Abstract
Ulcerative colitis is a chronic disease that specifically affects the mucosa of the rectum and colon. Although the etiology of this recurring inflammatory disorder remains essentially unknown, there have been significant advances in identifying the likely genetic and environmental factors that contribute to its pathogenesis. The clinical course of the disease typically manifests with remissions and exacerbations characterized by rectal bleeding and diarrhea. Since ulcerative colitis most commonly affects patients in their youth or early middle age, the disease can have serious long-term local and systemic consequences. There is no specific medical therapy that is curative. Although medical therapy can ameliorate the inflammatory process and control most symptomatic flares, it provides no definitive treatment for the disease. Proctocolectomy or total removal of the colon and rectum provides the only complete cure; however, innovative surgical alternatives have eliminated the need for a permanent ileostomy. The aim of this review is to provide a detailed account of the surgical management of ulcerative colitis.
Keywords: Ileostomy, proctocolectomy, ulcerative colitis
INTRODUCTION
Although Hippocrates described diarrheal diseases that were colitis-like well before 360 BC, it was not until the late 1800s that ulcerative colitis was distinguished clinically from common infectious enteritis. Ulcerative colitis has now been recognized as a distinct disease entity for nearly 150 years. The first medical account of colitis by Sir Samuel Wilks of London in 1859 described a 42-year-old woman who died after several months of diarrhea and fever. Postmortem examination revealed a transmural ulcerative inflammation of the colon and terminal ileum that was originally designated as “simple ulcerative colitis”, may in fact have been Crohn's disease. A subsequent case report in 1875, again by Wilks and Walter Moxon, that described ulceration and inflammation of the entire colon in a young woman who had succumbed to severe bloody diarrhea, was more likely the first detailed account of ulcerative colitis.[1] Despite our long knowledge of the existence of ulcerative colitis, a clear understanding of the factors that underlie its pathogenesis continues to elude investigators.
From a surgical perspective, after Burrill Crohn's landmark description of regional enteritis in the 1930s, distinguishing between ulcerative colitis and Crohn's disease of the large intestine appeared to be relatively uncomplicated. Although the two diseases initially appeared to have distinct pathologic features, a marked overlap is now appreciated not only pathologically, but also in anatomic distribution. The fact remains that the diagnosis is indeterminate in more than 10% of patients,[2] which can have significant therapeutic implications because the surgical approaches to ulcerative colitis and Crohn's disease are inherently quite different. As will be discussed further, the more recent surgical alternatives for ulcerative colitis are generally contraindicated in patients with Crohn's disease.
Epidemiology
Ulcerative colitis poses many challenges to the epidemiologist since the incidence of the disease is low and it is rarely fatal, its clinical presentation can be variable and often insidious, the interval between the initiating event and the diagnosis can be decades, and there are no universal diagnostic criteria.[3] Despite these limitations, epidemiological studies can provide invaluable insight into numerous potential etiologic factors. Although the age of onset of ulcerative colitis is bimodal and it typically occurs between the ages of 15 and 40 years and again after the age 60, the disease can present at any age from infancy to the elderly. In fact, nearly 5% of new cases reportedly occur after age 60. Throughout the age range, males and females are affected almost equally. The mortality rate from ulcerative colitis has steadily declined worldwide, especially in the US, not only as a result of improved medical therapy, but also due to earlier surgical intervention.
Pathophysiology
Although our understanding of the role of familial and genetic factors in the etiology of ulcerative colitis has increased considerably, the pathogenesis of ulcerative colitis remains poorly understood due to complex environmental or extrinsic factors that can significantly influence susceptibility. As mentioned, ulcerative colitis is a chronic inflammatory disease characterized by recurring episodes of intestinal inflammation followed by partial healing. These repetitious inflammatory cycles eventually lead to chronically disrupted bowel function. The clinical manifestations of this pathological process are the result of a series of overlapping interactions between extrinsic environmental factors, genetic intrinsic factors, and mucosal barrier function. Although a single etiological factor has yet to be identified, strong evidence suggests that the disease is perpetuated by a sustained mucosal inflammatory response that the host is unable to downregulate. The failure to attenuate this response enhances the recruitment and activation of numerous immune and inflammatory cells, and coupled with the release of proinflammatory mediators, perpetuates inflammation and facilitates damage to intestinal tissues. Recent research has focused on the role of the mucosal immune system in the pathogenesis of ulcerative colitis. Immune-mediated inflammatory events include the dysregulation of humoral and cell-mediated immunity and enhanced reactivity against intestinal bacterial antigens. It is currently thought that loss of tolerance against indigenous enteric flora is the fundamental event in the pathogenesis of ulcerative colitis.[4,5] The intestinal mucosa is continually exposed to an immense environmental challenge. Optimal mucosal tolerance lies in the tight regulation of an intricate network of mucosal immune and nonimmune cells, which is orchestrated by a finely tuned network of autocrine and paracrine mediators. Chronic dysregulation of mucosal immunity can initiate an uncontrolled inflammatory response and may be an underlying immunopathological mechanism of ulcerative colitis. Immunoregulatory and proinflammatory cytokines also play key roles in the modulation of intestinal inflammation. Cytokines can have paracrine and autocrine as well as endocrine functions that mediate both local and systemic manifestations of intestinal inflammation. Proinflammatory cytokines such as interleukin (IL 1), IL-6, IL-8, and tumor necrosis factor-alpha, and prostaglandins such as prostaglandin E2 and leukotriene B4 also have been implicated in exacerbating mucosal inflammation, while IL-4 and IL-10 play a pivotal role in suppressing intestinal inflammation as well as initiating repair and healing mechanisms. While the roles of these immunoregulatory and proinflammatory cytokines have yet to be completely defined, it appears that ulcerative colitis is mediated by a Th2-like cytokine pattern.
Pathology
On gross inspection, the colonic mucosa appears swollen and congested even in mild cases. As the disease progresses, the mucosa begins to erode leaving only small islands of mucosa that resemble polyps but are actually pseudopolyps. The mucosal erosions often coalesce to form linear ulcers and superficial fissures that undermine the remaining mucosa, which becomes friable and erythematous with reduced haustral folds. The recurrent nature of the disease frequently leaves healed granular superficial ulcers superimposed on a friable and thickened mucosa with increased vascularity. This appearance sharply contrasts with the transmural inflammatory changes found in Crohn's disease of the colon, in which all layers of the colonic wall may be involved in a granulomatous inflammatory process.
