Abstract
Background:
Hepatorenal syndrome (HRS) is known as development of acute renal failure in a patient who usually has advanced liver disease. The aim of the present study was to determine the safety and the efficacy of noradrenalin in comparison with midodrine-octreotide in patients with HRS.
Methods:
This study was registered to the Iranian Registry of Clinical trials (IRCT). This study was a single-center, randomized, clinical trial that performed in Alzahra hospital, Isfahan, Iran. Since March 2011 to January 2012, twenty-three patients were enrolled in the study. Eligible patients were allocated in 2 groups. In the first group, patients received infusion of NA with the dose of 0.1–0.7 μg/kg/min, and in the other groups, patients received octreotide 100-200 μg subcutaneously 3 times daily and midodrine 5-15 mg orally 3 times daily. In both study groups, patient received albumin infusion in addition to noradrenalin or midodrine-octreotide.
Results:
Complete response of HRS was observed in 8 of the 11 patients (73%) treated with noradrenalin and in 9 of the 12 patients (75%) treated with midodrine-octreotide (P > 0.05). HRS recurred after treatment withdrawal in 2 of 11 in NA and 3 of 12 in MO group. That shows no significant difference between 2 groups (P > 0.05).
Conclusion:
We deduce that NA has the same efficacy and safety with MO and can induce a complete response in high percentage of the patients. Moreover, we observed no significant differences in the recurrence rate and outcomes after 3 months among the patients in both study groups; this result could support the use of NA in HRS management. The IRCT ID is: IRCT201107217085N1.
Keywords: Clinical trial, hepatorenal syndrome, midodrine, noradrenalin, octreotide
INTRODUCTION
Hepatorenal syndrome (HRS) is known as development of acute renal failure in a patient who usually has advanced liver disease.[1,2] HRS has 2 clinical patterns: Type 1 HRS is an acute form of HRS, characterized by rapidly progressive renal failure with a median survival of 4 weeks; Type 2 HRS has slower course of renal failure, with a median survival of about 6 months.[3]
The criteria for HRS are based on the following mechanisms: Renal failure in HRS is functional and caused by marked intra-renal arteriolar vasoconstriction and extra-renal vasodilatation, and plasma volume expansion does not improve renal failure,[4]
Liver transplantation is still the treatment of choice in patients with HRS.[5] However, achievement to this ideal management even in the advanced care settings has major obstacles. Accordingly, several attempts have been made recently for obtaining some effective, rapid strategies for improving renal function and extending the patients’ survival before transplantation[6,7] Arterial vasodilatation, mainly in the splanchnic circulation, has an important role in the hemodynamic changes and in renal function in cirrhosis, so using vasoconstrictor drugs has made a big stride in this path as they have brought about promising outcomes.[1] Their effectiveness in increasing mean arterial pressure (MAP) and improvement in renal function has been significant, which may also be enhanced by administration of plasma expanders, especially human albumin.
As it is mentioned above, vasoconstrictors are effective drugs in management of HRS. There are previous data that suggest that midodrine plus octreotide are highly effective and safe in HRS management.[8–10] Moreover, there is only one pilot study, which evaluated the efficacy of noradrenalin (NA) in management of HRS.[11] Considering that NA is a cheap and widely available drug,[12] designing a randomized clinical trial to evaluate the safety and efficacy of NA in comparison with midodrine-octreotide (MO) seemed to be necessary.
The aim of the present study was to determine the safety and efficacy of NA in comparison with MO in patients with HRS.
METHODS
Patients and settings
This single-center, randomized, clinical trial was performed in Alzahra hospital, Isfahan, Iran. During the period of 9 months since March 2011 to January 2012, 23 patients who met the inclusion criteria were enrolled in the study. Eligibility: Patients’ with hepatorenal syndrome and without heart failure and without respiratory failure and without coronary and peripheral artery disease that admitted to the adult gastroenterology and hepatology department of Alzahra hospital were enrolled in the study. Patients were excluded if any of the following criteria were present: Evidences of hepatocellular carcinoma,[6] recent history of related complications of cirrhosis,[7] and decline to participate in research project.
