Abstract
Background
Budesonide/formoterol used for both maintenance and reliever therapy has been shown to benefit patients with persistent asthma. We evaluated patient satisfaction and asthma control among Malaysian patients prescribed budesonide/formoterol as single maintenance and reliever therapy in a real-life clinical practice.
Methods
Adult patients diagnosed with partially controlled or uncontrolled asthma were recruited in a 6-month, prospective, open-label study involving ten hospital-based chest clinics in Malaysia. Patients were prescribed one or two inhalations of budesonide/formoterol Turbuhaler (160/4.5 μg per inhalation) twice daily as maintenance therapy and additional inhalation as reliever therapy. Maintenance doses were decided by physicians based on Global Initiative for Asthma-defined treatment objectives. The primary outcome measure was the change in mean Satisfaction with Asthma Treatment Questionnaire (SATQ) scores from baseline to an average of 3 months and 6 months. Secondary outcome was the change in mean Asthma Control Questionnaire 5-item version (ACQ-5) scores from baseline to an average of 3 months and 6 months and the proportion of patients achieving the minimum clinically important difference.
Results
Of 201 eligible patients recruited, 195 completed the study. Overall, SATQ mean (standard deviation) score was significantly improved from 5.1 (0.76) at baseline to 5.5 (0.58) (P < 0.001). The increase was observed in all domains of SATQ and had occurred at 3 months for most patients. ACQ-5 mean (standard deviation) score was significantly reduced from 2.2 (1.13) at baseline to 1.2 (0.95) (P < 0.001). A total of 132 (67.7.1%) patients had achieved the minimal clinically important difference (≥0.5) of ACQ-5 scores at study end.
Conclusion
In a nationwide study, budesonide/formoterol maintenance and reliever therapy achieved greater patient satisfaction and better asthma control compared with previous conventional asthma regimes among Malaysian patients treated in a real-life practice setting. Such an approach may represent an important treatment alternative for our local patients with persistent asthma.
Keywords: asthma, asthma control, Malaysia, maintenance and reliever therapy, satisfaction, Symbicort, budesonide/formoterol
Introduction
Asthma remains a disease that brings considerable morbidity and mortality worldwide despite modern effective treatment.1 Over recent years, there has been increasing evidence to support an approach of using budesonide/formoterol as both maintenance and reliever therapy (Symbicort SMART®, AstraZeneca AB, Sodertalje, Sweden) in chronic persistent asthma. Several studies involving both adult2–4 and pediatric5 patients have shown that such an approach reduces severe asthma exacerbations and improves day-to-day asthma control, compared with conventional maintenance inhaled corticosteroid (ICS) or combination ICS and long-acting β2-agonist (LABA) with as-needed bronchodilator. Although there is a sound scientific basis in support of budesonide/formoterol maintenance and reliever therapy,6 acceptability and practicality can be best addressed by a real-life effectiveness study. Furthermore, the sociocultural and local healthcare systems may play a significant role here.
Studies have shown that patient satisfaction is greater when ICS and LABA are administered in a single inhaler than separately for maintenance therapy.7,8 Although there are two studies reporting on patient satisfaction with such an approach,9,10 there is currently no published prospective multicenter study that specifically examines patient satisfaction with budesonide/formoterol maintenance and reliever therapy and its potential for improving treatment adherence and acceptability. Although real-life effectiveness of budesonide/formoterol maintenance and reliever therapy has been well studied elsewhere,11,12 it is prudent to evaluate whether such benefit can be translated into a multiethnic population like Malaysia.
With this purpose in mind, we tested our hypothesis in the local Malaysian patient population in a prospective, open-label, single-cohort study in ten major hospitals across Peninsular Malaysia. The primary outcome was change in mean score from baseline in patient-reported satisfaction level using the Satisfaction with Asthma Treatment Questionnaire (SATQ)13 to the average of 3 months and 6 months. The secondary outcomes were change in mean score from baseline in asthma control level as defined by Asthma Control Questionnaire 5-item Version (ACQ-5)14 to the average of 3 months and 6 months after starting budesonide/formoterol maintenance and reliever therapy. Physician-assessed asthma control levels defined by Global Initiative for Asthma (GINA),15 frequency of reliever medication use, and asthma exacerbation rates were also determined.