Histologically, the typical early lesion consists of an infiltration of inflammatory cells, primarily polymorphonuclear leukocytes, into the crypts at the base of the mucosa, forming crypt abscesses. As the lesions progress, there is a coalescence of crypt abscesses and desquamation of overlying cells to form an ulcer. This cryptitis is associated with undermining of adjacent, relatively normal mucosa, which becomes edematous and assumes a polypoid configuration as it becomes isolated between adjacent ulcers. A whole mount section of a colon from a patient with severe disease shows these broad-based undermined ulcers. The absence of fibrosis and the lack of transmural inflammation in part rule out Crohn's disease. Collagen and granulation tissue often occupy the areas of ulceration, which extend down to, but rarely through, the muscularis. On higher magnification, an ulcer edge is shown overhanging inflamed mucosa. Although ulcerative colitis is generally confined to the mucosa and submucosa, in the most severe forms of the disease, such as fulminant colitis or toxic megacolon, the disease process may extend to the deeper muscular layers of the colon and even to the serosa. For example, we have noted that colectomy specimens from some patients with severe chronic active colitis contain superficial fissuring-type ulcers that extend into the inner half of the muscularis propria of the colon.[6] Aside from the fissures, the pathological features of these colons appear typical of ulcerative colitis. These ulcers appear as knife like, vertically oriented defects lined by actively inflamed granulation tissue and are often associated with marked chronic inflammation in the vicinity of the ulcer. Although deep fissuring ulcers are normally associated with Crohn's disease, some pathologists believe that superficial fissuring ulcers may be seen in severe cases of ulcerative colitis as well. This type of presentation can certainly complicate the differential diagnosis. Rarely, crypt abscesses penetrate the muscularis propria, often extending along a blood vessel, eventually leading to perforation.
Clinical features
Patients with a relatively mild episode of ulcerative colitis typically present with bloody diarrhea, abdominal pain, and fever. Although the disease may be initially limited to the rectosigmoid, it eventually progresses proximally in most cases. A smaller percentage (25%) present with a moderate attack in which bloody diarrhea is the major symptom. In a small number of patients (15%), ulcerative colitis can present rapidly with a fulminating course. These patients develop the relatively sudden onset of frequent, bloody bowel movements, high fever, weight loss, and diffuse abdominal tenderness.
Physical findings are generally associated with the duration, extent, and severity of disease. Weight loss and pallor usually accompany acute flares, along with detectable alterations in numerous metabolic functions. During active periods, the abdomen, especially in proximity to the colon, is tender to palpation. Acute attacks or fulminating forms of the disease can present much like an acute surgical abdomen, with accompanying fever and decreased bowel sounds. In patients with toxic megacolon, abdominal distention may be identified.
Extra-intestinal manifestations of ulcerative colitis are observed in a number of organ systems.[7] Thus, careful examination of the skin, oral cavity and tongue, joints, and eyes can be a vital component of the initial diagnosis because the presence of extra-intestinal disease suggests that Inflamatory Bowl Disease is the likely cause of the underlying diarrheal illness. Many extra-intestinal manifestations of ulcerative colitis are closely related to disease activity and respond to therapy with steroids, immunosuppressive agents, or surgical treatment.[8] Liver and biliary tract disorders also commonly afflict patients with ulcerative colitis. Up to 80% of patients, especially those with pancolitis, show some hepatic involvement. Sclerosing cholangitis, one of the most difficult extra-intestinal complications associated with ulcerative colitis, is observed in 1-4% of patients. Although some patients respond to colectomy, most show progression of their hepatic disease even after colon resection. Patients with progressive liver failure ultimately require liver transplantation. Affected patients are also at greater risk of developing carcinoma of the bile duct, although this may also develop de novo in patients with ulcerative colitis.
DIAGNOSTIC MODALITIES
Endoscopy
There are no specific laboratory, radiographic, or histologic tests that definitively establish the diagnosis of ulcerative colitis; thus the final diagnosis is generally one of exclusion. However, endoscopy with biopsy can play an integral role in the diagnosis, management, and surveillance of ulcerative colitis.[9] Endoscopy can be very valuable in establishing the final diagnosis, excluding other potential etiologies in patients presenting with bloody diarrhea, delineating the extent and activity of mucosal inflammation, and obtaining mucosal biopsies for histologic evaluation. For the surgeon, endoscopy can be particularly useful in differentiating ulcerative colitis from Crohn's colitis, which can have a significant impact on surgical decisions and on the management of disease-related complications. The major distinguishing clinical characteristics of ulcerative colitis and Crohn's disease are shown in Table 1.
Table 1.
Pathologic, clinical, endoscopic and radiographic features of ulcerative colitis and Crohn's disease
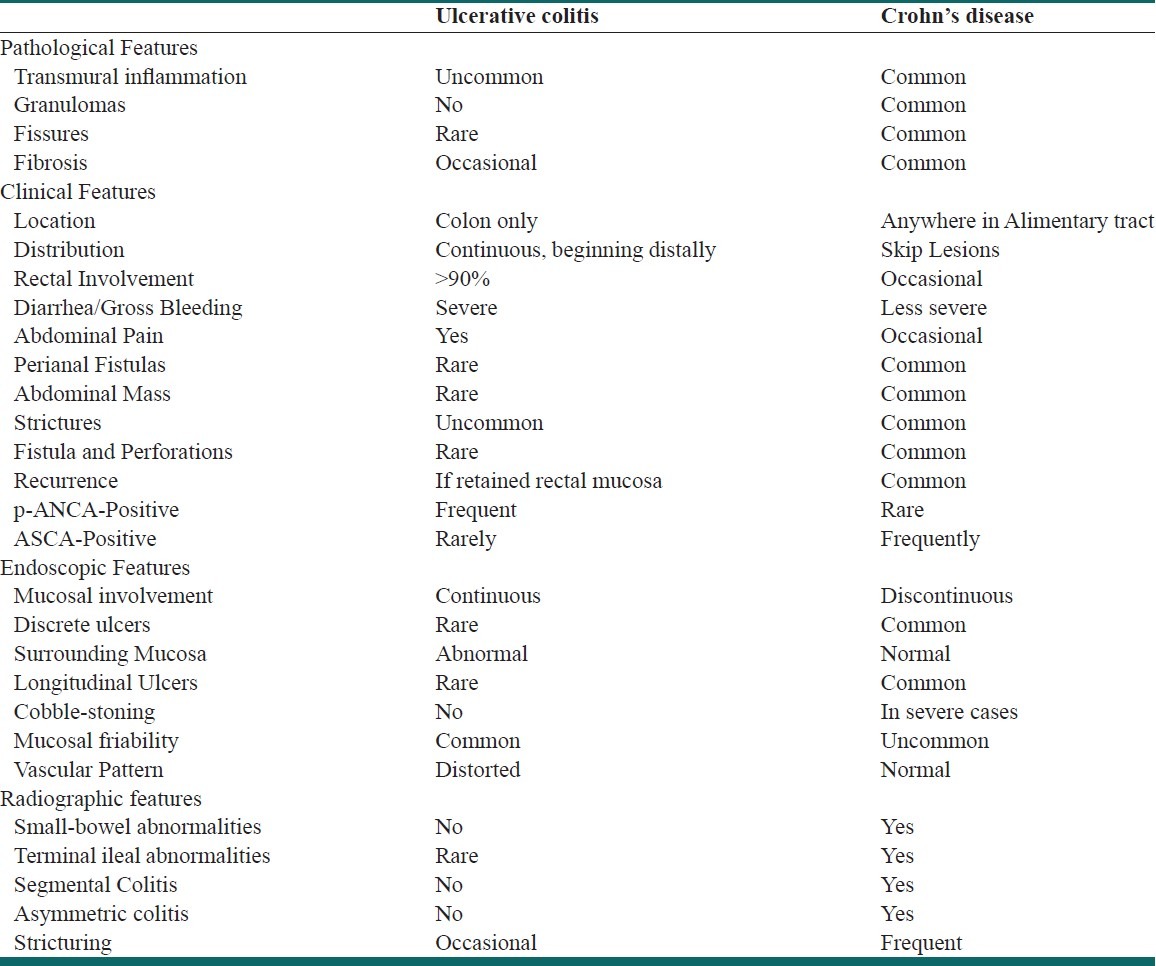
Since ulcerative colitis involves the rectum in 90–95% of cases, flexible sigmoidoscopy is the first step in diagnosis. Mild cases may only show a loss of normal vascular pattern, a granular texture, and micro hemorrhages when the friable mucosa is touched or wiped. When the disease is moderately active, the mucosa becomes more grossly pitted, and spontaneous bleeding is often present. In severe cases, there is macroulceration and profuse bleeding, usually accompanied by a purulent exudate. Chronic ulcerative colitis is frequently associated with the appearance of small pseudopolyps, which represent areas of regenerating mucosa in the midst of diffuse mucosal destruction. The use of flexible sigmoidoscopy as well as other imaging modalities has greatly improved diagnostic accuracy and patient acceptability. Colonoscopy may be useful in determining the extent and activity of the disease, particularly in patients in whom the diagnosis is unclear or cancer is suspected.