Intervention
In patients who enrolled in the study, a central venous line and urinary bladder catheter were inserted and continuous cardiac rhythm monitoring was also assigned. Intravenous albumin (20 g/100 mL) was initiated to maintain central venous pressure (CVP) between 10 and 15 cm H2O. The measurement of arterial blood pressure was performed manually every 4 hours while CVP and urine output were checked every 8 hours.
In all patients’ serum and urine sodium, blood coagulation states were checked. Blood and urine samples were provided prior to the initiation of treatment and daily during the study. We also checked plasma rennin activity (PRA) and plasma aldosterone by radioactive immune assay (RIA) at the initiation and at the end of treatment.
In the first group, patients received a continuous infusion of NA at an initial dose of 0.1 μg/kg/min, aimed to attain an increase in MAP of at least 10 mm Hg. In case of lack of increase in baseline MAP of at least 10 mmHg, noradrenalin was increased every 4 hours in steps of 0.05 μg/kg/min up to the maximum dose of 0.7 μg/kg/min.
NA was administered either until HRS reversal or for a maximum of 15 days. NA doses were subsequently tapered to 0 over 3 days.[12]
In the other group, octreotide was administered subcutaneously at an initial dose of 100 μg 3 times daily and then, if necessary, increased to 200 μg 3 times daily. Midodrine was administered orally at an initial dose of 5 mg 3 times daily, and in case of lack of increase in baseline MAP of at least 15 mmHg, midodrine was increased every 24 hours in steps of 5 mg 3 times daily up to the maximum dose of 15 mg 3 times daily, if needed. In addition, an amount of 20 to 60 g/d of albumin was infused in all patients to maintain CVP in the range of 10 to 15 mmHg.[10]
Assessments and outcomes
HRS was diagnosed according to the International Ascites Club (Club criteria): Cirrhotic patients with serum creatinine >1.5 mg/dL without improvement following diuretic withdrawal and plasma volume expansion with intravenous albumin and in the absence of (a) shock, (b) renal or gastrointestinal fluid loss, (c) recent management with nephrotoxic drug, (d) recent ongoing bacterial infection, (e) proteinuria ≥500 mg/dL, and (f) ultra sonographic evidences of parenchymal renal disease or urinary tract obstruction.[4,5]
Patients considered as complete response (reversal of HRS) to the therapy when the decrease of 30% or greater of serum creatinine level compared with the baseline value to a final value of 1.5 mg/dL (133 μmol/L) or lower during treatment were observed.
HRS recurrence diagnosed by increase in serum creatinine level of 50% or more with respect to the lowest value after therapy in patients with complete response with a final value above 1.5 mg/dL (133 lmol/L) during the follow-up period.
Sample size and randomization
Sample size was calculated to be 11 in each group, using statistical formula with considering that α =0.05 and β =0.2 (power =80%). Enrolled patients randomized into 2 groups using random allocation software. Informed consents were obtained from all patients for authorized use of their medical records for research purposes with approval of the protocol by ethical committee of our university.
Statistical analysis
Statistical analysis was performed using the statistical software SPSS version 16.0. Comparisons between groups were made by Chi-square, Mann-witheny, Fisher test, and Wilcoxon test as needed. Statistical difference considered significant if P was <0.05.
RESULTS
A total number of 23 patients were enrolled in the study. Eleven were allocated in NA group, and 12 were in MO group. Fifteen were men, and 8 were women. Patients’ enrollment, allocation, follow-up, and analysis are shown in Diagram 1.
Diagram 1.