Methods
Patients
All adult patients ≥18 years old with an asthma history of ≥6 months treated with at least 3 months of daily ICS were considered for the study. Patients who remained partially controlled or uncontrolled as defined by the GINA15 guidelines and on daily doses of 200–1000 μg beclomethasonedipropionate (or equivalent) for at least 4 weeks prior to study entry were eligible. Patients currently on budesonide/formoterol maintenance and reliever therapy; those who had used oral, rectal, or parenteral glucocorticoids; those who had respiratory tract infection within 30 days prior or had known or suspected hypersensivity to budesonide, formoterol, or inhaled lactose; those who were pregnant or lactating; and those diagnosed with chronic obstructive pulmonary disorder or another chronic respiratory or significant medical condition were excluded. Patients were able to discontinue from the study treatment and assessments at any time. Specific reasons for discontinuing from the study were voluntary discontinuation, safety reasons, severe noncompliance, incorrect enrolment, lost to follow-up, or pregnancy. The study protocol was approved by the ethics and research committees of participating centers and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines (Clinical Trial Registration Number: NCT00576316). Written informed consent was obtained from all recruited patients.
Study design
This was a 6-month, open-label, single-cohort, multicenter study conducted in chest clinics of ten urban-based hospitals across Peninsular Malaysia. The study involved a screening/baseline visit where eligibility was assessed and informed consent obtained. Relevant baseline demographic data including educational level, baseline SATQ score, and ACQ-5 score were obtained prior to initiation of budesonide/formoterol maintenance and reliever therapy. Patients were followed up at 3 months and 6 months. Recruited patients were prescribed one or two inhalations of budesonide/formoterol Turbuhaler (160/4.5 μg per inhalation; AstraZeneca AB) twice daily as maintenance treatment, and additional inhalation of budesonide/formoterol Turbuhaler (160/4.5 μg per inhalation) as needed for breakthrough symptoms. Initial dose for maintenance treatment and its subsequent adjustment were left to the clinical judgment of the treating physicians based on GINA-defined treatment objectives.15 In all visits, relevant data on treatment satisfaction, asthma control level, asthma symptoms, asthma exacerbations, frequency of rescue treatment, current medications, and occurrence of adverse events were collected in a standard case record form.
Patient satisfaction assessment
Patient satisfaction with their current treatment was assessed using the self-administered SATQ,13 which consisted of an overall score plus individual scores from four domains of satisfaction (ie, effectiveness of treatment, ease of use, medication burden, and side effects or worries). A higher score indicates greater satisfaction. Changes in mean scores from baseline to the average of the two follow-up visits were used as our primary outcome measure of patient satisfaction.
Asthma control assessment
The self-administered ACQ-5 was used for assessing asthma control.14 Lower scores indicate better control, and a change of −0.5 or more (the minimal clinically important difference [MCID]) represents clinically important improvement. In our study, the secondary outcome of asthma control was based on changes in mean scores from baseline to the average of the two follow-up visits, and the proportion of patients achieving MCID at 3 months and 6 months. Asthma control was also assessed by treating physicians in accordance with GINA guidelines at each visit.
Data analysis
The data were analyzed on an intention-to-treat basis. Changes in mean scores of SATQ and ACQ-5 were assessed using two-sided paired t-tests, and a P-value of ≤0.05 was considered significant. The Wilcoxon rank-sum test was used for comparisons of nonparametric data.
Calculation of SATQ
Scores for negatively phrased questions were reversed before calculation of the overall and domain scores, such that for all questions a higher score indicated greater satisfaction.
The overall (26 questions) score was calculated for each subject using the following formula:
| (1) |
The domain (relevant questions) score was calculated using the following formula:
| (2) |
The average of SATQ at months 3 and 6 for each subject was calculated by using the following formula:
| (3) |
The overall and domain group mean scores were calculated by the sum of mean scores divided by 195 subjects.
For subjects who discontinued before month 6, data up to the point of discontinuation were used to estimate response to treatment. No MCID has yet been established for the SATQ.