Radiographic studies
In patients presenting with fulminant or severe ulcerative colitis, a plain abdominal radiograph may be useful initially, especially since more invasive imaging techniques can have serious risks. An abdominal film may demonstrate colonic dilation or toxic megacolon in 3-5% of patients. Although this dilation is most frequently observed in the transverse colon, it can occur anywhere in the colon. A plain radiograph is also useful for detecting free air within the peritoneal cavity, indicating a potential perforation of the diseased colon.
A lower Gastro intestinal series or barium enema examination of the colon is useful in most patients, although potentially dangerous in those with toxic megacolon. As ulcerative colitis develops, mucosal granularity and microhemorrhages produce a diffusely reticulated pattern, on which are superimposed countless punctate collections of contrast material lodged in microulcerations. More mild cases of acute ulcerative colitis may be manifested by a diffusely granular appearance, which can also be seen in more detail on air-contrast barium enema. In more advanced cases, the colon develops irregular margins with spiculated and undermining collar-button ulcers that can be observed on full-column barium enema. End-stage or “burned-out” ulcerative colitis is characterized by shortening of the colon, loss of normal redundancy in the sigmoid region and at the splenic and hepatic flexures, disappearance of the haustral pattern, a featureless mucosa, absence of discrete ulceration, and narrowed caliber of the bowel.
Medical management
Surgeons are now becoming involved in the management of potential surgical candidates much earlier in the course of their disease, so a general understanding of the medical management of the various presentations and stages of ulcerative colitis is required. Medical therapy for ulcerative colitis is not curative. It is primarily intended to control the patient's symptoms or manage their underlying inflammatory process in order to induce remission. Once the diagnosis of ulcerative colitis has been established, the decision regarding the implementation of medical therapy depends on the severity of symptoms, the patients’ clinical history, and on their endoscopic and radiographic studies. The optimal treatment plan, which may eventually include surgery, is often devised with input from the patient as well as their gastroenterologist and surgeon. Although adherence to individualized treatment plans can result in better long-term outcomes, a significant percentage of patients will eventually become refractory to even the most aggressive treatment regimens or experience other complications that require surgical intervention.[10]
The choice of agents commonly used to induce remission in patients with ulcerative colitis depends on both the extent and severity of the disease and its anatomic location, and can include oral and topical regimens alone or in combination.[11] Drugs commonly used in the treatment of various stages of ulcerative colitis include sulfasalazine and its aminosalicylate analogues, corticosteroids, immunomodulators, suppressive antimetabolites, anti-tumor necrosis factor-alpha, biologics including infliximab, and in some cases antibiotics. Symptomatic anti-diarrheals and antispasmodic agents can also be used in combination therapy as needed [Table 2].
Table 2.
Medical management of ulcerative colitis

SURGICAL MANAGEMENT OF ULCERATIVE COLITIS
Indications for surgery
Emergency indications
Fulminant colitis: The clinical course of ulcerative colitis is one of a chronic inflammatory state characterized by sporadic symptomatic flares. However, in a small percentage of patients, the initial presentation can be of a fulminant nature.[12] Fulminant colitis is characterized by the rapid onset of severe symptoms including bloody diarrhea, severe abdominal pain, and dehydration. These patients are often extremely ill, generally anemic and tachycardic, and present with a high fever. They can require immediate and aggressive medical therapy that can include high-dose intravenous steroids, fluid resuscitation, correction of electrolyte abnormalities, and in some cases, blood transfusions. If severe colonic distention is present, nasogastric tube decompression may be required. Approximately 10% of ulcerative colitis patients initially present with fulminant colitis;[13] however, if the patient's history is not known or if the diagnosis of ulcerative colitis is unclear, flexible sigmoidoscopy of the rectum and sigmoid colon should be performed as soon as possible. The gastroenterologist and the surgeon should closely monitor the patient's condition for 24–48 h and, if there is no improvement or conditions worsen, surgical treatment is recommended. If there is any indication of perforation or peritonitis, the patient should be operated upon immediately.
Toxic megacolon: Toxic megacolon is a rare but devastating complication occurring in up to 2.5% of patients with ulcerative colitis.[14] Acute toxic megacolon may be the initial presentation of the disease or may represent a flare-up in a patient with chronic disease.[13] Usually, an isolated segment of the transverse or the left colon is dilated more than 5.5 cm; however, the entire colon can be involved. Because of the associated high morbidity and mortality, early recognition and aggressive, often surgical management is of vital importance. Medical therapy for toxic megacolon is similar to that for fulminant colitis and includes intravenous fluid and electrolyte resuscitation, nasogastric suction, broad-spectrum antibiotics, and total parenteral nutrition to improve nutritional status.[14] Although the therapeutic role of high-dose steroids in toxic megacolon is controversial, most patients presenting with a severe attack of ulcerative colitis are most likely undergoing steroid therapy and thus need stress doses of corticosteroids to avert adrenal crisis. The medical and surgical teams must also monitor these patients very closely, and if there is no substantial clinical improvement after 24–36 h of aggressive medical therapy or if there is evidence of perforation, emergency surgery is indicated. Any delay in performing surgery significantly increases the risk of perforation, which raises mortality from under 5% to nearly 30%.[13] Although prompt and aggressive medical therapy can postpone emergency surgery, nearly 50% of these patients will require total proctocolectomy within a year. This observation suggests that more conservative surgery is appropriate in the acute setting. With the popularity of anal sphincter-sparing procedures, the surgeon should always weigh the possibility of the need for later surgery for restoration of continence. Specifically, leaving the rectum intact allows its use for subsequent mucosal proctectomy and ileoanal anastomosis. Historically, operations such as Turnbull's blow-hole colostomy with loop ileostomy[15] are rarely used because of improved medical care during emergencies and better surgical options. However, the blow-hole colostomy-ileostomy procedure may still be indicated for select patients with toxic megacolon, large bowel obstruction, severe Clostridium difficile colitis, adult Hirschsprung's disease, and pancreatitis with obstructing pseudocysts. The procedure may also act as a bridge to a more definitive operation for toxic patients with benign disease and palliates those with malignant obstructions and metastasis.[16]
Massive hemorrhage: Massive unrelenting hemorrhage severe enough to result in hemodynamic instability is also a rare surgical complication of ulcerative colitis, occurring in less than 1% of patients and accounting for about 10% of urgent colectomies. Initial treatment should consist of aggressive fluid, electrolyte and blood-product resuscitation. If the hemorrhage is continuous but the patient is hemodynamically stable, a 2–3-day course of high-dose steroids may be tried prior to surgical intervention. However, in most cases prompt surgical intervention is indicated, but only after other causes of bleeding such as gastric or duodenal ulcers are ruled out. Uncontrollable hemorrhage from the entire colorectal mucosa may be the one clear indication for emergency proctocolectomy. If possible, the rectum should be spared for later mucosal proctectomy with ileoanal anastomosis, realizing that about 12% of patients will have continued hemorrhage from the retained rectal segment.
