Abstract
Objectives
To estimate nevirapine pharmacokinetics and examine its association with rash and/or hepatotoxicity in women starting antiretroviral treatment in the ACTG A5208/OCTANE study in Africa.
Design
In HIV-infected, non-pregnant women with screening CD4<200 cells/mm3 randomized to nevirapine (twice daily, after 14-day once-daily lead-in period) plus tenofovir/emtricitabine, single nevirapine blood samples were collected 14 and 28 days following randomization. Rash and hepatotoxicity that occurred during therapy, or within 7 days after the last dose of nevirapine, were defined as toxicity.
Methods
Nevirapine pharmacokinetics were modeled by population pharmacokinetic analysis. Individual Bayesian pharmacokinetic estimates were used to calculate clearance, 24-hour area under the curve, and predicted plasma concentrations.
Results
Median week 4 nevirapine clearance was 2.0 L/hr. Among the 359 women, 194 (54%) developed a rash of any grade; 82 (23%) had grade 2+ and 9 (3%) had grade 3+ rash. Median clearance was 1.7L/hr for subjects exhibiting 3+ rash versus 2.0 L/hr in women without 3+ rash (p=0.046). The odds of developing 3+ rash was 50% higher for every 20% decrease in clearance (p=0.046). Nevirapine discontinuation due to rash/liver toxicity was significantly more common among women with pretreatment CD4 count > 250 cells/mm3 (p=0.003).
Conclusions
In this study, HIV-infected African women starting a nevirapine-based antiretroviral regimen had a lower nevirapine clearance compared to previous reports. Severe rash, but not hepatotoxicity, was associated with higher NVP exposure. Albeit observed in a small number of women, baseline CD4≥250 cells/mm3 was significantly associated with NVP toxicity.
Keywords: nevirapine, pharmacokinetics, rash, hepatotoxicity, drug toxicity
Introduction
Nevirapine (NVP), a non-nucleoside reverse transcriptase inhibitor, is widely prescribed in resource limited settings as part of initial antiretroviral therapy (ART). As the CD4 cell count threshold for initiating ART rises globally[1], it is important to understand the risks of NVP, particularly in women, and among patients with higher CD4 counts (>250 cells/mm3 in women and > 400 cells/mm3 in men).[2-7] NVP can be associated with serious and sometimes life threatening rash and and/or hepatotoxicity during the first 6 to 18 weeks of therapy.
The exact etiology of NVP toxicity remains unclear. A potential relationship between NVP pharmacokinetic (PK) exposure and increased risk of toxicity has been proposed but no definitive association has been established. [8-14] Studies linking NVP PK and toxicity have enrolled predominantly male subjects, whereas for women, similar investigations have not been formally conducted. Moreover, women appear to exhibit higher NVP drug exposure compared to men as a result of lower drug clearance.[15] Additionally, the PK disposition of NVP may be influenced by several factors, including body weight, ethnicity, pre-existing liver disease, and pharmacogenetics.[14, 16-18] Therefore, this study was conducted to clarify the association of NVP PK and the risk of rash and/or liver toxicity in non-pregnant women residing in seven sub-Saharan African countries who started NVP-based antiretroviral treatment in the ACTG A5208/OCTANE clinical trial.
Primary study objectives were to: 1) determine NVP PK based on sparse PK sampling from a large cohort of women and 2) examine the association between NVP PK and rash and liver toxicity. A secondary objective was to evaluate the association between NVP toxicity and pre-treatment CD4 count and weight.
Methods
The demographics of 745 HIV+ women from 10 African sites enrolled in ACTG 5208/OCTANE, a phase III, open label trial, have been described in detail by Lockman et al. [19]. In brief, women were antiretroviral-naïve, apart from use of zidovudine or single dose NVP for reducing the risk of mother-to-child HIV transmission (MTCT) (in a subset of participants), and had a CD4 count at screening of less than 200 cells/mm3. Women were randomized 1:1 to 400 mg lopinavir/100mg ritonavir BID or NVP 200mg daily for 14 days then 200mg BID, each in combination with tenofovir (TDF) 300 mg + emtricitabine (FTC) 200 mg, administered once daily as the fixed co-formulation, Truvada®. All 370 women who were randomized to receive NVP (including 121 with prior single-dose NVP exposure for prevention of MTCT and 249 without prior single-dose NVP exposure) were eligible to participate in the NVP PK portion of the study. For the PK study, single random NVP blood samples were collected at 14 (±7) days following randomization (i.e. before NVP BID dose escalation), and again at 28 (±7) days if no NVP doses were missed during the previous three days. All women received treatment and were followed until the last woman randomized had completed 48 weeks of follow-up.
NVP plasma concentrations were quantified by liquid chromatography mass spectrometry (LC-tandem-MS) using a method developed and validated within the Drug Research Unit (DRU) of the Department of Clinical Pharmacy, UCSF. The method was approved through the Clinical Pharmacology Quality Assurance (CPQA) Program funded by the Division of AIDS and utilizes reverse phase HPLC separation coupled with tandem-MS detection. NVP and the internal standard were extracted from EDTA plasma using percholoric acid precipitation of plasma proteins followed by centrifugation. The supernatant was neutralized with ammonium hydroxide and injected onto a Zorbax-Eclipse XDB- C8 reverse phase column and separated using a gradient elution. Ion pairs 267.4/226.8 for NVP and 628.6/421.2 for internal standard were selected for tandem mass detection in multiple reaction monitoring mode. The calibration curve concentration range was 50 to 5000 ng/mL and lower limit of quantification (LLOQ) was 50 ng/mL. Inter-assay precision (CV%) ranged from 2.14 to 4.43. Intra-assay CV% ranged from 2.34 to 13.03%.