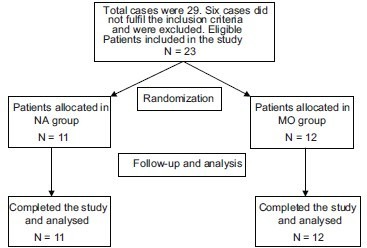
Patients’ enrollment, allocation, follow-up, and analysis in both study groups
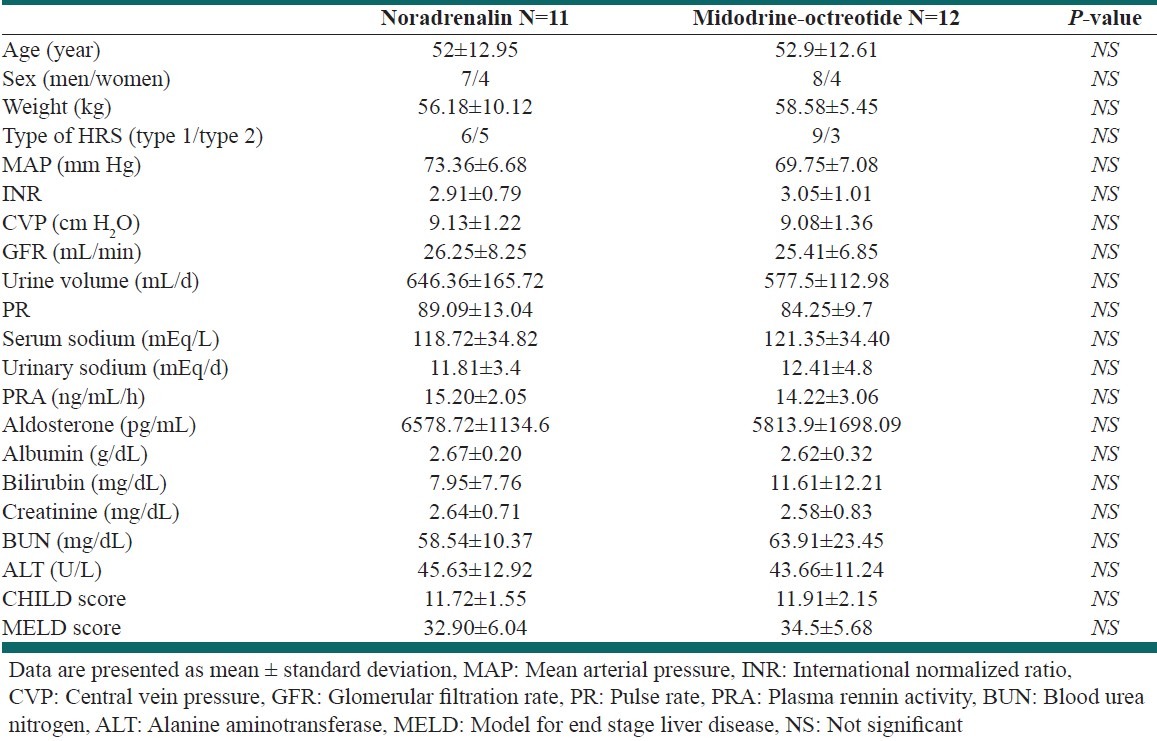
The mean age was 52 ± 12.95 and 52.9 ± 12.61 for NA and MO group respectively. Detailed data about the participants and the comparisons between the groups are shown in the Table 1; none of the parameters were significant among two groups.
Table 1.
Baseline demographic, clinical, and laboratory data in the patients at the beginning of the study
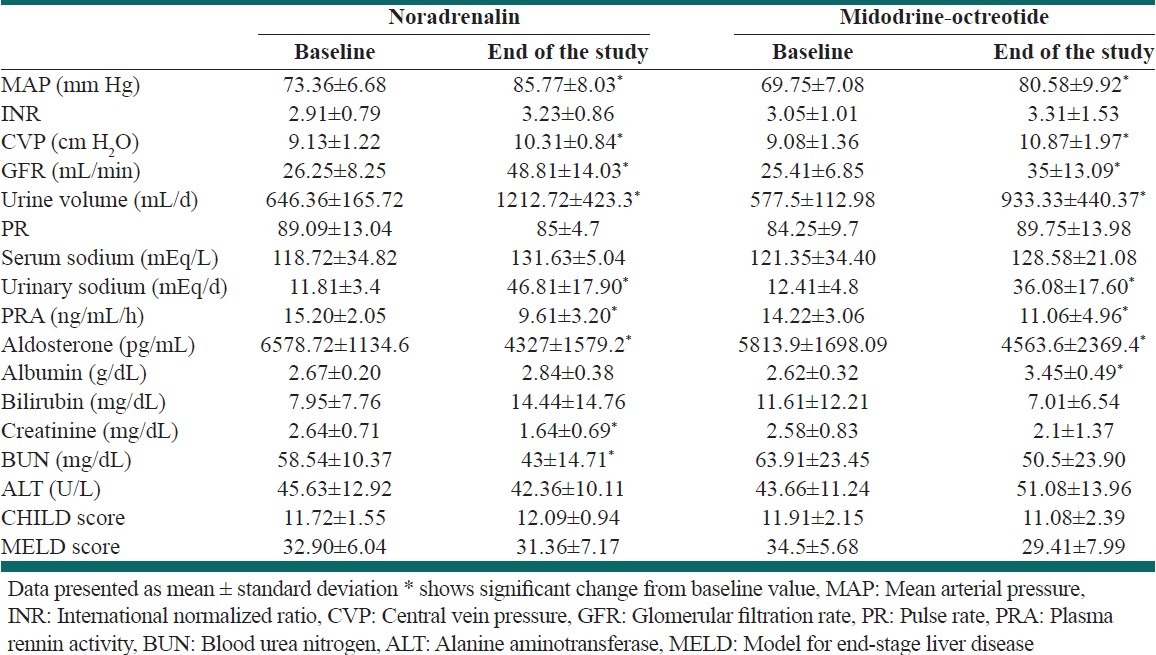
The parameters that explained above also evaluated between study groups at the end of study. Significant changes were observed in MAP, CVP, GFR, urine sodium and volume, aldosterone, BUN, and creatinine in both study groups. Detailed data are shown in [Table 2].
