metabolism to favor SM accumulation decreased sensitivity to BAX-mediated death (Yang et al., 2006).
Earlier observations suggested that CL is required for MOMP, however the presence of this lipid in the OMM is controversial (Kuwana et al., 2002; Lutter et al., 2000; Ott et al., 2009). Our studies suggest that mitochondria interact with distinct cellular components to establish the sphingolipid milieu that is compatible with MOMP. Indeed, we have found that lipid vesicles lacking CL can be permeabilized by the combination of BID and BAX, provided hex is present. Several proteins (e.g., DRP-1, MTCH2, OPA-1, and TOM22) are also implicated in orchestrating MOMP, by coordinating BAX and/or BID function at the OMM or by directly regulating the mitochondrial network, and it will be necessary to determine how S1P and hex potentially cooperate with the above proteins to fully appreciate the mechanisms that control BAK and BAX activation (Bellot et al., 2007; Cassidy-Stone et al., 2008; Montessuit et al., 2010; Yamaguchi et al., 2008; Zaltsman et al., 2010). Structural and biochemical studies suggest that BAK/BAX activation involves a displacement of helix a1, leading to distal exposure of the BH3 region of these molecules (Dewson et al., 2008; Gavathiotis et al., 2008). The binding of S1P to BAK, or hex to BAX, may act to lower the thermodynamic constraints on these conformational changes. In support of this idea, we found that BID-induced exposure the 6A7 epitope on BAX is facilitated by hex, which directly binds to BAX.
TNF- and UV-induced apoptosis are reported to signal through N-SMase-regulated pathways (Clarke et al., 2011; Dai et al., 2004; Luberto et al., 2002). We also observed that several such inhibitors act to preserve cell survival following treatments that normally induce apoptosis. These observations support the idea that downstream components of the pathway, such as S1P and hex, promote MOMP and cell death. Our finding that S1P lyase inhibitors supported cell survival (where potentially BAK activation, facilitated by S1P could still occur) is consistent with the protective effects of bax deletion in cells (Zhang et al., 2000). Interestingly, S1P lyase deficiency is described to promote DNA damage resistance and enhanced tumor xenograft formation (Colie et al., 2009; Oskouian et al., 2006). The expression of S1P lyase is also reduced in colon cancer cells, which are suggested to primarily utilize BAX to promote apoptosis highlighting the potential cooperation between BAX and SPH lyase function and its products (Oskouian et al., 2006).
Ceramide production can occur in cells independently of the N-SMases, such as by acidic SMase or ceramide synthase, both of which can be activated by stress conditions (Kolesnick and Fuks, 2003; Santana et al., 1996). Increased ceramide production by these mechanisms may contribute to MOMP by providing precursors at the OMM. S1P lyase appears to directly impact on cellular survival following DNA damage, and studies suggest that hex coordinates apoptosis through BID, BIM, and BAX (Kumar et al., 2011a; Kumar et al., 2011b). Finally, SMPD3 is lost or mutated in a subset of human lymphomas (Kim et al., 2008), and the SMPD3 promoter is shown to be hyper-methylated in aggressive breast tumors (Demircan et al., 2009). It is tempting to speculate that such changes can facilitate cell survival by restricting MOMP through the mechanisms we describe.
EXPERIMENTAL PROCEDURES
Heavy Membrane Fractions, Mitochondrial Purification, and Cyto-c Release Assay
Heavy membrane fractions were purified from murine liver using dounce homogenization and differential centrifugation in mitochondrial isolation buffer. To purify mitochondria from the HM fraction, the HM fraction was prepared in the absence of BSA, and 1 μg/mg of proteomics grade trypsin was added and incubated on ice for 10 min. Soybean trypsin inhibitor (10 mg/mg trypsin) was added, incubated on ice for 30 min, and the trypsinized fraction was loaded on top of a 26%/60% Percoll gradient. Mitochondria at the 26%/60% Percoll interface were removed with an 18 gauge syringe, and washed twice in MIB. For MOMP assays, HM fractions or mitochondria were incubated in MIB supplemented to 110 mM KCl (mitochondrial assay buffer, MAB), plus or minus proteins and lipids for 1 hr at 37°C. Reactions were then centrifuged at 5,500 × g for 10 min, the resulting pellets and supernatants were analyzed for cyto c by SDS-PAGE and western blot. For high throughput, total cyto c was occasionally determined by a sample containing mitochondria solubilized in 1% CHAPS instead of analyzing the pellet fractions.
