Abstract
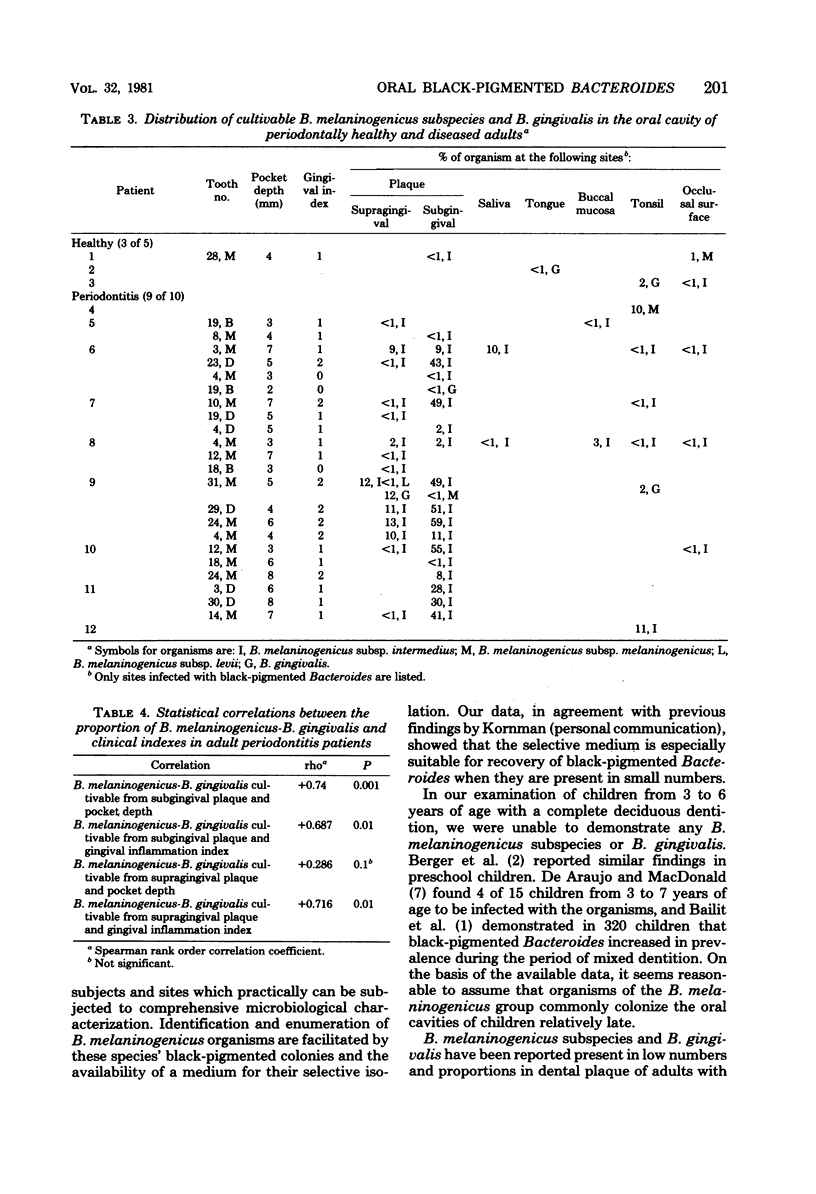
Five healthy children under 6 years of age, five healthy adults, and 10 adult periodontitis patients were examined for the prevalence and distribution of black-pigmented Bacteroides in the oral cavity. A total of 13 samples was obtained from each individual, including four supragingival and four subgingival dental plaques, dental occlusal surface, buccal mucosa, dorsal tongue, tonsil, and whole saliva. Black-pigmented Bacteroides were recovered from nine adult periodontitis patients. Healthy adults harbored the organisms in low incidence and proportions, whereas the children exhibited no cultivable black-pigmented Bacteroides. The organisms were isolated in highest proportions from dental plaque, especially subgingival plaque, and from the tonsil area, indicating that these sites constitute the organisms' primary ecological niche in the oral cavity. The predominant isolate was Bacteroides melaninogenicus subsp. intermedius followed by Bacteroides gingivalis and B. melaninogenicus subsp. melaninogenicus. B. melaninogenicus subsp. levii constituted low proportions of supragingival microflora of one adult periodontitis patient. A positive correlation was demonstrated between the proportion of black-pigmented Bacteroides (mainly B. melaninogenicus subsp. intermedius) and both the severity of gingival inflammation and the periodontal pocket depth, suggesting that these organisms may contribute to the pathogenesis of certain forms of periodontal disease.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAILIT H. L., BALDWIN D. C., HUNT E. E., Jr THE INCREASING PREVALENCE OF GINGIVAL BACTEROIDES MELANINOGENICUS WITH AGE IN CHILDREN. Arch Oral Biol. 1964 Jul-Aug;9:435–438. doi: 10.1016/0003-9969(64)90028-7. [DOI] [PubMed] [Google Scholar]
- DE ARAUJO W. C., MACDONALD J. B. THE GINGIVAL CREVICE MICROBIOTA IN FIVE PRESCHOOL CHILDREN. Arch Oral Biol. 1964 Mar-Apr;9:227–228. doi: 10.1016/0003-9969(64)90013-5. [DOI] [PubMed] [Google Scholar]
- Hausmann E., Raisz L. G., Miller W. A. Endotoxin: stimulation of bone resorption in tissue culture. Science. 1970 May 15;168(3933):862–864. doi: 10.1126/science.168.3933.862. [DOI] [PubMed] [Google Scholar]
- Ingham H. R., Sisson P. R., Tharagonnet D., Selkon J. B., Codd A. A. Inhibition of phagocytosis in vitro by obligate anaerobes. Lancet. 1977 Dec 17;2(8051):1252–1254. doi: 10.1016/s0140-6736(77)92662-9. [DOI] [PubMed] [Google Scholar]