Population Pharmacokinetic Analysis
NVP concentration time data were analyzed using the nonlinear mixed-effects modeling program NONMEM® Version 7 (Icon Development Solutions, Ellicott City, MD). Variables incorporated in NONMEM® included measured NVP plasma concentration, dosage, administration time of the last three doses of NVP before sample collection, age, weight, and study site. The first order conditional estimation method with interaction between inter-individual variability (IIV) and residual variability was used throughout the model building and evaluation process. Model selection was based on change in the NONMEM objective function value (ΔOFV), precision of the parameter estimates, and improvements in standard diagnostic plots. Models were compared statistically with a significance set at P < 0.05 (ΔOFV = -3.84). To evaluate the accuracy and stability of the final PK model, a non-parametric bootstrap re-sampling method was performed using the NONMEM® interface PLT Tools (Version 3.2, PLTsoft, San Francisco, CA). A total of 1000 bootstrap datasets were generated from the original data set by repeated sampling with replacement. The median parameter estimates obtained from the bootstrap data set were compared with those obtained by the population PK model.
A one-compartment PK structural model with first-order absorption and elimination was selected. IIV of pharmacokinetic parameters was incorporated using an exponential (log normal) model and residual (intra-subject) variability was assumed to be a proportional error model. Since only a single NVP blood sample was obtained at week 2 and week 4 for each subject, a simple additive clearance (CL) model was used to capture any induction of CL after week 2 (CLweek4 = CLweek2 + CLadditional)
The influence of age and bodyweight on PK parameters was investigated assuming an exponential relationship: where θj and weightj are the value of the parameter and weight, respectively, for the j-th individual, θ the typical parameter value in the population standardized to an individual with the median weight at enrollment, and θ is the exponent describing the relationship between the parameter and weight .
Definitions of Toxicity
All liver or rash events occurring while receiving NVP, or within 7 days after the last dose of NVP and before starting another ART regimen, and deemed to be potentially related to NVP, were designated as toxicity endpoints. Liver toxicity was defined as any grade 2+ transaminase elevation or grade 3+ total bilirubin or alkaline phosphatase elevation according to the DAIDS Adverse Events Grading Tables (Division of AIDS table for grading the severity of adult and pediatric adverse events. December 2004). Laboratory and safety assessments occurred on six separate occasions during the first 24 weeks, every 12 weeks thereafter, and additionally if clinically indicated. NVP was discontinued if subjects experienced a grade 3 or 4 rash (or rash of any grade accompanied by other signs/symptoms or laboratory abnormalities suggestive of hypersensitivity reaction), grade 2 or higher transaminase elevations, or increases in liver enzymes by one grade or more (or rash) along with concomitant signs or symptoms of hepatitis.
Statistical analysis
Wilcoxon rank sum test, Wilcoxon signed rank test, and Fisher's exact test were used in the univariate comparisons, and logistic regression was used to fit the multivariable models. In the multivariable models for toxicity endpoints, pre-treatment CD4 count was categorized as ≥250 cells/mm3 versus <250 cells/mm3 and PK parameters (log transformed) were analyzed as continuous variables. In the model for efficacy outcomes, a variable indicating pre-treatment single dose NVP exposure was also included since women with such exposure had a higher rate of virologic failure/death than non-exposed women. For grade 3+ rash, due to the small number of events (n=9), the univariate exact logistic regression including only PK parameter was performed. The following sensitivity analyses were conducted: only including events determined by site investigators as definitely, probably, or possibly NVP related; only including events occurring 6 weeks after treatment initiation; and only including events occurring 18 weeks after treatment initiation. All p-values were two-sided and not adjusted for multiple comparisons.
Results
Baseline Demographics
Among the 370 women randomized to a NVP-based regimen, eleven (6 with no PK data and 5 with zero concentrations at both PK time points) were excluded, leaving 359 subjects included in the final analysis. Baseline median characteristics of the 359 women were: age 33 years (IQR 28, 38); weight 57 kg (IQR 52, 67), CD4 128 cells/mm3 (IQR 80,176) and HIV-1 RNA 158,489 copies/mL (IQR 50,119, 316,228). Although the screening CD4 count had to be ≤200 cells/mm3 (within 90 days of enrollment), there was no restriction on the baseline CD4 count (obtained from a sample drawn at the time of treatment initiation); and a total of 47 women with CD4>200 cells/mm3 were enrolled. Of these 47 women, 15 had CD4 >250 cells/mm3 (median CD4 262 cells/mm3, range 250-386 cells/mm3). Women weighing < 50 kg vs ≥ 50 kg were significantly younger (median 30 vs. 34 years, p=0.012), had higher HIV-1 RNA levels (median 199,526 vs.125,893 copies/mL, p=0.010) and more advanced WHO Stage (44% vs. 28% at stage III/IV, p=0.013). No significant differences in demographics were evident for the 11 women excluded from PK analysis.
Adherence was considered excellent: 83% of women had an adherence index (number of pills actually consumed/number of pills expected to consume) ≥0.95, and 87% reported not missing any dose of medication during the past month.
NVP Plasma Concentrations
Of the 359 women with available NVP concentrations, 330 women (92%) had valid NVP measurements at both study weeks 2 and 4; 18 women had week 2 measurements only; and 11 had week 4 measurements only. The majority of the samples were obtained within 6 hours of NVP administration (87% at week 2 and 93% at week 4) and all except two (at 19 and 25 hours) were obtained within 12 hours. The mean ± standard deviation time of sample collection following the daily 2 week dose was 4.06 hr ± 4.18; CV=103%; following the 4 week dose was 3.02± 2.18; CV 72%.
NVP Pharmacokinetics
NVP concentrations were adequately described by a one-compartment model with first order absorption and elimination. The IIV for volume and oral absorption (ka) could not be reliably estimated and removal of these random effects did not change the OFV or goodness of fit plots. Therefore, only IIV for CL was incorporated into the final model. Neither age nor body weight was found to be associated with PK parameters. The final population PK parameters estimates and their confidence intervals are presented in Table 1. The parameter estimates as found by bootstrap were in agreement with those obtained by the final population PK model (Table 1).