Calculation of ACQ
The ACQ-5 score was the mean of five responses. At least four out of the five questions had to be answered for a valid score. The mean group ACQ-5 scores at baseline, 3 months, and 6 months were calculated using the following formula:
| (4) |
Results
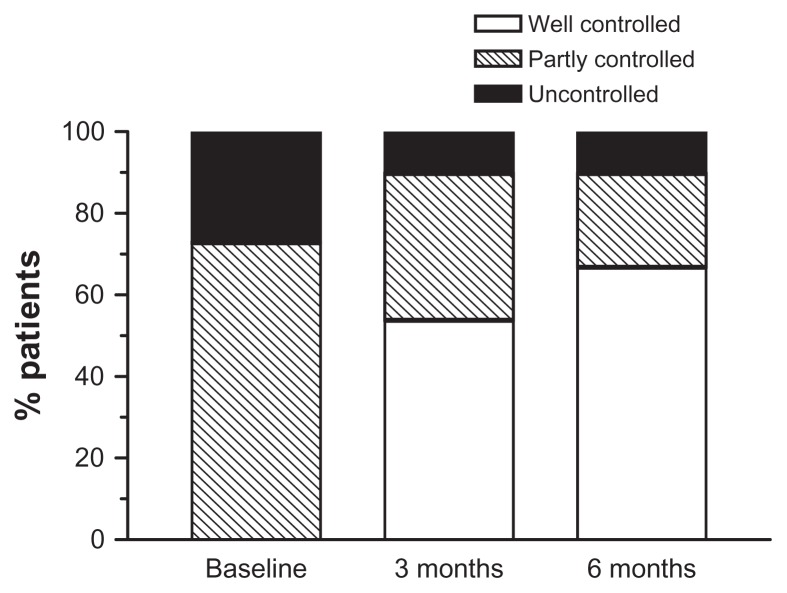
A total of 195 patients of the 201 eligible patients who were recruited completed the study. Most of the patients were female, 79% were middle aged (mean age 46 years), and 76% were Malays. More than half the patients were in step three asthma severity requiring two or more controller medications. Thirty-seven percent of patients were on ICS/LABA combination therapy. Slightly over a quarter of these patients were considered by their attending physician to have uncontrolled asthma (Table 1). The proportions of patients judged to have controlled, partly controlled, or uncontrolled asthma at study entry were 0%, 73%, and 27%, respectively.
Table 1.
Clinic-demographic characteristics (n = 195)
| Characteristics | n (%)a |
|---|---|
| Male | 42 (21.5) |
| Mean age, yrs (range) | 46 (18–79) |
| Ethnicity | |
| Malay | 148 (76) |
| Chinese | 12 (6.6) |
| Indian | 34 (17.4) |
| Education level | |
| Primary | 41 (21) |
| Secondary | 106 (54.3) |
| Diploma | 29 (14.8) |
| University degree | 19 (9.7) |
| Medication types* | |
| ICS | 92 (47.18) |
| ICS + LABA (2 separate inhalers) | 28 (14.36) |
| ICS + LABA (1 combined inhaler) | 72 (36.92) |
| Others | 3 (1.54) |
| Asthma control levelb | |
| Controlled | 0 (0) |
| Partially controlled | 142 (72.82) |
| Uncontrolled | 53 (27.18) |
Notes:
ICS = inhaled corticosteroids; LABA = inhaled long-acting β2-agonist; Others includes theophylline and montelukast;
Unless otherwise specified;
Global Initiative for Asthma (GINA) 2008 Guidelines.15
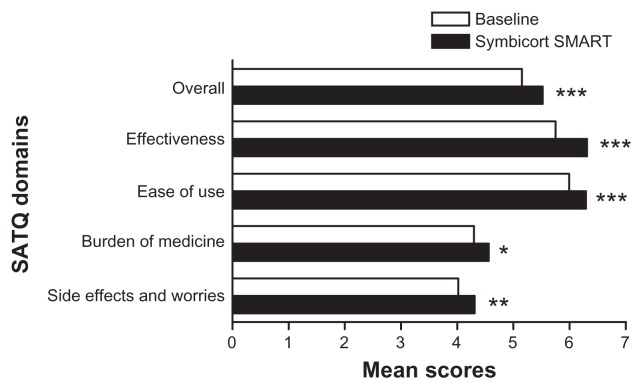
The average SATQ mean (standard deviation) score between 3 months and 6 months had also significantly improved to 5.5 (0.58) (P < 0.001) from 5.1 (0.76) at baseline. The mean SATQ score was overall significantly improved to 5.4 (0.63) in 3 months (P < 0.001) and to 5.5 (0.63) at 6 months (P < 0.001). There were also significant improvements of the mean SATQ scores in the four SATQ domains. The effectiveness score increased from 5.7 (1.00) to 6.3 (0.73), P < 0.001. The ease of use score increased from 6.0 (0.87) to 6.3 (0.65), P < 0.001. The burden of medicine score increased from 4.3 (1.13) to 4.6 (0.79), P < 0.05. The side effects or worries score increased from 4.0 (1.40) to 4.3 (1.21), P < 0.001 (Figure 1). There was no significant difference between the 3 months and 6 months for both the overall and individual domain mean SATQ scores; most improvement in overall satisfaction was evident by 3 months.