- LOE H., SILNESS J. PERIODONTAL DISEASE IN PREGNANCY. I. PREVALENCE AND SEVERITY. Acta Odontol Scand. 1963 Dec;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- Listgarten M. A., Helldén L. Relative distribution of bacteria at clinically healthy and periodontally diseased sites in humans. J Clin Periodontol. 1978 May;5(2):115–132. doi: 10.1111/j.1600-051x.1978.tb01913.x. [DOI] [PubMed] [Google Scholar]
- Nisengard R. J. The role of immunology in periodontal disease. J Periodontol. 1977 Sep;48(9):505–516. doi: 10.1902/jop.1977.48.9.505. [DOI] [PubMed] [Google Scholar]
- SILNESS J., LOE H. PERIODONTAL DISEASE IN PREGNANCY. II. CORRELATION BETWEEN ORAL HYGIENE AND PERIODONTAL CONDTION. Acta Odontol Scand. 1964 Feb;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- SOCRANSKY S. S., GIBBONS R. J. REQUIRED ROLE OF BACTEROIDES MELANINOGENICUS IN MIXED ANAEROBIC INFECTIONS. J Infect Dis. 1965 Jun;115:247–253. doi: 10.1093/infdis/115.3.247. [DOI] [PubMed] [Google Scholar]
- Slots J., Hausmann E. Longitudinal study of experimentally induced periodontal disease in Macaca arctoides: relationship between microflora and alveolar bone loss. Infect Immun. 1979 Feb;23(2):260–269. doi: 10.1128/iai.23.2.260-269.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. Subgingival microflora and periodontal disease. J Clin Periodontol. 1979 Oct;6(5):351–382. doi: 10.1111/j.1600-051x.1979.tb01935.x. [DOI] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable microflora of advanced periodontitis. Scand J Dent Res. 1977 Jan-Feb;85(2):114–121. doi: 10.1111/j.1600-0722.1977.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Socransky S. S. Relationship of bacteria to the etiology of periodontal disease. J Dent Res. 1970 Mar-Apr;49(2):203–222. doi: 10.1177/00220345700490020401. [DOI] [PubMed] [Google Scholar]
- Spiegel C. A., Hayduk S. E., Minah G. E., Krywolap G. N. Black-pigmented Bacteroides from clinically characterized periodontal sites. J Periodontal Res. 1979 Sep;14(5):376–382. doi: 10.1111/j.1600-0765.1979.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Tanner A. C., Haffer C., Bratthall G. T., Visconti R. A., Socransky S. S. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979 Oct;6(5):278–307. doi: 10.1111/j.1600-051x.1979.tb01931.x. [DOI] [PubMed] [Google Scholar]