Table 1.
Final population PK model parameter estimates and bootstrap results
| Final Model | Bootstrap Runs (n=1000) | |||
|---|---|---|---|---|
| Population PK | Estimate | 95% CI* | Median | 95% CI** |
| Parameters ka, (h-1) | 1.32 | 0.77-1.87 | 1.29 | 0.82-2.03 |
| Week 2 CL/F (L/hr) | 1.57 | 1.50-1.64 | 1.57 | 1.49-1.65 |
| Additional CL/F at week 4 (L/hr)# | 0.4 | 0.33-0.47 | 0.4 | 0.33-0.47 |
| Vd/F (L) | 72.8 | 58.8-86.8 | 70.1 | 59.0-87.2 |
| IIV+ for CL | 29.6 | 22.4-35.4 | 29.7 | 25.7-33.3 |
| RES§ | 22.3 | 20.1-24.2 | 22.2 | 20.4-24.2 |
CI = confidence intervals
95% Confidence intervals corresponding to parameter estimates at the 2.5th and 97.5th percentiles of bootstrap runs.
CL/FWk4 (total CL) = 2 wk CL + additional CL=1.57 + 0.40 = 1.97 L/hr Vd/F = volume
Inter-individual variability
Residual unexplained variability
From the final population PK model, individual Bayesian estimates of oral clearance (CL/F), volume (Vd/F), and ka were used to calculate steady state 24 hr area under the plasma concentration versus time curve (AUC24), maximum (Cmax) and minimum (Cmin) plasma concentrations for each subject at 200 mg daily (week 2) and 200 mg bid (week 4) (Table 2). As anticipated, a significant difference between weeks 2 and 4 estimated PK parameters (p< 0.001 by signed rank test), including an increase in CL/F, was observed which coincided with dose escalation from 200 mg daily to 200 mg BID at week 2 (to compensate for cytochrome P450 enzyme induction). No significant differences in PK parameters were noted by CD4 count, weight, virologic response, or study site (p> 0.12). Therapeutic estimated NVP Cmin levels > 3 mcg/mL were achieved in 86% of subjects at week 2 and in all subjects at week 4. Median NVP Cmin was 7.2 mcg/mL (IQR 6.1, 8.3) with 200 mg bid (Table 2).
Table 2.
Individual Predicted Median NVP Pharmacokinetic Parameters from the Final Model (n=359)
| NVP PK | Week 2 (IQR)* | Week 4 (IQR)* | p-value** |
|---|---|---|---|
| CL/F(L/hr) | 1.6 (1.4, 1.8) | 2.0 (1.8, 2.3) | <0.001 |
| AUC24 hr (mcg*hr/mL) | 124.2 (109.0, 144.7) | 200.9 (174.4, 227.5) | <0.001 |
| Cmin (mcg/mL) | 4.0 (3.4, 4.8) | 7.2 (6.1, 8.3) | <0.001 |
| Cmax (mcg/mL) | 6.3 (5.7, 7.2) | 9.3 (8.2, 10.4) | <0.001 |
IQR= Interquartile range
Wilcoxon signed rank test
CL/F = oral clearance
AUC24 = steady state 24 hr area under the curve
Cmin = minimum plasma concentrations
Cmax= maxiumum plasma concentrations
NVP Toxicity Outcomes
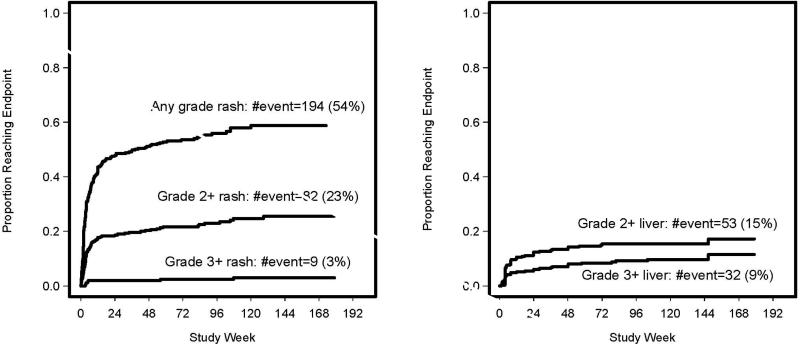
We classified NVP toxicity into two major categories: 1) rash of any grade and 2) hepatotoxicity of grade ≥ 2+ with or without rash (Table 3). Forty-six (13%) subjects discontinued NVP due to liver/rash toxicity (Table 3). Kaplan-Meier plots show time to first rash and liver toxicity, respectively. (Figures 1a and 1b). The majority of NVP related rash of any grade (n=164, 84.5%) and hepatotoxicity (n=39, 73.6%) developed within the first 18 weeks of therapy (Figure 1). However, grade 3+ rash occurred earlier by week 5 (7/9 subjects).
Table 3.
Overall Rates of Rash and Liver Toxicities by Pretreatment CD4 Count
| Toxicity Events | Subjects (n=359) | CD4 <250 (n=344) | CD4 ≥250* (n=15) | Fisher's Exact Test p-value |
|---|---|---|---|---|
| Any grade rash | 194 (54.0%) | 186 (54.1%) | 8 (53.3%) | 1.00 |
| Grade 2+ rash | 82 (22.8%) | 77 (22.4%) | 5 (33.3%) | 0.35 |
| Grade 3+ rash | 9 (2.5%) | 8 (2.3%) | 1 (6.7%) | 0.32 |
| Any liver/rash | 224(62.4%) | 213(61.9%) | 11(73.3%) | 0.43 |
| Grade 3+ liver/rash | 40 (11.1%) | 35 (10.2%) | 5(33.3%) | 0.02 |
| Grade 3+ liver | 32 (8.9%) | 28 (8.1%) | 4(26.7%) | 0.04 |
| Grade 2+ liver | 53 (14.8%) | 47(13.7%) | 6(40.0%) | 0.01 |
| NVP Stopped due to liver/rash toxicity | 46(12.8%) | 41(11.9%) | 5(33.3%) | 0.03 |
Range 250-386 cells/mm3
Figure 1a and 1b.