Perforation: Although acute colonic perforation occurs infrequently in the absence of toxic megacolon, the incidence is usually directly related to both the severity of the initial episode and extent of the disease. Although the overall incidence of perforation during a first attack is less than 4%, if the attack is severe, the incidence rises to about 10%. If the patient has pancolitis, the perforation rate can rise to 15%; if the pancolitis is associated with a clinically severe attack, the perforation rate rises to nearly 20%. Perforation may not always be directly associated with the underlying ulcerative colitis, and other causes such as gastric or duodenal ulcers from steroid use or even Crohn's disease might be other causes of perforation. However, since perforation is the most lethal complication of ulcerative colitis, there is no role for medical therapy, and the patient should undergo surgery immediately. Although free colon perforation occurs much more frequently in the presence of toxic megacolon than in its absence, it is important to remember that toxic megacolon is not a prerequisite for the development of perforation. In the presence of colonic perforation, the operation should be definitive without being overly aggressive. Abdominal colectomy with ileostomy and Hartmann closure of the rectum is the procedure of choice.
Obstruction: Complete obstructions caused by benign strictures occur in 11% of patients, with 34% of the strictures occurring in the rectum. Strictures are usually the result of submucosal fibrosis and occasionally mucosal hyperplasia. Although they do not usually cause acute obstruction, the lesions must be differentiated from carcinoma by biopsy or excision, and particular attention should be given to ruling out Crohn's disease. Strictures caused by carcinoma are less common than those caused by benign disease and are more prone to perforate. Many surgeons now believe that any colonic stricture that causes obstructive symptoms, even if it appears benign on endoscopy, should be treated surgically.[12]
Elective indications for surgery: Many patients with chronic ulcerative colitis are choosing to undergo elective proctocolectomy much earlier in the course of their disease,[17] now that there are restorative procedures that offer low complication rates and excellent outcomes. The patient usually decides on elective surgery in consultation with the gastroenterologist and surgeon, and although ulcerative colitis is a chronic disease, the indications for elective surgery may occur early in the course of a patient's disease or after years of fairly mild diseaseof fairly mild disease. The major indications to surgical treatment of ulcerative colitis are:
-
a)
Intractable disease: The failure of medical management as indicated by chronic physical disability and physiologic dysfunction is by far the most common indication for elective surgery in chronic ulcerative colitis. Intractability can be characterized as the severe and persistent impairment of a patient's quality of life, caused by the underlying disease or the therapy. Since intractability is clinically defined, it can occur in patients with either acute or chronic disease. Acutely, intractability generally refers to the inability to control a patient's symptoms despite maximal medical therapy. Conversely, in the chronic state, intractability refers either to the inability to taper medications without relapse, especially steroids, to a tolerable maintenance dose, or to the development of severe drug-related side-effects.[12] There are numerous elective operations for medically intractable ulcerative colitis which are discussed below.
-
b)
Dysplasia, Malignancy of the colon or rectum, or cancer prophylaxis: Patients with ulcerative colitis are clearly at an increased risk for the development of dysplasia and colorectal cancer.[18] Most surgeons agree that significant dysplasia, suspected cancer, or frank malignancy are clear indications for colectomy. Despite the fact that colorectal cancer complicating ulcerative colitis only accounts for approximately 2% of all cases of colorectal cancer in the general population, it is considered a serious complication and accounts for approximately 15% of all deaths associated with inflammatory bowel diseases.[18] Ulcerative colitis increases the risk of colon cancer by approximately 0.5-1.0% annually after 10 years. Early age at diagnosis, and increased duration and extent of disease appear to increase the risk substantially.[19] Thus, by the time the patient has had the disease for 20 years, the risk of colon cancer may be as high as 20%, rising to over 30% in patients who have had even quiescent disease for longer than 35 years. This increased risk clearly emphasizes the importance of performing complete colonoscopies with numerous biopsies from the entire colon and rectum at regular intervals in order to detect mucosal dysplasia and to identify possible candidates for prophylactic colectomy.[20] Although the question of the timing of surgery for cancer prophylaxis remains controversial, there are few patients in whom this is the sole indication for operation. The role of rectal or colonic biopsy in directing the timing of colectomy also remains controversial. Patients with longstanding colitis, unequivocal high-grade dysplasia, or a Dysplasia associated lesions or mass (DALM) are candidates for colectomy. Dysplasia in ulcerative colitis may be classified as flat or elevated (DALM). Patients with flat high-grade dysplasia are generally candidates for colectomy.[21] Since dysplasia is an unreliable marker for the detection of synchronous carcinoma, some surgeons now advocate that even low-grade dysplasia, if verified by an experienced IBD pathologist, is an indication for colectomy. Dysplasia of any grade increases the probability of coexistent cancer and even low-grade dysplasia has a high positive predictive value.[22] Since advanced cancer has been found in association with dysplastic changes of any grade, confirmed dysplasia of any grade is now an indication for colectomy.[22] The presence of carcinoma is not a contraindication to mucosal proctectomy with ileoanal anastomosis, unless the tumor is found to be of an advanced stage or is located within the rectum. Mucosal proctectomy with ileoanal anastomosis is contraindicated for rectal tumors located in the middle and lower thirds of the rectum. In these patients, a standard proctocolectomy and permanent Brooke ileostomy is recommended. Since these tumors are prone to local recurrence, subsequent radiation therapy may be required which contributes to very poor function. In contrast, patients with tumors located in the upper third of the rectum may safely undergo mucosal proctectomy with ileoanal anastomosis, except in cases in which the tumor is large or advanced, when proctocolectomy with Brooke ileostomy is a safer option. If there is uncertainty about the stage of the tumor at the time of the initial operation, subtotal colectomy with ileostomy and Hartmann closure of the rectum can be performed. This operation would allow subsequent conversion to ileoanal anastomosis if the patient remains disease-free. Patients with relatively early-stage colon cancers have several options including mucosal proctectomy with ileoanal anastomosis or continent or Brooke ileostomy, as discussed below. Colon cancers that have metastasized to the liver should be treated with proctocolectomy with Brooke ileostomy or abdominal colectomy and ileorectal anastomosis, as discussed below. Proctocolectomy and Brooke ileostomy is a safer option than mucosal proctectomy with ileoanal anastomosis in patients presenting with lymph node-positive tumors unless the patient is averse to a permanent stoma.