Figure 1.
Satisfaction with Asthma Treatment Questionnaire (SATQ) domains and mean scores at baseline and after Symbicort SMART. The mean score on Symbicort SMART is calculated from the average of 3- and 6-month scores.
Notes: *P < 0.05; **P < 0.01; ***P < 0.001 vs baseline.
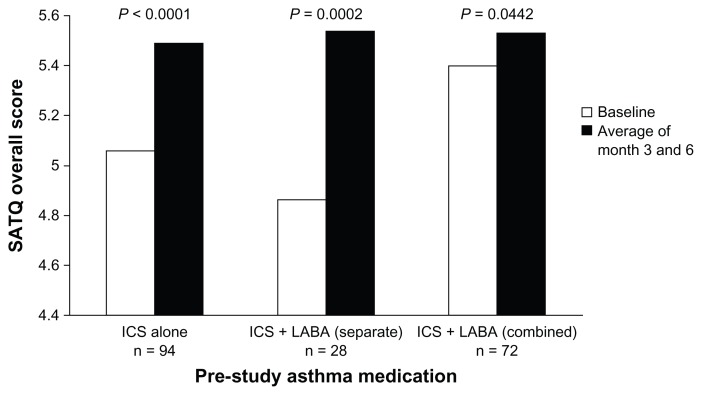
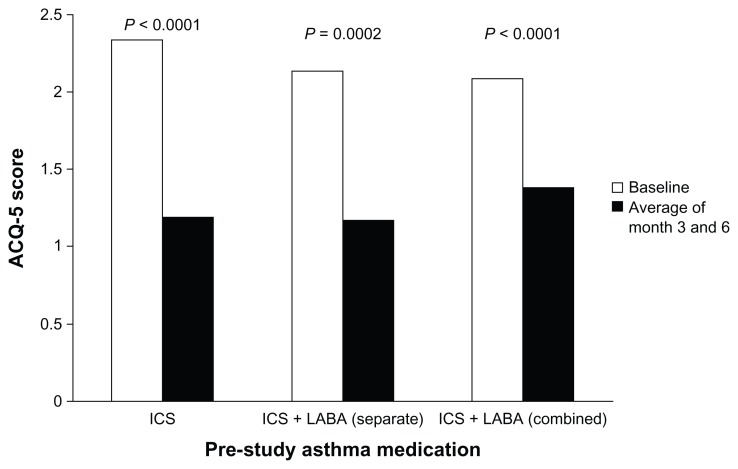
The mean SATQ overall scores improved significantly on Symbicort single inhaler therapy whether subjects were previously on ICS inhaler alone (5.06 vs 5.49, P < 0.0001), ICS/LABA as separate (4.86 vs 5.54, P = 0.0002), or combination inhaler (5.40 vs 5.53, P = 0.0442) (Figure 2).
Figure 2.
Change in SATQ overall score by pre-study asthma medication.
Abbreviations: ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; SATQ, Satisfaction with Asthma Control Questionnaire.