Kaplan–Meier plot for proportion of women experiencing rash and liver related events over time from starting NVP-based therapy.
Among the 359 women, 194 (54%) developed rash of any grade possibly related to NVP as determined by site investigators; 82 (23%) had grade 2+ and 9 (3%) had grade 3+ rashes (one of which also had 3+ liver toxicity). An association between week 4 (but not week 2) NVP PK exposures (i.e. lower week 4 CL/F, higher Cmin, AUC24hr, and Cmax) and a significantly increased odds of grade 3+ rash was observed (Table 4). Median CL/F was 1.7 L/hr for subjects exhibiting grade 3+ rash compared to 2.0 L/hr in women without 3+ rash (p=0.046). The odds of developing grade 3+ rash were estimated to be approximately 50% greater for every 20% decrease in NVP CL/F (p=0.046) which corresponds to a 20% increase in NVP AUC24hr, Cmax, and Cmin concentrations (Table 4).
Table 4.
Association of Grade 3+ Rash with Week 4 NVP PK**
| Week 4 Median PK (IQR)+ | 3+ Rash (n=9) | No 3+ Rash (n=350) | Odds Ratio# (95% CI) | p-value |
|---|---|---|---|---|
| CL/F(L/hr) | 1.7 (1.3, 2.0) | 2.0 (1.8, 2.3) | 1.51 (1.01, 2.21) | 0.046 |
| AUC24 hr (mcg*hr/mL) | 235 (203, 298) | 200 (174, 223) | 1.51 (1.01, 2.21) | 0.047 |
| Cmin (mcg/mL) | 8.7(7.3, 11.3) | 7.2 (6.1, 8.2) | 1.45 (1.01, 2.04) | 0.046 |
| Cmax (mcg/mL) | 10.7 (9.4, 13.3) | 9.3 (8.2, 10.2) | 1.56 (1.01, 2.35) | 0.047 |
+ IQR = interquartile range
no significant association observed between 3+ rash, week 2 PK, or baseline CD4
per 20% increase in AUC, Cmax, Cmin or 20% decrease in CL/F
All patients with hepatotoxicity exhibited transaminase elevations except for two women: one with grade 3 alkaline phosphatase and one with grade 3 total bilirubin elevations. Grade ≥2+ hepatotoxicity developed in 53 women (15%); of whom 32 (9%) also had grade 3+ hepatotoxicity (Table 3; Figure 1b). In 23 women with grade 2+ hepatotoxicity, various grades of rash also occurred. However, only 1 participant with grade 3+ rash also experienced hepatotoxicity. No significant association was demonstrated between any NVP PK parameters and liver toxicity (data not shown).
Although the number of women with higher pretreatment CD4 count was small, these women exhibited an increased risk of hepatotoxicity. Specifically, within the first 18 weeks of treatment and following adjustment for body weight and week 4 CL/F, women with screening CD4 ≥250 versus <250 cells/mm3 had higher odds of experiencing combined grade 3+ liver/rash toxicity, any liver toxicity, and NVP discontinuation due to liver or rash toxicities (Table 3). In addition, a greater odds of developing any rash/grade 2+ liver toxicity (OR 3.20, p=0.038) and a trend toward greater odds of grade 2+ rash (p=0.046) were observed within six weeks but did not persist at 18 weeks. No significant association was observed between pretreatment CD4 ≥ 250 versus <250 cells/mm3 with rash of any grade. Due to the small number of women with grade 3+ rash, multivariate analysis was not undertaken.
Consistent results were observed across our sensitivity analyses, except that in the models including only NVP-related events as determined by site investigators, the women with pretreatment CD4 count ≥ 250 cells/mm3 had higher odds for rash of any grade or liver toxicity/rash of any grade.
Discussion
NVP PK is well characterized in men, but its disposition in women -- especially those of sub-Saharan African descent -- is less well defined. In this large cohort of sub-Saharan African women, we observed a week 4 median NVP CL/F of 2.0 L/hr, that is approximately 40% lower than CL/F reported among women from the US and Europe.[16, 18, 20] This lower-than-expected CL/F in sub-Saharan African women receiving standard NVP dosing led to higher NVP exposures and an increased risk of grade 3+ rash but not other grades of rash or hepatotoxicity. The odds of developing grade 3+ rash were estimated to be approximately 50% greater for every 20% reduction in week 4 NVP CL/F and 20% increase in NVP exposures (AUC24, Cmin, and Cmax). NVP rash has been reported to be sex specific, being higher in women than men after adjusting for CD4 count.[6, 7, 11, 21, 22] In our study, 54% of subjects developed rash compared to previous reports of 6.5-32%. [7, 11, 13, 21-23] The overall lower NVP clearance in African women along with the greater occurrence of grade 3+ rash associated with higher NVP exposures in our study suggests that lower NVP doses might be appropriate in this population. However, further trials incorporating NVP pharmacogenetic testing in conjunction with PK studies are warranted before dose reductions can be recommended.