-
c)
Extra-intestinal manifestations: Other than for extreme growth and development retardation in children and adolescents, extra-intestinal manifestations and complications of ulcerative colitis seldom provide the sole indication for surgical management. However, in some cases colectomy can bring dramatic benefits to children with ulcerative colitis. Joint-, eye-, and skin-associated extra-intestinal manifestations often respond to colectomy; however, other more serious manifestations such as ankylosing spondylitis and liver dysfunction or failure may remain unresponsive. The progression of primary sclerosing cholangitis (PSC), a chronic cholestatic syndrome characterized by fibrosing inflammation in the intra- and extra-hepatic bile ducts, appears to bear no relation to the presence or absence of the colon or to the degree of the inflammatory process within the diseased mucosa. The epidemiology of PSC and its relationship to ulcerative colitis has become much clearer recently.[8] In fact, ulcerative colitis patients with PSC may represent a distinct subset of IBD patients in which colorectal cancer develops in a significant fraction and overall survival is worse.[23] Therefore, patients with this rare complication require careful and more frequent surveillance prior to colectomy. Another extra-intestinal manifestation of ulcerative colitis that occasionally emerges as a potential surgical indication is progressively destructive pyoderma gangrenosum, which generally resolves in approximately 50% of patients following colectomy. A rare but more urgent extra-colonic indication for colectomy is massive hemolytic anemia, usually Coombs’ test-positive, that is unresponsive to steroid and immunosuppressive therapy. Under these circumstances, colectomy is generally accompanied by splenectomy.
Other indications for elective surgery for ulcerative colitis can include anorectal complications, which are more common than generally appreciated and can occasionally confound the differential diagnosis between Crohn's colitis and ulcerative colitis. Most rectal symptoms occur within the first year of onset of symptoms, and in part correlate with the severity of disease. Overall, up to 18% of patients with ulcerative colitis develop perirectal or ischiorectal abscesses and associated anal fistulas that require surgical intervention.
The most common extra-intestinal manifestations that present as an emergency include thromboembolic events, ocular complications, and hepatobiliary disease.[24] Hence, for most patients with ulcerative colitis, a colectomy is performed when the disease enters an intractable, chronic phase and becomes a physical and social burden. Again, with sphincter-sparing operations available for patients with ulcerative colitis, it is vitally important to avoid standard proctectomy whenever possible.
Surgical options
Proctocolectomy and ileostomy: Since ulcerative colitis is essentially cured once the colon and rectum are removed, a single-stage total proctocolectomy with permanent ileostomy has historically been the procedure of choice, especially in elective situations.[25] Though this procedure eliminates all diseased tissue and the risk of malignant transformation, and requires a single operation providing patients with a predictable functional result, it remains poorly accepted by patients and their physicians and is usually performed only after other operations have failed or under special circumstances. The reluctance to undergo this operation is primarily associated with the permanent abdominal ileostomy, which is required after a standard proctocolectomy. Although the use of a Brooke ileostomy facilitates the immediate maturation of the stoma and eliminates many of the functional problems previously associated with a permanent ileostomy, patients receiving even the most carefully constructed ileostomies are incontinent and must continuously wear an external collecting device.
Significant postoperative complications are also associated with this operation. A 20% overall morbidity rate is reported for elective, 30% for urgent, and 40% for emergency proctocolectomy. The risks are primarily hemorrhage, contamination, sepsis, and neural injury. Up to 25% of patients require stoma revision and experience perineal wound problems after a standard abdominal perineal proctectomy. Fifteen to twenty percent of patients experience small-bowel obstruction at some point in the postoperative period. Of major concern are bladder and sexual dysfunction associated with parasympathetic nerve injury. Impotence is reported to occur in to up to 5% of male patients after proctectomy.
Despite the fact that the majority of patients with a Brooke ileostomy eventually adjust to the stoma, nearly half experience some level of appliance-related problems including skin irritation or excoriation, discomfort, leakage and odour, or just the time, effort, and financial burden of caring for an ileostomy. Perhaps more central than these problems are the significant psychological and psychosocial implications of a permanent ileostomy, particularly for young and physically active patients. It was for these reasons that surgeons sought alternatives to total proctocolectomy and ileostomy.
Subtotal Colectomy and Ileorectal Anastomosis: Subtotal colectomy and ileorectal anastomosis, has been utilized in the surgical treatment of ulcerative colitis for over 50 years.[26] An ileorectal anastomosis eliminates the need for an abdominal stoma and since the pelvic autonomic nerves are not disturbed, the risk for impotence and bladder dysfunction are very low. Although abdominal colectomy with ileorectal anastomosis is a less extensive procedure that usually leaves the patient with full continence, it is not performed extensively except under certain circumstances because it is not curative. Mucosal inflammation can persist in the retained rectum and there is an ongoing risk of malignancy that increases with time. Approximately 20% of patients require subsequent proctectomy for uncontrollable proctitis or poor function. Even in the absence of disease recurrence or malignancy, function in the early postoperative period can be poor, averaging four or five stools per 24 h. Ileorectal anastomosis can also be associated with a number of postoperative complications, including small-bowel obstruction in up to 20% of patients. In addition, there is the potential for leakage of the anastomosis between the ileum and the disease-bearing rectum. Subtotal colectomy with ileorectal anastomosis is clearly a compromise operation, except for specific indications, and is obviously contraindicated in patients with anal sphincter dysfunction, severe rectal disease, rectal dysplasia, or malignancy. With the availability and success of definitive mucosal proctectomy and ileoanal anastomosis, ileorectal anastomosis is indicated in very few patients. As discussed below, with the recent concern over infertility in young women following Ileal pouch anal anastomosis, subtotal colectomy with or without ileorectal anastomoses became more popular in this patient population.
Continent ileostomy: Patient dissatisfaction due to mechanical and functional problems with the ileostomy and the associated incontinence has motivated surgeons to seek alternatives to preserving continence. Early attempts at continence, such as the continent ileostomy or Kock pouch, however, were fraught with technical complications.[27] Kock first constructed the continent ileostomy entirely of terminal ileum with an ileal pouch that served as a reservoir for stool and an ileal conduit connecting the pouch to a cutaneous stoma. Despite poor functional results, patients undergoing total proctocolectomy could, for the first time, be offered an option for continence. The operation was later modified to include an intestinal nipple valve between the pouch and the stoma.
Typically, 45–50 cm of terminal ileum is required to surgically construct the pouch and the nipple valve. The proximal 30–35 cm is fashioned into a pouch; whereas intussuscepting the outflow tract from the pouch and then securing it with sutures or staples forms the nipple valve. The ileal reservoir is sutured to the peritoneum and fascia, and the efferent limb is externalized through the abdominal wall as a flush stoma. Passing a soft plastic tube through the nipple valve via the stoma can then empty the ileal pouch. The significance of this operation for its time was that patients could finally be offered an operation that was curative and did not require the use of an external appliance.