Table 2.
Effect of therapy on study parameters between two groups
Response to therapy: Eight of the 11 patients (72.72%) treated with NA plus albumin and 9 of the 12 patients (75%) treated with MO plus albumin showed a complete response to therapy. Recurrence was occurred in 2 of 8 in NA group and 3 of 9 in MO group. Detailed data about the response, recurrence, and outcome after 3 months are shown in [Table 3]. Further data on individual values of serum creatinine levels in study groups are shown in [Figures 1 and 2].
Table 3.
Response to therapy, recurrence, and the outcome after 3 months among the patients
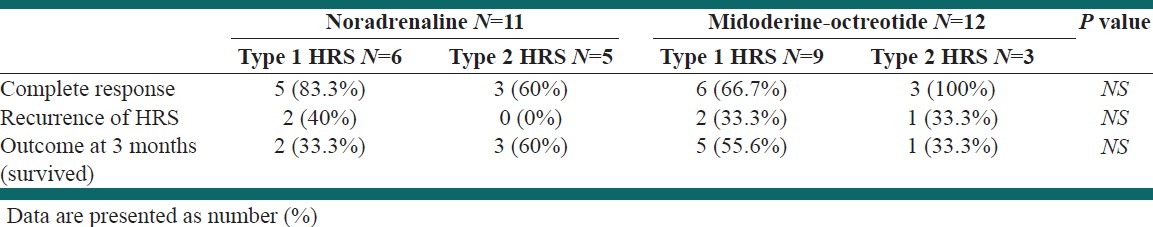
Figure 1.
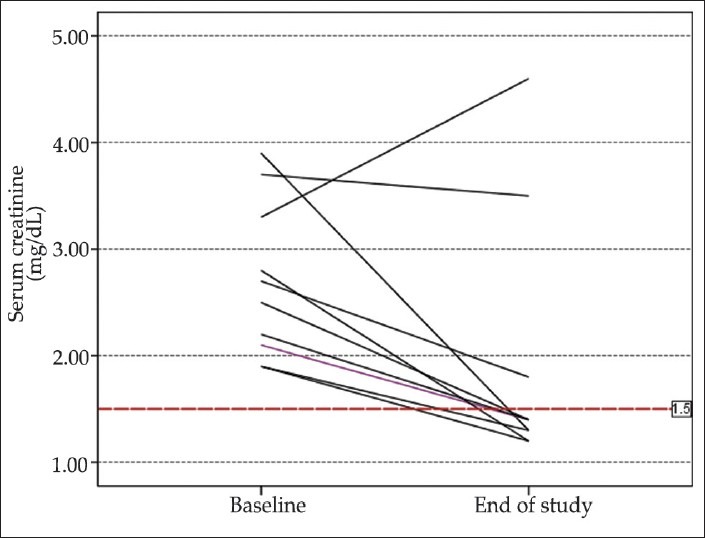
Individual values of serum creatinine before and after the study period in patients treated with NA
Figure 2.