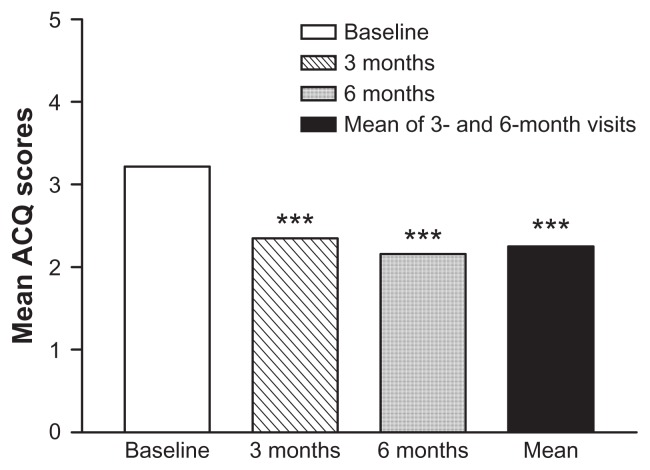
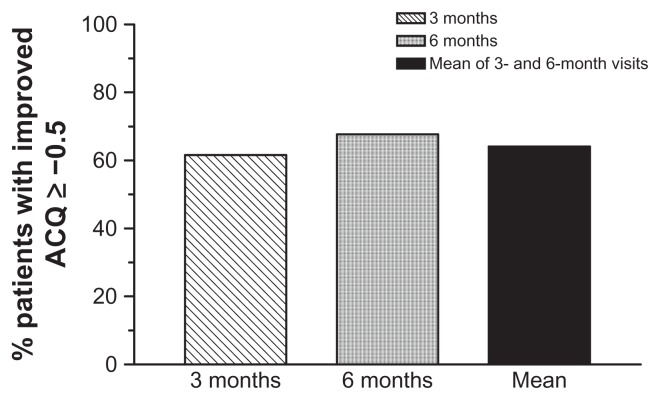
ACQ-5 score between 3 months’ and 6 months’ mean scores had significantly reduced to 1.2 (0.95) (P < 0.001) from 2.2 (1.13) at baseline. ACQ-5 mean score was also significantly reduced to 1.3 (1.01) at 3 months (P < 0.001) and to 1.1 (1.03) at 6 months (P < 0.001) (Figure 3). This reduction translates into 120 (61.6%) patients achieving MCID at 3 months and 132 (67.7%) patients achieving MCID at 6 months. A total of 121 (62.1%) patients achieved MCID of improved asthma control at mean of months 3 and 6 (Figure 4).
Figure 3.
Asthma Control Questionnaire (ACQ) mean scores at baseline and after Symbicort SMART.
Note: ***P < 0.001 vs baseline (NB: P < 0.001 between 3 and 6 months).
Figure 4.
Proportion of patients achieving minimal clinically important improvement in Asthma Control Questionnaire (ACQ) Scores, as defined by ACQ scores ≥ −0.5.
The mean ACQ scores improved significantly on Symbicort single inhaler therapy whether subjects were previously on ICS inhaler alone (2.34 vs 1.19, P < 0.0001), ICS/LABA as separate (2.14 vs 1.17, P = 0.0002), or combination inhaler (2.09 vs 1.38, P < 0.0001) (Figure 5).
Figure 5.
Change in ACQ-5 score by pre-study asthma medication.
Abbreviations: ACQ-5, Asthma Control Questionnaire 5-item; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist.
At 3 months, the proportion of controlled, partly controlled, and uncontrolled asthma had changed to 54%, 36%, and 10%, respectively, with a shift in favor of better control. At 6 months, the proportions were 67%, 23%, and 10%, respectively, with a further shift toward better control (Figure 6).
Figure 6.
Proportion of patients assessed by physicians as having uncontrolled, partly controlled and controlled asthma (according to Global Initiative for Asthma, GINA) at baseline and after Symbicort SMART at 3 and 6 months.