Acidic and N-SMase Assay
For acidic SMase (asm) analysis, samples were prepared in 0.2 M sodium acetate pH 5.0 and 0.1% Triton X-100. For N-SMase analysis, samples were prepared in 25 mM Tris-HCl (pH 7.4), 5 mM EDTA and 0.2% Triton X-100. Samples were incubated for 30 min at 37°C, subjected to Folch extraction and the amount of choline-methyl-14C release was measured by scintillation counting.
Survival Assays
HeLa cells and MEFs were transfected, then treated with TNF and CHX, cultured for 24 hr, harvested by trypsinization, stained with AnnexinV and analyzed by flow cytometry. For pharmacological inhibitor studies, HeLa cells were pretreated with indicated pharmacological inhibitors of the SM pathway for 6 hr before apoptotic inducers (anti-Fas/CD95 antibody TNF or ActD) were added. For clonogenic survival studies, HeLa cells were pretreated with indicated pharmacological inhibitors of the SM pathway for 6 hr, apoptotic inducers added, and cells cultured for 12 hr before changing the media. Colonies were stained with methylene blue 12 days after treatment.
Please see the online Extended Experimental Procedures for more details.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Dr. Ronald Gordon (MSSM) for TEM analysis; Dr. Richard Kolesnick (Memorial Sloan-Kettering) for the asm-/- liver; Dr. Joseph Opferman (SJCRH) for the MxCre bak-/-bax-/f animals; all members of the Obeid and Hannun laboratories (MUSC) for excellent discussion and training in sphingolipid biology, especially Drs. K. Alexa Orr Gandy, Stefka Spassieva, and Xingjun Wu; The Hartwell Center for Bioinformatics and Biotechnology; and members of the Kuwana Laboratory for technical assistance. This work was supported by: NIH CA157740 (to J.E.C.), pilot project from NIH P20AA017067 (to J.E.C.), NIH AI52735 (to D.R.G.), CA69381 (to D.R.G.), NIH AG024478 (to T.K.), an NCI Cancer Center Core Grant P30CA21765 (SJCRH), VA Merit (to L.M.O.), VA CDA-2 Award (to L.J.S), pilot project from the VA REAP (to L.J.S., and L.M.O.), the ACS IRG #IRG-97-219-11 (subaward to L.J.S.), Lipidomics Shared Resource of the Hollings Cancer Center supported by a Cancer Center Support Grant (P30 CA138313), pilot project (to L.J.S) from the NIH/NCRR P20 RR17677 COBRE in Lipidomics and Pathobiology, and the American Lebanese Syrian Associated Charities. This work was also supported in part by a Research Grant 5-FY11-74 from the March of Dimes Foundation (to J.E.C.).
Footnotes
SUPPLEMENTAL INFORMATION Supplemental Information includes Extended Experimental Procedures and seven figures and can be found with this article online at doi:10.1016/j.cell. 2012.01.038.