Studies comprising >80% men report NVP CL/F to be approximately 3.5 L/hr. [17, 20, 23-30] Overall, women have lower CL/F than men although the reasons are not clear; differences in body weight and liver metabolizing capacity have been suggested.[17, 18, 25, 26] Changes in bioavailability are not likely since NVP absorption is high. In ACTG 241, sex significantly affected NVP CL/F, averaging 3.97 L/hr for men compared to 3.02 L/hr for women, but women only comprised 10 out of 82 subjects.[20] In the 2NN study (n=1091) with 36% female and 36% of subjects from South Africa, steady state median NVP CL/F among all subjects was 2.8 L/hr but was 13.8% lower in women.[24,25] Lower NVP clearances and higher NVP exposures have also been reported in Asians and African Americans.[16, 17, 23, 31] Additionally, NVP CL/F may be influenced by geographical region and concomitant hepatitis B and C infection [16, 25] although no such relationship was observed in our study. However, our cohort included no women with HCV and small numbers of HBV infected women (n=25). Median week 2 and week 4 CL/F were similar (data not shown) between hepatitis B infected and non-infected women (p=0.6). In an intensive NVP PK study of US HIV infected women, NVP exposure was increased by renal insufficiency and hepatic inflammation and reduced by crack cocaine, high fat diets, and amenorrhea > 12 months but not age or exogenous administered hormone. [16] Lower clearance rates of ritonavir and saquinavir have also been reported in women compared to men in other studies conducted by the ACTG. [32, 33]
Our results relating NVP exposure and grade 3+ rash in women have not been previously reported. Although a relationship between higher NVP PK exposure and rash and hepatic events has been proposed, no association had been established in either men or women. [11, 13, 34, 35] Moreover, no specific drug concentration predicting NVP toxicity has been identified.[10, 13, 30, 36] The risk of rash was previously found to be more than two-fold higher in subjects with NVP concentrations greater than 5.3 mg/L, however, only 10% were female. [11] A higher Cmax has also been associated with liver enzyme elevations.[37] Other studies have not shown such a consistent relationship.[11, 13] Additionally, pharmacogenetics may play an important role in NVP PK and toxicity and may be responsible for the lower CL/F observed in this study. Significantly higher NVP PK exposure and greater risk of skin toxicity were observed in African patients expressing the CYP2B6526TT genotype.[14, 38-41] In Cambodian HIV-infected patients with the CYP2B6526TT genotype, a lower CL/F of 1.86 L/hr was found, similar to our results. Preliminary findings also suggest the contribution of the ABCB1 c.3435C>T SNP in NVP hepatotoxicity.[42, 43]
An increased risk of hepatotoxicity was observed in a few women (n=15) with higher pretreatment CD4 >250 cells/mm3 but screening CD4<200 cells/mm3. Some prior studies have reported increased risks of hepatotoxicity and rash for women with CD4 >250 cells/mm3 [44, 45], while others have not. [4, 46-50] Of note, for participants with pretreatment CD4>200 cells/mm3 but entry CD4<200 cells/mm3; we believe these CD4 counts are accurate despite the intra-patient variability, since CD4 testing was performed in monitored labs that successfully met the requirements of the DAIDS Immunology Quality Assurance program. Furthermore, considerable intra-subject CD4 count variability (as much as 200 cells/mm3 obtained a few weeks apart) has been reported previously. [51-54]
World Health Organization antiretroviral treatment guidelines recommend NVP as one possible component of initial antiretroviral treatment regimens among persons (including women) with CD4 < 350 cells/mm3 [1]. Our results add to previous data suggesting that vigilance and close monitoring for NVP toxicity are required for all African women starting NVP at any CD4 count, but toxicity is more likely in those with CD4 ≥250 cells/mm3.
Some study limitations should be noted. Oral NVP CL/F was estimated by NONMEM® using two or fewer collected samples after self-reported dosing rather than an intensive PK study. However, the population model appeared to adequately characterize the drug levels. An important limitation is the lack of pharmacogenetic data to correlate with our PK results since patient consent was not initially obtained. Since only sub-Saharan African women were studied, our findings may not be generalizable to other ethnicities. In addition our findings based on differences between screening and pretreatment CD4 counts deserve cautious interpretation due to the small number of women and the CD4 count within-subject fluctuations that might occur between the two measurements.
Conclusions
In our cohort of HIV+ African women, NVP CL/F was substantially lower than previous reports, resulting in higher NVP plasma levels and drug exposure. Grade 3+ rash but not liver toxicity was significantly associated with a lower week 4 NVP CL/F. Further PK studies with incorporation of pharmacogenetic testing are recommended, particularly in resource limited settings, to determine optimal NVP dosages in African women that will maintain efficacy while minimizing toxicity. Consistent with previous findings, baseline CD4≥250 in a small number of women was significantly associated with NVP liver toxicity and drug discontinuation.
Acknowledgements
The authors wish to thank the African site investigators and study participants who contributed to this study.
Sources of Funding: The project described was supported in part by the AIDS Clinical Trials Group, funded by the National Institute of Allergy and Infectious Diseases (awards U01AI068636, AI38838, A1068636, A1022763, and SDMC AI68634). It also was supported in part by the General Clinical Research Center Units funded by the National Center for Research, and NIH grants R01 AI50587, GM26696. The following companies donated study drug: Boehringer-Ingelheim (nevirapine), Gilead (tenofovir/emtricitabine), Abbott (lopinavir/ritonavir), GlaxoSmithKline (zidovudine), Bristol-Myers Squibb (didanosine). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, the Walter Reed Army Institute of Research, the U.S. Army, the U.S. Department of Defense, the Kenya Medical Research Institute, or the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. Dr. Currier is supported in part by K24 AI56933
Footnotes
Part of this study has been presented during the 18th Conference on Retroviruses and Opportunistic Infections Retroconference, Feb 27-March 2, 2011 in Boston, MA (Oral Themed Presentation Abstract 647).
Potential Conflicts of Interest: Dr Hughes reports being a paid member of Data Monitoring Committees for Boehringer Ingelheim, Medicines Development, Pfizer and Tibotec. For remaining authors none were declared.
Dong contributed to study design, data evaluation and interpretation, and writing of the manuscript.
Zheng and Hughes contributed to statistical analysis, data evaluation and interpretation, and writing of the manuscript.