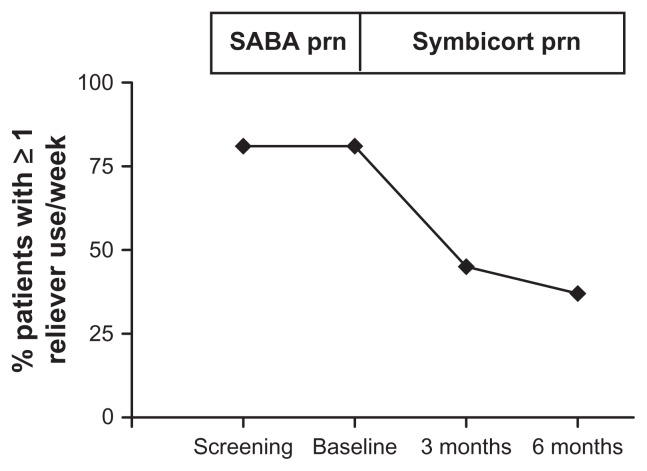
At baseline, 161 (80%) patients reported one or more asthma exacerbations in the preceding 12 months. At 3 months and 6 months, only 30 (15%) patients reported one or more asthma exacerbations over the past 3 months, respectively. At baseline, 163 (81%) patients reported use of rescue medication one or more times per week. At 3 months and 6 months, only 90 (45%) and 74 (37%), respectively, had used rescue medication one or more times per week (Figure 7). A total of 146 (73%) patients were treated with two inhalations of budesonide/formoterol twice a day at study entry. At 3 months and 6 months, 33% and 34% of patients were stepped down by treating physicians to one inhalation of budesonide/formoterol twice a day as maintenance therapy.
Figure 7.
Proportion of patients reporting ≥1 use of their reliever medication in the preceding week.
Abbreviations: SABA, short-acting β2-agonist; prn, when required.
Adverse events that were considered causally related to use of Symbicort SMART were mostly local side effects of IHC. They were pharyngitis (n = 8), palpitations (n = 5), voice hoarseness (n = 1), dry throat (n = 1), oral thrush (n = 1), disturbed taste in the mouth (n = 1), sneezing (n = 1), and skin pruritis (n = 1).
Discussion
In this open-label, real-life practice study, we found that our patients with persistent asthma were overall more satisfied with budesonide/formoterol maintenance and reliever therapy than with their previous treatment regime. Satisfaction was observed in all the individual domains of treatment effectiveness, ease of use, burden of medicine, and side effects or worries. The level of asthma control based on ACQ-5 was also significantly improved. Physician-assessed asthma control, asthma exacerbations, frequency of reliever use, and maintenance dosing were similarly reported to be better after budesonide/formoterol maintenance and reliever therapy. These achievements were apparent at 3 month follow-up and occurred in the local context of multiethnic Malaysian patients of different hospitals and varying educational levels. The assessment of control by physician discretion reflects real-life practice in using international guidelines.
Our mean change in overall SATQ score at 6 months was 0.4. This compares favorably with previous studies using such an approach.10,11 Although all individual domains of satisfaction were significantly increased compared with previous treatment regimes, the greatest increase occurred in the domain of effectiveness. This subjective perception of improved efficacy is supported by objective measures of improved asthma control in our study, such as reliever use and ACQ scores. Improvement in satisfaction may be attributable to the improved efficacy of treatment by the addition of an LABA. However, more than half of the subjects were already receiving LABA either as combination or as add-on prior to initiation of budesonide/formoterol maintenance and reliever therapy. This group still demonstrated significant improvement in SATQ overall scores (Figure 2). In nearly 10% of the patients, maintenance treatment was successfully reduced from two inhalations twice a day to one inhalation twice a day at 3 months and 6 months, respectively. This observation lends support to the clinical efficacy of budesonide/formoterol maintenance and reliever therapy as a treatment strategy, consistent with other findings.
With regard to increased satisfaction on ease of use and burden of medicine, budesonide/formoterol maintenance and reliever therapy are likely to have contributed to this, because the treatment requires only a single inhaler, not multiple inhalers, as the treatment regime. Studies that looked at patient preference have shown that, for asthmatic patients, a rapid and effective relief of symptoms is their highest concern.16,17 Our findings of improved ease of use and reduced burden of medicine with such an approach suggest that such a “simplified” approach does not compromise on patient preference for rapid and effective treatment of asthma symptoms. Formoterol, the long-acting bronchodilator component, has been shown to provide symptom relief as rapid as any other short-acting β2-agonist.18 Our findings also suggest that budesonide/formoterol maintenance and reliever therapy can successfully be taught and learnt in patients of varying levels of education who were previously on conventional regimes that used separate inhalers for regular and rescue use. Patient satisfaction was demonstrated early in the course of treatment, and it is therefore important that practitioners recognize this to avoid treatment discontinuation and to improve compliance.