The continent ileostomy has been associated with a number of complications, the most significant being related to dislodgment of the nipple valve, which results in fecal incontinence and difficulty in intubating and emptying the pouch. Nipple valve failure requiring revision reportedly occurs in up to 60% of patients and approximately 20% of patients will experience small-bowel obstruction, primarily due to adhesions.[28] The risk of bladder dysfunction, impotence, and perineal wound problems are similar to those of standard proctocolectomy and ileostomy. Several dysfunction syndromes associated directly with the continent ileostomy include stagnant loop syndrome, enteritis, nonspecific ileitis, and pouchitis. Clinically, these patients often present with diarrhea, fat and vitamin B12 malabsorption, bacterial overgrowth, and mucosal inflammation of the pouch and incontinence. Patients may also develop fistulae between the pouch and the skin or other enteric organs. Crohn's disease is a clear contraindication to performing this operation.
Although the continent ileostomy has clear theoretical advantages over the Brooke ileostomy, especially with respect to continence, its high rate of functional complications has restricted its clinical utility. The continent ileostomy may be useful in patients who have already undergone total proctocolectomy and ileostomy, and after careful counseling, wish to undergo a continence-restoring procedure. This operation also remains an option for patients who wish to remain continent but are either not candidates for or have failed IPAA,[29] or who for other reasons prefer a permanent ileostomy. In major centers that offer all surgical alternatives to patients with ulcerative colitis, the Kock pouch has limited clinical usefulness and few such pouches are currently being constructed despite recent reports of satisfactory long-term function in more than two-thirds of patients for up to 30 years.[28] Although surgical revisions may be needed to restore proper function, the continent ileostomy appears to have good durability. In a more recent study, patients reported adequate function, high satisfaction, and a health-related quality of life similar to that of the general population.[30]
Total proctocolectomy with ileal pouch-anal anastomosis: As mentioned, until about 25 years ago, proctocolectomy with a Brooke ileostomy was the only viable surgical option that surgeons could offer patients with ulcerative colitis requiring colectomy. Even though this procedure eliminated all diseased tissue and the subsequent risk of malignant transformation, patients and their physicians were averse to this option because it required a permanent abdominal ileostomy. It is for this reason that surgeons sought alternatives to total proctocolectomy and ileostomy that could provide the patient with continence and acceptable function. While the options discussed above were being performed, many surgeons were developing more innovative, functional, and acceptable procedures.
Although the first ileoanal anastomosis was reportedly performed by Nissen in Germany in the early 1930s,[31] it was the pioneering efforts of two surgeons, Mark Ravitch and David Sabiston, who more than half a century ago proposed the novel concept of restorative proctocolectomy with anal sphincter preservation.[32] Instead of ablating the entire rectum, anus, and anal sphincter as occurs during a standard proctocolectomy, they purported that since ulcerative colitis is a mucosal disease, the disease-bearing rectal mucosa could be dissected completely down to the dentate line of the anus, and in theory preserve the rectal muscular cuff and the anal sphincter apparatus. The subsequent extension of the terminal ileum into the pelvis endorectally, and suturing it circumferentially to the anus in an end-to-end fashion would re-establish the continuity of the intestinal tract. This novel surgical advance incorporated a number of potential advantages including preservation of parasympathetic innervation to the bladder and genitalia, elimination of the abdominal perineal proctectomy, and if performed carefully, preservation of the anorectal sphincter. Most importantly, the permanent abdominal ileostomy was eliminated and continence was maintained. Early on, poor functional results forced the operation to be largely abandoned, due in part to an inadequate understanding of anal sphincter physiology at the time. The pioneering efforts of these surgeons, however, set the stage for what has become the definitive procedure for patients seeking surgical intervention for ulcerative colitis.
Although a few surgeons continued to experiment with the ileoanal anastomosis procedure throughout the 1950s and 1960s, there were no more human trials until the late 1970s when Martin, LeCoultre, and Schubert reported on a cohort of 17 patients with ulcerative colitis in whom they successfully performed a total colectomy and mucosal proctectomy and straight ileoanal anastomosis.[33] Despite significant postoperative complications and high stool frequency, others who performed them concluded that ileoanal anastomosis is a viable alternative for patients requiring proctocolectomy.[34,35] However, it was the physiologic studies of Heppell and associates in 1982 that showed an inverse relationship between ileal compliance and stool frequency in patients after an end-to-end or straight ileoanal anastomosis.[36] These studies led to perhaps the most significant technical refinement in the evolution of the IPAA procedure, which was the surgical construction of an ileal pouch or reservoir proximal to the ileoanal anastomosis. Increasing the capacity for storage greatly improved function, reduced stool frequency, and led to increased patient satisfaction.
Although the above reports are historically relevant to the revitalization of the ileoanal anastomosis procedure, it was probably the reports by Parks and Nicholls[37] and Utsunomiya and colleagues[38] that motivated the resurgence of the modern ileal pouch-anal anastomosis procedure. They independently developed and were among the first to successfully utilize an ileal reservoir or pelvic pouch proximal to the ileoanal anastomosis to improve the functional outcome following total colectomy and mucosal proctectomy. Subsequent follow-up studies comparing functional outcomes between the straight ileoanal anastomosis and the ileal pouch-anal anastomosis concluded that inclusion of the ileal pouch significantly improved continence, function, quality of life,[39] and overall clinical outcome, due primarily to the increased distensibility of the neorectum.[40]
Since the addition of the ileal pouch, there has been a dramatic increase in the use of restorative proctocolectomy, especially as surgeons became more familiar with the technical aspects of the procedure. Despite the controversies surrounding methodological issues such as mucosectomy, stapled versus hand-sewn anastomoses, diverting loop ileostomy, pouch configurations, and staged procedures, most surgeons agree that restorative proctocolectomy with IPAA is the definitive operation for the surgical treatment of patients with ulcerative colitis. This procedure is also the choice for patients with familial adenomatous polyposis and more recently in select patients with hereditary nonpolyposis colorectal cancer.[41] Although this procedure is generally contraindicated for patients with Crohn's disease, there are reports of acceptable long-term outcomes in select patients.[42]
Total proctocolectomy with ileal pouch-anal anastomosis is gold standard of operative treatment for most patients with ulcerative colitis. However poor resting tone or anal sphincter dysfunction and low rectal cancers are contraindications. Its advantages are:
Completely restorative
With mucosectomy, eliminates the disease
Good function, continence and quality of life
Disadvantages are:
Two-staged procedure
reduced fertility in females
technically more demanding
abdominal adhesion following IPAA
Types of Pouch:
J-pouch
W-pouch
S-pouch
Lateral side-to-side isoperistaltic pouch
CONTROVERSIES
-
a)
One-stage or two-stage procedure: One-stage i.e., without a temporary diverting ileostomy has an advantage of single operation and no complication of ileostomy, disadvantage being increased risk of pelvic sepsis and increased risk of ileoanal anastomosis or pouch leak. Two-stage i.e., with a diverting ileostomy which is generally closed after eight weeks in a second-stage operation. It reduces the potential for leakage from anastomotic sites and reduces the risk of pelvic sepsis.