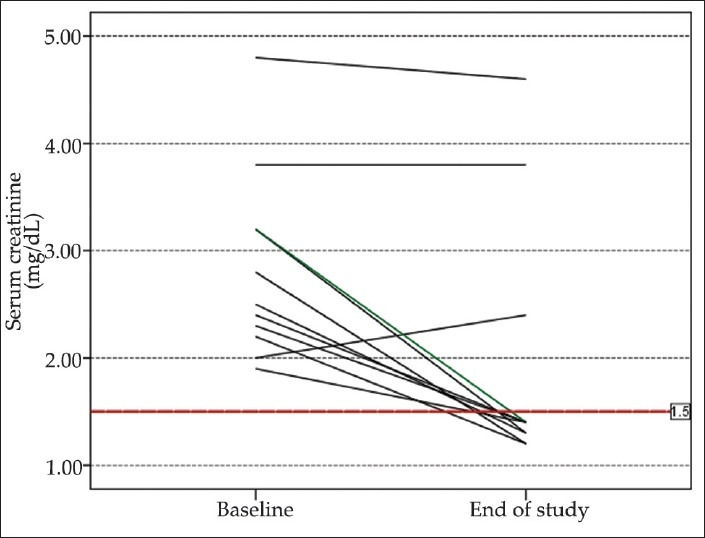
Individual values of serum creatinine before and after the study period in patients treated with MO
Adverse effects
No patient in any group developed signs of myocardial, intestinal, or finger ischemia.
DISCUSSION
The aim of the present study was to determine the efficacy and safety of NA versus the combination of midodrine and octreotide in patients with HRS. As it is explained in [Table 3], NA led to HRS reversal in 8 of 11 (72.72%) patients; on other hand, MO led to complete response in 9 of 12 (75%). There was no significant difference in complete response and the outcome after 3 months, which shows the same efficacy of these two methods. This finding is in line with previous studies in this field.[11,12] One of them that performed by Alessandria et al. compared NA and Terlipresin in 22 patients with HRS. They revealed that NA is as effective and safe as Terlipresin. They also conclude that since NA is a cheap and widely-available drug, it can be used in HRS management.
HRS recurred after treatment withdrawal in 2 of 11 in NA and 3 of 12 in MO group. That shows no significant difference between 2 groups. This low percentage of recurrence in patients who treated with NA is in agreement with previous studies.[12]
As it is shown in [Table 2], the parameters including MAP, CVP, GFR, urine volume, urine sodium, Aldostrone, creatinine, and PRA showed significant change before and after the study in both study groups.
We also observed significant change in BUN and creatinine in NA group, and on other hand, albumin in MO group. These changes didn’t observe in the other group. Glomerular filtration rate and sodium excretion remained below normal values despite the response to therapy. The reasons why renal function fails to normalize in these patients is unknown. The same results were obtained in previous researches in this field.[9,13–15]
Although NA can worsen the liver function due to decrease in liver blood flow, our results didn’t show significant changes in ALT, MELD, or CHILD score. In contrast with our study, previous data showed that treatment with telmipresin can significantly reduce MELD score in patients with HRS.[12]
Our study also had some limitations; we suggest further multicenter and multinational, blinded, controlled trials to confirm the results of the present study.
In conclusion, we deduce that NA has the same efficacy and safety with MO and can induce a complete response in high percentage of the patients. We also observed the same recurrence rate and outcomes after 3 months among the patients in both study groups; this result could support the use of NA in HRS management.
Footnotes
Source of Support: This study was supported by a grant from Isfahan University of Medical Sciences (Grant No: 390210)
Conflict of Interest: None declared.