The use of patient reported satisfaction level and asthma control as key outcome measures in an open-label study such as ours warrants careful interpretation. This is highlighted by a recent asthma study showing that subjective measurements based on patients’ perspectives does not reliably differentiate between active and inert treatments.19,20 This is particularly relevant in our study where patient satisfaction can be affected in more ways than the active treatment alone. Furthermore, the absence of a comparator treatment in our study can compromise further the validity of our findings.
Although there are three studies that reported previously on patient satisfaction with such an approach, to our knowledge, our study is the first major multicenter study that specifically looked at patient satisfaction on budesonide/formoterol maintenance and reliever therapy. In a small, local, Malaysian, real-life, effectiveness study of severe persistent asthmatics, Loh et al9 demonstrated that most patients have found that budesonide/formoterol maintenance and reliever therapy met their expectations for effectiveness, simplification of treatment, and ease of use. In the same study, the patients on budesonide/formoterol maintenance and reliever therapy were also shown to experience reduced frequency of rescue treatment, hospital admission rates, and increased forced expiratory volume in 1 second, compared with the conventional approach of using separate maintenance and reliever inhalers. A recently published Swiss postmarketing survey10 of 420 physicians and 2035 patients also reported that general patient satisfaction was high and that mean ACQ-5 score of asthma control improved by more than three-fold the defined MCID.
Although patient satisfaction is often assumed by means of an effective treatment regime, our findings clearly show such an association also exists with regard to ease of use and reduced burden of medicine. From the patients’ perspectives, there was also reduced concern about side effects and worries using this approach compared with previous treatment regimes, indicating that the approach is likely to be well received. This is highly relevant because medication nonadherence is a serious problem among asthmatic patients, where patient satisfaction and preference have significant influence.21 Furthermore, our study suggests the acceptance of budesonide/formoterol maintenance and reliever therapy in a real-life busy clinical setting of an Asian multiethnic country like Malaysia and in patients with varying educational achievements. This is important because cultural influences play important roles in overall asthma management,22 and studies conducted in the West should not be assumed to be translational in their findings. Currently, studies on real-life effectiveness of such an approach in other Asian countries are being pursued,23 and the increasing bulk of evidence will help to confirm or refute the consistency of the effectiveness of budesonide/formoterol maintenance and reliever therapy across cultures and countries.
Acknowledgments
Roslina AM and Loh LC were responsible for the writing of the manuscript. Roslina AM, Ismail T, Abdul Razak M, George KS, Rosalind BH, Norhaya MR, Noor Aliza MT, Che Wan AH, Mohammad Fauzi AR, and Aziah AM were responsible for the implementation of the study and review of the manuscript.
Footnotes
Disclosure
This study was fully supported and funded by AstraZeneca SdnBhd, Malaysia. AstraZeneca was not involved in data collection. Study monitoring and analysis were done independently by a third-party research organization. Writing the manuscript was the responsibility of the authors.
References
- 1.Braman SS. The global burden of asthma. Chest. 2006;130(1 suppl):4S–12S. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 2.O’Byrne PM, Bisgaard H, Godard PP, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Cri Care Med. 2005;171(2):129–136. doi: 10.1164/rccm.200407-884OC. [DOI] [PubMed] [Google Scholar]
- 3.Rabe KF, Atienza T, Magyar P, et al. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double-blind study. Lancet. 2006;368(9537):744–753. doi: 10.1016/S0140-6736(06)69284-2. [DOI] [PubMed] [Google Scholar]
- 4.Kuna P, Peters MJ, Manjra AI, et al. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract. 2007;61(5):725–736. doi: 10.1111/j.1742-1241.2007.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bisgaard H, Le Roux P, Bjamer D, et al. Budesonide/formoterol maintenance plus reliever therapy: a new strategy in pediatric asthma. Chest. 2006;130:1733–1743. doi: 10.1378/chest.130.6.1733. [DOI] [PubMed] [Google Scholar]