-
b)
With mucosectomy or double-stapled anastomosis: Mucosectomy, i.e., removal of complete rectal mucosa from the anal transition zone with hand-sewn ileal pouch anastomosis to anus at dentate line. Its advantage is complete removal of disease but is technically demanding and there is a risk of damage to underlying smooth muscle and needs prolonged retraction of anal sphincter.
Double-stapled anastomosis, i.e., distal rectum is stapled and divided near the pelvic floor leaving the anal transition zone largely intact. Ileal pouch is anastomosed to the top of the anal canal using transanally placed circular EEA stapler. It is a much simpler procedure and preserves distal internal anal sphincter and T-zone, resulting in significant functional advantage by
Increasing anal resting tone
Preservation of rectoanal inhibitory reflex
Improved continence
Fewer septic complications.
But it has the disadvantage of the risk of malignant transformation and cuffitis which confound treatment.
Post IPAA
Barium radiographic study after four weeks to assess the integrity of the anastomosis.
Anal manometry at eight weeks to ensure anal sphincter ms have retained full functions.
Pending satisfactory outcome, loop ileostomy is closed manually or using a stapling technique.
Follow-up at 1, 3, 6, and 12 months, then yearly.
Anal manometry is repeated at one year.
Flexible fibreoptic pouchoscopy with pouch biopsy every five years.
Complications of IPAA
Short-term: Pelvic sepsis and pelvic abscessà primarily due to anastomotic leak
Long-term:-
Adhesive small-bowel obstruction is due to excessive adhesion formation and if it does not show any sign of improvement by conservative treatment then surgery should be considered.
Pouch failure: Pouch salvage surgery/use of total reconstruction are viable alternatives to permanent ileostomy. Alternatively, excision of pouch and conventional Brooke ileostomy can be done. Kock pouch is an option if patient wishes to remain continent.
Dysplasia and carcinoma of ileal pouch.
Crohn's disease of ileal pouch: Its predictors are: complex perianal or pouch fistula, ileitis proximal to pouch and afferent limb ulcers. Medical treatment should be offered first including Infliximab. If it fails then surgical options should be considered
Pouchitis: It is a nonspecific idiopathic inflammation and is the most common and significant late and long-term complication. It presents with colitis-like symptoms. Diagnosis is confirmed by flexible ileal pouchoscopy. Location of mucosal inflammation is important. Broad-spectrum antibiotics are the mainstay of treatment (viz. ciproflaxacin 250 bid + metronidazole 250 qid) for 10 days.
CONCLUSION
Surgical management of ulcerative colitis requires a comprehensive understanding of all the surgical options. While ileorectal anastomosis and proctocolectomy with Brooke ileostomy or Kock pouch have a role in the surgical management of select patients with ulcerative colitis, IPAA has become the definitive procedure in most cases. IPAA has evolved through many phases prior to arriving at the highly successful procedure currently utilized in major centers. Continued technical advances and greater surgeon experience can only further improve function, outcome, and patient satisfaction. Despite some opposition, under elective conditions, IPAA remains an excellent option for patients with ulcerative colitis once the decision for surgery has been mutually reached by the patient, gastroenterologist, and surgeon. With experience, mucosal proctectomy and IPAA can now be performed with a low complication rate, good functional results, and good quality of life, and with excellent long-term outcome. Optimal results can be obtained by careful patient selection, appropriate preoperative management, meticulous standardized surgical technique, appropriate postoperative education, and rigorous follow-up.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kirsner JB. Historical origins of current IBD concepts. World J Gastroenterol. 2001;7:175–84. doi: 10.3748/wjg.v7.i2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joossens S, Reinisch W, Vermeire S, Sendid B, Poulain D, Peeters M, et al. The value of serologic markers in indeterminate colitis: A prospective follow-up study. Gastroenterology. 2002;122:1242–7. doi: 10.1053/gast.2002.32980. [DOI] [PubMed] [Google Scholar]
- 3.Binder V. Epidemiology of IBD during the twentieth century: An integrated view. Best Pract Res Clin Gastroenterol. 2004;18:463–79. doi: 10.1016/j.bpg.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: Antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–33. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Wen Z, Fiocchi C. Inflammatory bowel disease: autoimmune or immune-mediated pathogenesis? Clin Dev Immunol. 2004;11:195–204. doi: 10.1080/17402520400004201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yantiss RK, Sapp HL, Farraye FA, El-Zammar O, O’Brien MJ, Fruin AB, et al. Histologic predictors of pouchitis in patients with chronic ulcerative colitis. Am J Surg Pathol. 2004;28:999–1006. doi: 10.1097/01.pas.0000126758.35603.8d. [DOI] [PubMed] [Google Scholar]
- 7.Orchard T. Extraintestinal complications of inflammatory bowel disease. Curr Gastroenterol Rep. 2003;5:512–7. doi: 10.1007/s11894-003-0042-6. [DOI] [PubMed] [Google Scholar]
- 8.Loftus EV., Jr Management of extraintestinal manifestations and other complications of inflammatory bowel disease. Curr Gastroenterol Rep. 2004;6:506–13. doi: 10.1007/s11894-004-0073-7. [DOI] [PubMed] [Google Scholar]
- 9.Fefferman DS, Farrell RJ. Endoscopy in inflammatory bowel disease: Indications, surveillance, and use in clinical practice. Clin Gastroenterol Hepatol. 2005;3:11–24. doi: 10.1016/s1542-3565(04)00441-0. [DOI] [PubMed] [Google Scholar]
- 10.Carter MJ, Lobo AJ, Travis SP. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53(Suppl 5):V1–6. doi: 10.1136/gut.2004.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanauer SB, Present DH. The state of the art in the management of inflammatory bowel disease. Rev Gastroenterol Disord. 2003;3:81–92. [PubMed] [Google Scholar]
- 12.Cima RR, Pemberton JH. Surgical indications and procedures in ulcerative colitis. Curr Treat Options Gastroenterol. 2004;7:181–90. doi: 10.1007/s11938-004-0039-8. [DOI] [PubMed] [Google Scholar]