Frymoyer and Verotta contributed to NONMEM analysis, data interpretation, and manuscript writing
Lizak contributed to NVP sample analysis and manuscript writing
Sawe contributed to data interpretation and manuscript writing
Currier contributed to data evaluation and interpretation and manuscript writing
Lockman contributed to data evaluation and interpretation and manuscript writing
Aweeka contributed to study design, data evaluation, and manuscript writing
References
- 1.World Health Organization [August 20, 2011];Antiretroviral therapy for HIV infection in adults and adolescents. Recommendations for a public health approach: 2010 revision. 2010:1-156. http://www.who.int/hiv/pub/arv/adult2010/en/index.html. [PubMed]
- 2.Hahn JA, Maier M, Byakika-Tusiime J, Oyugi JH, Bangsberg DR. Hepatotoxicity during nevirapine-based fixed-dose combination antiretroviral therapy in kampala, Uganda. J Int Assoc Physicians AIDS Care (Chic) 2007;6:83–86. doi: 10.1177/1545109707299356. [DOI] [PubMed] [Google Scholar]
- 3.Martinez E, Blanco JL, Arnaiz JA, Perez-Cuevas JB, Mocroft A, Cruceta A, et al. Hepatotoxicity in HIV-1-infected patients receiving nevirapine-containing antiretroviral therapy. AIDS. 2001;15:1261–1268. doi: 10.1097/00002030-200107060-00007. [DOI] [PubMed] [Google Scholar]
- 4.Peters PJ, Stringer J, McConnell MS, Kiarie J, Ratanasuwan W, Intalapaporn P, et al. Nevirapine-associated hepatotoxicity was not predicted by CD4 count >/=250 cells/muL among women in Zambia, Thailand and Kenya. HIV Med. 2010;11:650–660. doi: 10.1111/j.1468-1293.2010.00873.x. [DOI] [PubMed] [Google Scholar]
- 5.Sanne I, Mommeja-Marin H, Hinkle J, Bartlett JA, Lederman MM, Maartens G, et al. Severe hepatotoxicity associated with nevirapine use in HIV-infected subjects. J Infect Dis. 2005;191:825–829. doi: 10.1086/428093. [DOI] [PubMed] [Google Scholar]
- 6.Aaron E, Kempf MC, Criniti S, Tedaldi E, Gracely E, Warriner A, et al. Adverse events in a cohort of HIV infected pregnant and non-pregnant women treated with nevirapine versus non-nevirapine antiretroviral medication. PLoS One. 2010;5:e12617. doi: 10.1371/journal.pone.0012617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bersoff-Matcha SJ, Miller WC, Aberg JA, van Der Horst C, Hamrick HJ, Jr, Powderly WG, Mundy LM. Sex differences in nevirapine rash. Clin Infect Dis. 2001;32:124–129. doi: 10.1086/317536. [DOI] [PubMed] [Google Scholar]
- 8.De Requena DG, Jimenez-Nacher I, Soriano V. Changes in nevirapine plasma concentrations over time and its relationship with liver enzyme elevations. AIDS Res Hum Retroviruses. 2005;21:555–559. doi: 10.1089/aid.2005.21.555. [DOI] [PubMed] [Google Scholar]
- 9.Almond LM, Boffito M, Hoggard PG, Bonora S, Raiteri R, Reynolds HE, et al. The relationship between nevirapine plasma concentrations and abnormal liver function tests. AIDS Res Hum Retroviruses. 2004;20:716–722. doi: 10.1089/0889222041524670. [DOI] [PubMed] [Google Scholar]
- 10.Hall DB, Macgregor TR. Case-control exploration of relationships between early rash or liver toxicity and plasma concentrations of nevirapine and primary metabolites. HIV Clin Trials. 2007;8:391–399. doi: 10.1310/hct0806-391. [DOI] [PubMed] [Google Scholar]
- 11.de Maat MM, ter Heine R, Mulder JW, Meenhorst PL, Mairuhu AT, van Gorp EC, et al. Incidence and risk factors for nevirapine-associated rash. Eur J Clin Pharmacol. 2003;59:457–462. doi: 10.1007/s00228-003-0613-3. [DOI] [PubMed] [Google Scholar]
- 12.Barreiro P, Soriano V, Casas E, Estrada V, Tellez MJ, Hoetelmans R, et al. Prevention of nevirapine-associated exanthema using slow dose escalation and/or corticosteroids. AIDS. 2000;14:2153–2157. doi: 10.1097/00002030-200009290-00012. [DOI] [PubMed] [Google Scholar]
- 13.Kappelhoff BS, van Left F, Robinson PA, MacGregor TR, Baraldi E, Montella F, et al. Are adverse events of nevirapine and efavirenz related to plasma concentrations? Antivir Ther. 2005;10:489–498. [PubMed] [Google Scholar]
- 14.Schipani A, Wyen C, Mahungu T, Hendra H, Egan D, Siccardi M, et al. Integration of population pharmacokinetics and pharmacogenetics: an aid to optimal nevirapine dose selection in HIV-infected individuals. J Antimicrob Chemother. 2011;66:1332–1339. doi: 10.1093/jac/dkr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regazzi M, Villani P, Seminari E, Ravashi G, Cusato M, Marubbi F, et al. Sex differences in nevirapine disposition in HIV-infected patients. AIDS. 2003;17:2399–2400. doi: 10.1097/00002030-200311070-00018. [DOI] [PubMed] [Google Scholar]