- 6.Barnes PJ. Scientific rationale for using a single inhaler for asthma control. Eur Respir J. 2007;29:587–595. doi: 10.1183/09031936.00080306. [DOI] [PubMed] [Google Scholar]
- 7.Murphy K, Nelson H, Parasuraman B, et al. The effect of budesonide and formoterol in one pressurized metered-dose inhaler on patient-reported outcomes in adults with mild-to-moderate persistent asthma. Curr Med Res Opin. 2008;24(3):879–894. doi: 10.1185/030079908X273354. [DOI] [PubMed] [Google Scholar]
- 8.Chervinsky P, Baker J, Bensch G, et al. Patient-reported outcomes in adults with moderate to severe asthma after use of budesonide and formoterol administered via 1 pressurized metered-dose inhaler. Ann Allergy Asthma Immunol. 2008;101(5):463–473. doi: 10.1016/S1081-1206(10)60284-0. [DOI] [PubMed] [Google Scholar]
- 9.Loh LC, Lim BK, Raman S, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in moderate-to-severe asthma: a real-life effectiveness study of Malaysian patients. Med J Malaysia. 2008;63:188–192. [PubMed] [Google Scholar]
- 10.Steurer-Stey C, Courteheuse C, Taegtmeyer AB, et al. Real-life data on asthma control using budesonide/formoterol for both maintenance and relief: the Swiss experience. Praxis (Bern 1994) 2009;98(23):1335–1342. doi: 10.1024/1661-8157.98.23.1335. [DOI] [PubMed] [Google Scholar]
- 11.Louis R, Joos G, Michils A, et al. A comparison of budesonide/formoterol maintenance and reliever therapy vs conventional best practice in asthma management. Int J Clin Pract. 2009;63(10):1479–1488. doi: 10.1111/j.1742-1241.2009.02185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogelmeier C, D’Urzo A, Pauwels R, et al. Budesonide/formoterol maintenance and reliever therapy: an effective asthma treatment option? Eur Respir J. 2005;26(5):819–828. doi: 10.1183/09031936.05.00028305. [DOI] [PubMed] [Google Scholar]
- 13.Campbell JL, Kiebert GM, Patridge MR. Development of the satisfaction with inhaled asthma treatment questionnaire. Eur Resp J. 2003;22:127–134. doi: 10.1183/09031936.03.00097503. [DOI] [PubMed] [Google Scholar]
- 14.Juniper EF, O’Byrne PM, Guyatt GH, et al. Development and validation of a questionnaire to measure asthma control. Eur Resp J. 1999;14:902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 15.Global Initiative for Asthma (GINA) Global strategy for asthma management and prevention. 2006. [Accessed October 25, 2011]. Available from: http://www.ginasthma.com.
- 16.Johansson G, Ställberg B, Tornling G, et al. Asthma treatment preference study: a conjoint analysis of preferred drug treatments. Chest. 2004;125(3):916–923. doi: 10.1378/chest.125.3.916. [DOI] [PubMed] [Google Scholar]
- 17.Partridge MR, van der Molen T, Myrseth SE, et al. Attitudes and actions of asthma patients on regular maintenance therapy: the INSPIRE study. BMC Pulm Med. 2006;6:13. doi: 10.1186/1471-2466-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balanag VM, Yunus F, Yang PC, et al. Efficacy and safety of budesonide/formoterol compared with salbutamol in the treatment of acute asthma. Pulm Pharmaco Ther. 2006;19:139–147. doi: 10.1016/j.pupt.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler ME, Kelley JM, Boyd IOE, et al. Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N Engl J Med. 2011;365:119–126. doi: 10.1056/NEJMoa1103319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moerman DE. Meaningful placebos-controlling the uncontrollable. N Eng J Med. 2011;365:171–172. doi: 10.1056/NEJMe1104010. [DOI] [PubMed] [Google Scholar]
- 21.Bender BG, Long A, Parasuraman B, et al. Factors influencing patient decisions about the use of asthma controller medication. Ann Allergy Asthma Immunol. 2007;98(4):322–328. doi: 10.1016/S1081-1206(10)60877-0. [DOI] [PubMed] [Google Scholar]
- 22.Loh LC, Teh PN, Seth KD, et al. Ethnicity as a determinant of asthma-related quality of life in a multiracial country. Asia Pac J Public Health. 2006;18(1):49–55. doi: 10.1177/10105395060180010801. [DOI] [PubMed] [Google Scholar]
- 23.Study to Investigate Real Life Effectiveness of Symbicort Maintenance and Reliever Therapy in Asthma Patients Across Asia (SMARTA-SIA) [Accessed on October 25, 2011]. Available from: http://www.clinicaltrials.gov/ct2/show/study/NCT00939341.