REFERENCES
- 1.Gluud LL, Christensen K, Christensen E, Krag A. Systematic review of randomized trials on vasoconstrictor drugs for hepatorenal syndrome. Hepatology. 2010;51:576–84. doi: 10.1002/hep.23286. [DOI] [PubMed] [Google Scholar]
- 2.Fagundes C, Gines P. Hepatorenal syndrome: A severe, but treatable, cause of kidney failure in cirrhosis. Am J Kidney Dis. 2012;59:874–85. doi: 10.1053/j.ajkd.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 3.Gines P, Guevara M, Arroyo V, Rodes J. Hepatorenal syndrome. Lancet. 2003;362:1819–27. doi: 10.1016/S0140-6736(03)14903-3. [DOI] [PubMed] [Google Scholar]
- 4.Arroyo V, Gines P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23:164–76. doi: 10.1002/hep.510230122. [DOI] [PubMed] [Google Scholar]
- 5.Salerno F, Gerbes A, Gines P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–8. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genzini T, Torricelli FC. Hepatorenal syndrome: An update. Sao Paulo Med J. 2007;125:50–6. doi: 10.1590/S1516-31802007000100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagher L, Patch D, Marley R, Moore K, Burroughs A. Review article: Pharmacological treatment of the hepatorenal syndrome in cirrhotic patients. Aliment Pharmacol Ther. 2000;14:515–21. doi: 10.1046/j.1365-2036.2000.00740.x. [DOI] [PubMed] [Google Scholar]
- 8.Kalambokis G, Economou M, Fotopoulos A, Al Bokharhii J, Pappas C, Katsaraki A, et al. The effects of chronic treatment with octreotide versus octreotide plus midodrine on systemic hemodynamics and renal hemodynamics and function in nonazotemic cirrhotic patients with ascites. Am J Gastroenterol. 2005;100:879–85. doi: 10.1111/j.1572-0241.2005.40899.x. [DOI] [PubMed] [Google Scholar]
- 9.Angeli P, Volpin R, Gerunda G, Craighero R, Roner P, Merenda R, et al. Reversal of type 1 hepatorenal syndrome with the administration of midodrine and octreotide. Hepatology. 1999;29:1690–7. doi: 10.1002/hep.510290629. [DOI] [PubMed] [Google Scholar]
- 10.Esrailian E, Pantangco ER, Kyulo NL, Hu KQ, Runyon BA. Octreotide/Midodrine therapy significantly improves renal function and 30-day survival in patients with type 1 hepatorenal syndrome. Dig Dis Sci. 2007;52:742–8. doi: 10.1007/s10620-006-9312-0. [DOI] [PubMed] [Google Scholar]
- 11.Duvoux C, Zanditenas D, Hezode C, Chauvat A, Monin JL, Roudot-Thoraval F, et al. Effects of noradrenalin and albumin in patients with type I hepatorenal syndrome: A pilot study. Hepatology. 2002;36:374–80. doi: 10.1053/jhep.2002.34343. [DOI] [PubMed] [Google Scholar]
- 12.Alessandria C, Ottobrelli A, Debernardi-Venon W, Todros L, Cerenzia MT, Martini S, et al. Noradrenalin vs terlipressin in patients with hepatorenal syndrome: A prospective, randomized, unblinded, pilot study. J Hepatol. 2007;47:499–505. doi: 10.1016/j.jhep.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Wong F, Pantea L, Sniderman K. Midodrine, octreotide, albumin, and TIPS in selected patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2004;40:55–64. doi: 10.1002/hep.20262. [DOI] [PubMed] [Google Scholar]
- 14.Alessandria C, Venon WD, Marzano A, Barletti C, Fadda M, Rizzetto M. Renal failure in cirrhotic patients: Role of terlipressin in clinical approach to hepatorenal syndrome type 2. Eur J Gastroenterol Hepatol. 2002;14:1363–8. doi: 10.1097/00042737-200212000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Colle I, Durand F, Pessione F, Rassiat E, Bernuau J, Barriere E, et al. Clinical course, predictive factors and prognosis in patients with cirrhosis and type 1 hepatorenal syndrome treated with Terlipressin: A retrospective analysis. J Gastroenterol Hepatol. 2002;17:882–8. doi: 10.1046/j.1440-1746.2002.02816.x. [DOI] [PubMed] [Google Scholar]