- 16.Gandhi M, Benet LZ, Bacchetti P, Kalinowski A, Anastos K, Wolfe AR, et al. Nonnucleoside reverse transcriptase inhibitor pharmacokinetics in a large unselected cohort of HIV-infected women. J Acquir Immune Defic Syndr. 2009;50:482–491. doi: 10.1097/qai.0b013e31819c3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Maat MM, Huitema AD, Mulder JW, Meenhorst PL, van Gorp EC, Beijnen JH. Population pharmacokinetics of nevirapine in an unselected cohort of HIV-1-infected individuals. Br J Clin Pharmacol. 2002;54:378–385. doi: 10.1046/j.1365-2125.2002.01657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Hentig N, Carlebach A, Gute P, Knecht G, Klauke S, Rohrbacher M, et al. A comparison of the steady-state pharmacokinetics of nevirapine in men, nonpregnant women and women in late pregnancy. Br J Clin Pharmacol. 2006;62:552–559. doi: 10.1111/j.1365-2125.2006.02664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lockman S, Hughes MD, McIntyre J, Zheng Y, Chipato T, Conradie F, et al. Antiretroviral therapies in women after single-dose nevirapine exposure. N Engl J Med. 2010;363:1499–1509. doi: 10.1056/NEJMoa0906626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou XJ, Sheiner LB, D'Aquila RT, Hughes MD, Hirsch MS, Fischl MA, et al. Population pharmacokinetics of nevirapine, zidovudine, and didanosine in human immunodeficiency virus-infected patients. The National Institute of Allergy and Infectious Diseases AIDS Clinical Trials Group Protocol 241 Investigators. Antimicrob Agents Chemother. 1999;43:121–128. doi: 10.1128/aac.43.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taiwo BO. Nevirapine toxicity. Int J STD AIDS. 2006;17:364–369. doi: 10.1258/095646206777323346. [DOI] [PubMed] [Google Scholar]
- 22.Wong KH, Chan KC, Lee SS. Sex differences in nevirapine rash. Clin Infect Dis. 2001;33:2096–2098. doi: 10.1086/324088. [DOI] [PubMed] [Google Scholar]
- 23.de Maat MM, Nellen JF, Huitema AD, Wit FW, Mulder JW, Prins JM, Beijnen JH. Race is not associated with nevirapine pharmacokinetics. Ther Drug Monit. 2004;26:456–458. doi: 10.1097/00007691-200408000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Kappelhoff BS, Huitema AD, van Leth F, Robinson PA, MacGregor TR, Lange JM, et al. Pharmacokinetics of nevirapine: once-daily versus twice-daily dosing in the 2NN study. HIV Clin Trials. 2005;6:254–261. doi: 10.1310/B5VU-FU5F-QNWC-UDCK. [DOI] [PubMed] [Google Scholar]
- 25.Kappelhoff BS, van Leth F, MacGregor TR, Lange J, Beijnen JH, Huitema AD. Nevirapine and efavirenz pharmacokinetics and covariate analysis in the 2NN study. Antivir Ther. 2005;10:145–155. [PubMed] [Google Scholar]
- 26.Molto J, Valle M, Miranda C, Cedeno S, Miranda J, Santos JR, et al. Once- or twice-daily dosing of nevirapine in HIV-infected adults: a population pharmacokinetics approach. J Antimicrob Chemother. 2008;62:784–792. doi: 10.1093/jac/dkn268. [DOI] [PubMed] [Google Scholar]
- 27.Sabo JP, Lamson MJ, Leitz G, Yong CL, MacGregor TR. Pharmacokinetics of nevirapine and lamivudine in patients with HIV-1 infection. AAPS PharmSci. 2000;2:1–8. doi: 10.1208/ps020101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Heeswijk RP, Veldkamp AI, Mulder JW, Meenhorst PL, Wit FW, Lange JM, et al. The steady-state pharmacokinetics of nevirapine during once daily and twice daily dosing in HIV-1-infected individuals. AIDS. 2000;14:F77–82. doi: 10.1097/00002030-200005260-00001. [DOI] [PubMed] [Google Scholar]
- 29.Dailly E, Raffi F, Perre P, Martin J, Deslandes G, Jolliet P. Influence of darunavir coadministration on nevirapine pharmacokinetics in HIV-infected patients: a population approach. HIV Med. 2009;10:586–589. doi: 10.1111/j.1468-1293.2009.00721.x. [DOI] [PubMed] [Google Scholar]
- 30.Dailly E, Billaud E, Reliquet V, Breurec S, Perre P, Leautez S, et al. No relationship between high nevirapine plasma concentration and hepatotoxicity in HIV-1-infected patients naive of antiretroviral treatment or switched from protease inhibitors. Eur J Clin Pharmacol. 2004;60:343–348. doi: 10.1007/s00228-004-0769-5. [DOI] [PubMed] [Google Scholar]
- 31.Stohr W, Back D, Dunn D, Sabin C, Winston A, Gilson R, et al. Factors influencing efavirenz and nevirapine plasma concentration: effect of ethnicity, weight and co-medication. Antivir Ther. 2008;13:675–685. [PubMed] [Google Scholar]
- 32.Fletcher CV, Jiang H, Brundage RC, Acosta EP, Haubrich R, Katzenstein D, et al. Sex-based differences in saquinavir pharmacology and virologic response in AIDS Clinical Trials Group Study 359. J Infect Dis. 2004;189:1176–1184. doi: 10.1086/382754. [DOI] [PubMed] [Google Scholar]
- 33.Umeh OC, Currier JS, Park JG, Cramer Y, Hermes AE, Fletcher CV. Sex Differences in Lopinavir and Ritonavir Pharmacokinetics Among HIV-Infected Women and Men. J Clin Pharmacol. 2011;51:1665–1673. doi: 10.1177/0091270010388650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez de Requena D, Jimenenez-Nacher I, Soriano V. Changes in nevirapine plasma concentrations over time and its relationship with liver enzyme elevations. AIDS Res Hum Retroviruses. 2005;21:555–559. doi: 10.1089/aid.2005.21.555. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez de Requena D, Nunez M, Jimenez-Nacher I, Soriano V. Liver toxicity caused by nevirapine. AIDS. 2002;16:290–291. doi: 10.1097/00002030-200201250-00020. [DOI] [PubMed] [Google Scholar]
- 36.Crommentuyn KM, Huitema AD, Brinkman K, van der Ende ME, de Wolf F, Beijnen JH. Therapeutic drug monitoring of nevirapine reduces pharmacokinetic variability but does not affect toxicity or virologic success in the ATHENA study. J Acquir Immune Defic Syndr. 2005;39:249–250. [PubMed] [Google Scholar]