REFERENCES
- Alphonse G, Bionda C, Aloy MT, Ardail D, Rousson R, Rodriguez-Lafrasse C. Overcoming resistance to gamma-rays in squamous carcinoma cells by poly-drug elevation of ceramide levels. Oncogene. 2004;23:2703–2715. doi: 10.1038/sj.onc.1207357. [DOI] [PubMed] [Google Scholar]
- Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J. Lipid Res. Suppl. 2009;50:S91–S96. doi: 10.1194/jlr.R800080-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellot G, Cartron PF, Er E, Oliver L, Juin P, Armstrong LC, Bornstein P, Mihara K, Manon S, Vallette FM. TOM22, a core component of the mitochondria outer membrane protein translocation pore, is a mitochondrial receptor for the proapoptotic protein Bax. Cell Death Differ. 2007;14:785–794. doi: 10.1038/sj.cdd.4402055. [DOI] [PubMed] [Google Scholar]
- Birbes H, El Bawab S, Hannun YA, Obeid LM. Selective hydrolysis of a mitochondrial pool of sphingomyelin induces apoptosis. FASEB J. 2001;15:2669–2679. doi: 10.1096/fj.01-0539com. [DOI] [PubMed] [Google Scholar]
- Birbes H, Luberto C, Hsu YT, El Bawab S, Hannun YA, Obeid LM. A mitochondrial pool of sphingomyelin is involved in TNFalpha-induced Bax translocation to mitochondria. Biochem. J. 2005;386:445–451. doi: 10.1042/BJ20041627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer-membrane permeabilization. Dev. Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Fisher JC, Dillon CP, Kriwacki RW, Kuwana T, Green DR. Mechanism of apoptosis induction by inhibition of the antiapoptotic BCL-2 proteins. Proc. Natl. Acad. Sci. USA. 2008;105:20327–20332. doi: 10.1073/pnas.0808036105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol. Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke CJ, Cloessner EA, Roddy PL, Hannun YA. Neutral sphingomyelinase 2 (nSMase2) is the primary neutral sphingomyelinase isoform activated by tumour necrosis factor-alpha in MCF-7 cells. Biochem. J. 2011;435:381–390. doi: 10.1042/BJ20101752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colie S, Van Veldhoven PP, Kedjouar B, Bedia C, Albinet V, Sorli SC, Garcia V, Djavaheri-Mergny M, Bauvy C, Codogno P, et al. Disruption of sphingosine 1-phosphate lyase confers resistance to chemotherapy and promotes oncogenesis through Bcl-2/Bcl-xL upregulation. Cancer Res. 2009;69:9346–9353. doi: 10.1158/0008-5472.CAN-09-2198. [DOI] [PubMed] [Google Scholar]
- Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Q, Liu J, Chen J, Durrant D, McIntyre TM, Lee RM. Mitochondrial ceramide increases in UV-irradiated HeLa cells and is mainly derived from hydrolysis of sphingomyelin. Oncogene. 2004;23:3650–3658. doi: 10.1038/sj.onc.1207430. [DOI] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- Demircan B, Dyer LM, Gerace M, Lobenhofer EK, Robertson KD, Brown KD. Comparative epigenomics of human and mouse mammary tumors. Genes Chromosomes Cancer. 2009;48:83–97. doi: 10.1002/gcc.20620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Yin X, Allan R, Lu DD, Maurer CW, Haimovitz-Friedman A, Fuks Z, Shaham S, Kolesnick R. Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C. elegans. Science. 2008;322:110–115. doi: 10.1126/science.1158111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewson G, Kratina T, Sim HW, Puthalakath H, Adams JM, Colman PM, Kluck RM. To trigger apoptosis, Bak exposes its BH3 domain and homodimerizes via BH3:groove interactions. Mol. Cell. 2008;30:369–380. doi: 10.1016/j.molcel.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Ganesan V, Perera MN, Colombini D, Datskovskiy D, Chadha K, Colombini M. Ceramide and activated Bax act synergistically to permeabilize the mitochondrial outer membrane. Apoptosis. 2010;15:553–562. doi: 10.1007/s10495-009-0449-0. [DOI] [PubMed] [Google Scholar]
- Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, Tu HC, Kim H, Cheng EH, Tjandra N, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Kumagai K, Tomishige N, Yamaji T. CERT-mediated trafficking of ceramide. Biochim. Biophys. Acta. 2009;1791:684–691. doi: 10.1016/j.bbalip.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the Bcl-2 family. J. Biol. Chem. 1997;272:13829–13834. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- Kim WJ, Okimoto RA, Purton LE, Goodwin M, Haserlat SM, Dayyani F, Sweetser DA, McClatchey AI, Bernard OA, Look AT, et al. Mutations in the neutral sphingomyelinase gene SMPD3 implicate the ceramide pathway in human leukemias. Blood. 2008;111:4716–4722. doi: 10.1182/blood-2007-10-113068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene. 2003;22:5897–5906. doi: 10.1038/sj.onc.1206702. [DOI] [PubMed] [Google Scholar]
- Kumar A, Byun HS, Bittman R, Saba JD. The sphingolipid degradation product trans-2-hexadecenal induces cytoskeletal reorganization and apoptosis in a JNK-dependent manner. Cell. Signal. 2011a;23:1144–1152. doi: 10.1016/j.cellsig.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Oskouian B, Fyrst H, Zhang M, Paris F, Saba JD. S1P lyase regulates DNA damage responses through a novel sphingolipid feedback mechanism. Cell Death Dis. 2011b;2:e119. doi: 10.1038/cddis.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- Lee H, Rotolo JA, Mesicek J, Penate-Medina T, Rimner A, Liao WC, Yin X, Ragupathi G, Ehleiter D, Gulbins E, et al. Mitochondrial Ceramide-Rich Macrodomains Functionalize Bax upon Irradiation. PLoS ONE. 2011;6:e19783. doi: 10.1371/journal.pone.0019783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate Mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YY, Han TY, Yu JY, Bitterman A, Le A, Giuliano AE, Cabot MC. Oligonucleotides blocking glucosylceramide synthase expression selectively reverse drug resistance in cancer cells. J. Lipid Res. 2004;45:933–940. doi: 10.1194/jlr.M300486-JLR200. [DOI] [PubMed] [Google Scholar]
- Luberto C, Hassler DF, Signorelli P, Okamoto Y, Sawai H, Boros E, Hazen-Martin DJ, Obeid LM, Hannun YA, Smith GK. Inhibition of tumor necrosis factor-induced cell death in MCF7 by a novel inhibitor of neutral sphingomyelinase. J. Biol. Chem. 2002;277:41128–41139. doi: 10.1074/jbc.M206747200. [DOI] [PubMed] [Google Scholar]
- Lutter M, Fang M, Luo X, Nishijima M, Xie X, Wang X. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nat. Cell Biol. 2000;2:754–761. doi: 10.1038/35036395. [DOI] [PubMed] [Google Scholar]
- Mesicek J, Lee H, Feldman T, Jiang X, Skobeleva A, Berdyshev EV, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell. Signal. 2010;22:1300–1307. doi: 10.1016/j.cellsig.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milhas D, Clarke CJ, Idkowiak-Baldys J, Canals D, Hannun YA. Anterograde and retrograde transport of neutral sphingomyelinase-2 between the Golgi and the plasma membrane. Biochim. Biophys. Acta. 2010;1801:1361–1374. doi: 10.1016/j.bbalip.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montessuit S, Somasekharan SP, Terrones O, Lucken-Ardjomande S, Herzig S, Schwarzenbacher R, Manstein DJ, Bossy-Wetzel E, Basanez G, Meda P, et al. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell. 2010;142:889–901. doi: 10.1016/j.cell.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- Oskouian B, Sooriyakumaran P, Borowsky AD, Crans A, Dillard-Telm L, Tam YY, Bandhuvula P, Saba JD. Sphingosine-1-phosphate lyase potentiates apoptosis via p53- and p38-dependent pathways and is down-regulated in colon cancer. Proc. Natl. Acad. Sci. USA. 2006;103:17384–17389. doi: 10.1073/pnas.0600050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M, Norberg E, Zhivotovsky B, Orrenius S. Mitochondrial targeting of tBid/Bax: a role for the TOM complex? Cell Death Differ. 2009;16:1075–1082. doi: 10.1038/cdd.2009.61. [DOI] [PubMed] [Google Scholar]
- Pchejetski D, Golzio M, Bonhoure E, Calvet C, Doumerc N, Garcia V, Mazerolles C, Rischmann P, Teissie J, Malavaud B, et al. Sphingosine kinase-1 as a chemotherapy sensor in prostate adenocarcinoma cell and mouse models. Cancer Res. 2005;65:11667–11675. doi: 10.1158/0008-5472.CAN-05-2702. [DOI] [PubMed] [Google Scholar]