- 13.Becker JM. Surgical therapy for ulcerative colitis and Crohn's disease. Gastroenterol Clin North Am. 1999;28:371–90. doi: 10.1016/s0889-8553(05)70061-3. [DOI] [PubMed] [Google Scholar]
- 14.Gan SI, Beck PL. A new look at toxic megacolon: An update and review of incidence, etiology, pathogenesis, and management. Am J Gastroenterol. 2003;98:2363–71. doi: 10.1111/j.1572-0241.2003.07696.x. [DOI] [PubMed] [Google Scholar]
- 15.Turnbull RB, Jr, Hawk WA, Weakley FL. Surgical treatment of toxic megacolon. Ileostomy and colostomy to prepare patients for colectomy. Am J Surg. 1971;122:325–31. doi: 10.1016/0002-9610(71)90252-2. [DOI] [PubMed] [Google Scholar]
- 16.Remzi FH, Oncel M, Hull TL. Current indications for blow-hole colostomy: Ileostomy procedure. A single center experience. Int J Colorectal Dis. 2003;18:361–4. doi: 10.1007/s00384-002-0453-0. [DOI] [PubMed] [Google Scholar]
- 17.Cima RR, Pemberton JH. Protagonist: Early surgical intervention in ulcerative colitis. Gut. 2004;53:306–7. doi: 10.1136/gut.2003.001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munkholm P. Review article: The incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18(Suppl 2):1–5. doi: 10.1046/j.1365-2036.18.s2.2.x. [DOI] [PubMed] [Google Scholar]
- 19.Sharan R, Schoen RE. Cancer in inflammatory bowel disease. An evidence-based analysis and guide for physicians and patients. Gastroenterol Clin North Am. 2002;31:237–54. doi: 10.1016/s0889-8553(01)00014-0. [DOI] [PubMed] [Google Scholar]
- 20.Sjoqvist U. Dysplasia in ulcerative colitis-clinical consequences? Langenbecks Arch Surg. 2004;389:354–60. doi: 10.1007/s00423-003-0455-6. [DOI] [PubMed] [Google Scholar]
- 21.Odze R. Diagnostic problems and advances in inflammatory bowel disease. Mod Pathol. 2003;16:347–58. doi: 10.1097/01.MP.0000064746.82024.D1. [DOI] [PubMed] [Google Scholar]
- 22.Gorfine SR, Bauer JJ, Harris MT, Kreel I. Dysplasia complicating chronic ulcerative colitis: is immediate colectomy warranted? Dis Colon Rectum. 2000;43:1575–81. doi: 10.1007/BF02236742. [DOI] [PubMed] [Google Scholar]
- 23.Loftus EV, Jr, Harewood GC, Loftus CG, Tremaine WJ, Harmsen WS, Zinsmeister AR, et al. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91–6. doi: 10.1136/gut.2004.046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung O, Regueiro MD. Inflammatory bowel disease emergencies. Gastroenterol Clin North Am. 2003;32:1269–88. doi: 10.1016/s0889-8553(03)00095-5. [DOI] [PubMed] [Google Scholar]
- 25.Meagher AP, Farouk R, Dozois RR, Kelly KA, Pemberton JH. J ileal pouch-anal anastomosis for chronic ulcerative colitis: Complications and long-term outcome in 1310 patients. Br J Surg. 1998;85:800–3. doi: 10.1046/j.1365-2168.1998.00689.x. [DOI] [PubMed] [Google Scholar]
- 26.Aylett SO. Ileorectal anastomosis: review 1952-1968. Proc R Soc Med. 1971;64:967–71. [PMC free article] [PubMed] [Google Scholar]
- 27.Kock NG. Intra-abdominal “reservoir” in patients with permanent ileostomy. Preliminary observations on a procedure resulting in fecal “continence” in five ileostomy patients. Arch Surg. 1969;99:223–31. doi: 10.1001/archsurg.1969.01340140095014. [DOI] [PubMed] [Google Scholar]
- 28.Lepisto AH, Jarvinen HJ. Durability of Kock continent ileostomy. Dis Colon Rectum. 2003;46:925–8. doi: 10.1007/s10350-004-6686-y. [DOI] [PubMed] [Google Scholar]
- 29.Borjesson L, Oresland T, Hulten L. The failed pelvic pouch: conversion to a continent ileostomy. Tech Coloproctol. 2004;8:102–5. doi: 10.1007/s10151-004-0065-5. [DOI] [PubMed] [Google Scholar]
- 30.Berndtsson IE, Lindholm E, Oresland T, Hultén L. Health-related quality of life and pouch function in continent ileostomy patients: A 30-year perspective. Dis Colon Rectum. 2004;47:2131–7. doi: 10.1007/s10350-004-0719-4. [DOI] [PubMed] [Google Scholar]
- 31.Stryker SJ, Dozois RR. The ileoanal anastomosis: Historical perspectives. In: Dozois RR, editor. Alternatives of Conventional Ileostomy. Chicago, IL: Yearbook Medical; 1985. p. 255. [Google Scholar]
- 32.Ravitch MM, Sabiston DL., Jr Anal ileostomy with preservation of the sphincter: A proposed operation in patients requiring total colectomy for benign lesions. Surg Gynecol Obstet. 1947;84:1095–9. [PubMed] [Google Scholar]
- 33.Martin LW, Le Coultre C, Schubert WK. Total colectomy and mucosal proctectomy with preservation of continence in ulcerative colitis. Ann Surg. 1977;186:477–80. doi: 10.1097/00000658-197710000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beart RW, Jr, Dozois RR, Kelly KA. Ileoanal anastomosis in the adult. Surg Gynecol Obstet. 1982;154:826–8. [PubMed] [Google Scholar]
- 35.Pemberton JH, Heppell J, Beart RW, Jr, Dozois RR, Telander RL. Endorectal ileoanal anastomosis. Surg Gynecol Obstet. 1982;155:417–24. [PubMed] [Google Scholar]
- 36.Heppell J, Kelly KA, Phillips SF, Beart RW, Jr, Telander RL, Perrault J. Physiologic aspects of continence after colectomy, mucosal proctectomy, and endorectalileo-anal anastomosis. Ann Surg. 1982;195:435–43. doi: 10.1097/00000658-198204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parks AG, Nicholls RJ. Proctocolectomy without ileostomy for ulcerative colitis. BMJ. 1978;2:85–8. doi: 10.1136/bmj.2.6130.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Utsunomiya J, Iwama T, Imajo M, Matsuo S, Sawai S, Yaegashi K, et al. Total colectomy, mucosal proctectomy, and ileoanal anastomosis. Dis Colon Rectum. 1980;23:459–66. doi: 10.1007/BF02987076. [DOI] [PubMed] [Google Scholar]
- 39.Taylor BM, Beart RW, Jr, Dozois RR, Kelly KA, Phillips SF. Straight ileoanal anastomosis v ileal pouch-anal anastomosis after colectomy and mucosal proctectomy. Arch Surg. 1983;118:696–701. doi: 10.1001/archsurg.1983.01390060018004. [DOI] [PubMed] [Google Scholar]
- 40.Taylor BM, Cranley B, Kelly KA, Phillips SF, Beart RW, Jr, Dozois RR. A clinico-physiological comparison of ileal pouch-anal and straight ileoanal anastomoses. Ann Surg. 1983;198:462–8. doi: 10.1097/00000658-198310000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becker JM, Stucchi AF. Inherited colorectal polyposis syndromes. In: Cameron JL, editor. Current Surgical Therapy. 8th ed. Philadelphia, PA: Elsevier Mosby; 2004. pp. 200–11. [Google Scholar]
- 42.Panis Y. Is there a place for ileal pouch-anal anastomosis in patients with Crohn's colitis? Neth J Med. 1998;53:S47–51. doi: 10.1016/s0300-2977(98)00123-5. [DOI] [PubMed] [Google Scholar]