- 37.van Leth F, Phanuphak P, Ruxrungtham K, Baraldi E, Miller S, Gazzard B, et al. Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open-label trial, the 2NN Study. The Lancet. 2004;363:1253–1263. doi: 10.1016/S0140-6736(04)15997-7. [DOI] [PubMed] [Google Scholar]
- 38.Rotger M, Colombo S, Furrer H, Bleiber G, Buclin T, Lee BL, et al. Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet Genomics. 2005;15:1–5. doi: 10.1097/01213011-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Saitoh A, Sarles E, Capparelli E, Aweeka F, Kovacs A, Burchett SK, et al. CYP2B6 genetic variants are associated with nevirapine pharmacokinetics and clinical response in HIV-1-infected children. AIDS. 2007;21:2191–2199. doi: 10.1097/QAD.0b013e3282ef9695. [DOI] [PubMed] [Google Scholar]
- 40.Haas DW, Gebretsadik T, Mayo G, Menon UN, Acosta EP, Shintani A, et al. Associations between CYP2B6 polymorphisms and pharmacokinetics after a single dose of nevirapine or efavirenz in African americans. J Infect Dis. 2009;199:872–880. doi: 10.1086/597125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan J, Guo S, Hall D, Cammett AM, Jayadev S, Distel M, et al. Toxicogenomics of nevirapine-associated cutaneous and hepatic adverse events among populations of African, Asian, and European descent. AIDS. 2011;25:1271–1280. doi: 10.1097/QAD.0b013e32834779df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ciccacci C, Borgiani P, Ceffa S, Sirianni E, Marazzi MC, Altan AM, et al. Nevirapine-induced hepatotoxicity and pharmacogenetics: a retrospective study in a population from Mozambique. Pharmacogenomics. 2010;11:23–31. doi: 10.2217/pgs.09.142. [DOI] [PubMed] [Google Scholar]
- 43.Haas DW, Bartlett JA, Andersen JW, Sanne I, Wilkinson GR, Hinkle J, et al. Pharmacogenetics of nevirapine-associated hepatotoxicity: an Adult AIDS Clinical Trials Group collaboration. Clin Infect Dis. 2006;43:783–786. doi: 10.1086/507097. [DOI] [PubMed] [Google Scholar]
- 44.Baylor MS, Johann-Liang R. Hepatotoxicity associated with nevirapine use. J Acquir Immune Defic Syndr. 2004;35:538–539. doi: 10.1097/00126334-200404150-00014. [DOI] [PubMed] [Google Scholar]
- 45.Dieterich DT, Robinson PA, Love J, Stern JO. Drug-induced liver injury associated with the use of nonnucleoside reverse-transcriptase inhibitors. Clin Infect Dis. 2004;38(Suppl 2):S80–89. doi: 10.1086/381450. [DOI] [PubMed] [Google Scholar]
- 46.Coffie PA, Tonwe-Gold B, Tanon AK, Amani-Bosse C, Bedikou G, Abrams EJ, et al. Incidence and risk factors of severe adverse events with nevirapine-based antiretroviral therapy in HIV-infected women. MTCT-Plus program, Abidjan, Cote d'Ivoire. BMC Infect Dis. 2010;10:1–10. doi: 10.1186/1471-2334-10-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nunez M. Hepatotoxicity of antiretrovirals: incidence, mechanisms and management. J Hepatol. 2006;44:S132–139. doi: 10.1016/j.jhep.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 48.Ouyang DW, Brogly SB, Lu M, Shapiro DE, Hershow RC, French AL, et al. Lack of increased hepatotoxicity in HIV-infected pregnant women receiving nevirapine compared with other antiretrovirals. AIDS. 2010;24:109–114. doi: 10.1097/QAD.0b013e3283323941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antela A, Ocampo A, Gomez R, Lopez MJ, Marino A, Losada E, et al. Liver toxicity after switching or simplifying to nevirapine-based therapy is not related to CD4 cell counts: results of the TOSCANA study. HIV Clin Trials. 2010;11:11–17. doi: 10.1310/hct1101-11. [DOI] [PubMed] [Google Scholar]
- 50.Knobel H, Guelar A, Montero M, Carmona A, Luque S, Berenguer N, et al. Risk of side effects associated with the use of nevirapine in treatment-naive patients, with respect to gender and CD4 cell count. HIV Med. 2008;9:14–18. doi: 10.1111/j.1468-1293.2008.00513.x. [DOI] [PubMed] [Google Scholar]
- 51.Hughes MD, Stein DS, Gundacker HM, Valentine FT, Phair JP, Volberding PA. Within-Subject variation in CD4 lymphocyte count in asymptomatic human immunodeficiency virus infection: implications for patient monitoring. J Infect Dis. 1994;169:28–36. doi: 10.1093/infdis/169.1.28. [DOI] [PubMed] [Google Scholar]
- 52.Malone JL, Simms TE, Gray GC, Wagner KF, Burge JR, Burke DS. Sources of variability in repeated T-helper lymphocyte counts from human immunodeficiency virus type 1-infected patients: total lymphocyte count fluctuations and diurnal cycle are important. J Acquir Immune Defic Syndr. 1990;3:144–151. [PubMed] [Google Scholar]
- 53.Raboud JM, Haley L, Montaner JS, Murphy C, Januszewska M, Schechter MT. Quantification of the variation due to laboratory and physiologic sources in CD4 lymphocyte counts of clinically stable HIV-infected individuals. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10(Suppl 2):S67–73. [PubMed] [Google Scholar]
- 54.Williams BG, Korenromp EL, Gouws E, Schmid GP, Auvert B, Dye C. HIV infection, antiretroviral therapy, and CD4+ cell count distributions in African populations. J Infect Dis. 2006;194:1450–1458. doi: 10.1086/508206. [DOI] [PubMed] [Google Scholar]