- Pyne NJ, Long JS, Lee SC, Loveridge C, Gillies L, Pyne S. New aspects of sphingosine 1-phosphate signaling in mammalian cells. Adv. Enzyme Regul. 2009;49:214–221. doi: 10.1016/j.advenzreg.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lafrasse C, Alphonse G, Aloy MT, Ardail D, Gerard JP, Louisot P, Rousson R. Increasing endogenous ceramide using inhibitors of sphingolipid metabolism maximizes ionizing radiation-induced mitochondrial injury and apoptotic cell killing. Int. J. Cancer. 2002;101:589–598. doi: 10.1002/ijc.10652. [DOI] [PubMed] [Google Scholar]
- Santana P, Pena LA, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, Cordon-Cardo C, Schuchman EH, Fuks Z, Kolesnick R. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86:189–199. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- Sawai H, Okamoto Y, Luberto C, Mao C, Bielawska A, Domae N, Hannun YA. Identification of ISC1 (YER019w) as inositol phosphosphingolipid phospholipase c in Saccharomyces cerevisiae. J. Biol. Chem. 2000;275:39793–39798. doi: 10.1074/jbc.M007721200. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, Korsmeyer SJ. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev. Cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- Siskind LJ, Kolesnick RN, Colombini M. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J. Biol. Chem. 2002;277:26796–26803. doi: 10.1074/jbc.M200754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ, Kolesnick RN, Colombini M. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion. 2006;6:118–125. doi: 10.1016/j.mito.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiban J, Caputo L, Colombini M. Ceramide synthesis in the endoplasmic reticulum can permeabilize mitochondria to proapoptotic proteins. J. Lipid Res. 2008;49:625–634. doi: 10.1194/jlr.M700480-JLR200. [DOI] [PubMed] [Google Scholar]
- Taha TA, Kitatani K, El-Alwani M, Bielawski J, Hannun YA, Obeid LM. Loss of sphingosine kinase-1 activates the intrinsic pathway of programmed cell death: modulation of sphingolipid levels and the induction of apoptosis. FASEB J. 2006a;20:482–484. doi: 10.1096/fj.05-4412fje. [DOI] [PubMed] [Google Scholar]
- Taha TA, Mullen TD, Obeid LM. A house divided: ceramide, sphingosine, and sphingosine-1-phosphate in programmed cell death. Biochim. Biophys. Acta. 2006b;1758:2027–2036. doi: 10.1016/j.bbamem.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T, Rafael J. Biochemical properties of liver megamitochondria induced by chloramphenicol or cuprizone. Exp. Cell Res. 1977;107:1–13. doi: 10.1016/0014-4827(77)90379-2. [DOI] [PubMed] [Google Scholar]
- Walter L, Hajnoczky G. Mitochondria and endoplasmic reticulum: the lethal interorganelle cross-talk. J. Bioenerg. Biomembr. 2005;37:191–206. doi: 10.1007/s10863-005-6600-x. [DOI] [PubMed] [Google Scholar]
- Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu BX, Rajagopalan V, Roddy PL, Clarke CJ, Hannun YA. Identification and characterization of murine mitochondria-associated neutral sphingomyelinase (MA-nSMase), the mammalian sphingomyelin phosphodiesterase 5. J. Biol. Chem. 2010;285:17993–18002. doi: 10.1074/jbc.M110.102988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabu T, Shimuzu A, Yamashita M. A novel mitochondrial sphingomyelinase in zebrafish cells. J. Biol. Chem. 2009;284:20349–20363. doi: 10.1074/jbc.M109.004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi R, Lartigue L, Perkins G, Scott RT, Dixit A, Kushnareva Y, Kuwana T, Ellisman MH, Newmeyer DD. Opa1-mediated cristae opening is Bax/Bak and BH3 dependent, required for apoptosis, and independent of Bak oligomerization. Mol. Cell. 2008;31:557–569. doi: 10.1016/j.molcel.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Khoury C, Jean-Baptiste G, Greenwood MT. Identification of mouse sphingomyelin synthase 1 as a suppressor of Bax-mediated cell death in yeast. FEMS Yeast Res. 2006;6:751–762. doi: 10.1111/j.1567-1364.2006.00052.x. [DOI] [PubMed] [Google Scholar]
- Zaltsman Y, Shachnai L, Yivgi-Ohana N, Schwarz M, Maryanovich M, Houtkooper RH, Vaz FM, De Leonardis F, Fiermonte G, Palmieri F, et al. MTCH2/MIMP is a major facilitator of tBID recruitment to mitochondria. Nat. Cell Biol. 2010;12:553–562. doi: 10.1038/ncb2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.