Abstract
Tomato (Lycopersicon esculentum Miller) fruit discs fed with [2,3-14C]1-aminocyclopropane-1-carboxylic acid (ACC) formed 1-malonyl-ACC (MACC) as the major conjugate of ACC in fruit throughout all ripening stages, from immature-green through the red-ripe stage. Another conjugate of ACC, γ-glutamyl-ACC (GACC), was formed only in mature-green fruit in an amount about 10% of that of MACC; conjugation of ACC into GACC was not detected in fruits at other ripening stages. No GACC formation was observed from etiolated mung bean (Vigna radiata [L.] Wilczek) hypocotyls, etiolated common vetch (Vicia sativum L.) epicotyls, or pea (Pisum sativum L.) root tips, etiolated epicotyls, and green stem tissue, where active conversion of ACC into MACC was observed. GACC was, however, formed in vitro in extracts from fruit of all ripening stages. GACC formation in an extract from red fruit at pH 7.15 was only about 3% of that at pH 8.0, the pH at which most assays were run. Our present in vivo data support the previous contention that MACC is the major conjugate of ACC in plant tissues, whereas GACC is a minor, if any, conjugate of ACC. Thus, our data do not support the proposal that GACC formation could be more important than MACC formation in tomato fruit.
The biosynthesis of ethylene in plants has been established to occur via the following pathway: Met → S-adenosylMet → ACC → ethylene (Adams and Yang, 1979; Yang and Hoffman, 1984). As part of the regulation of ethylene production, ACC can be conjugated to form MACC (Yang, 1987). MACC has been isolated and identified from buckwheat hypocotyls (Amrhein et al., 1981), wheat leaves (Hoffman et al., 1982), and peanut seed (Hoffman et al., 1983) and measured in many other tissues (Amrhein et al., 1982).
It is generally believed that ACC is mainly conjugated into MACC catalyzed by ACC N-malonyl transferase, which is widely present in plant tissues. Recently, Martin et al. (1995) reported the in vitro production of another conjugate of ACC, GACC, in extracts of tomato (Lycopersicon esculentum) fruit, and the enzyme responsible for this in vitro formation of GACC, GGT, has been purified (Martin and Slovin, 1997). Comparing the in vitro GACC-formation activity with that for the in vitro MACC-formation activity via N-malonyltransferase, Martin and Saftner (1995) concluded that GACC formation could be more important than MACC formation in the regulation of ethylene production in tomato fruit. The authors stated that GACC was formed in vivo when tomato fruit discs were fed with [14C]ACC, but no data were presented. The objective of our work was to examine the relative importance of the in vivo formation of GACC versus MACC in tomato discs fed with [14C]ACC.
MATERIALS AND METHODS
Plant Material and Chemicals
Tomato (Lycopersicon esculentum Miller cv Match and Trust) plants were grown in a greenhouse. Fruit were harvested at the indicated developmental stage. The fruit stages used were: immature green, about one-half full size; mature green, fruit green throughout and full size with slight formation of jelly and some seed cut with a knife; breaker, 5 to 15% of surface colored red; turning, 15 to 30% of surface colored red; light red, 60 to 90% of surface colored red; and red, all of surface colored red (Kader and Morris, 1976). Etiolated mung bean (Vigna radiata [L.] Wilczek), common vetch (Vicia sativa L.), and pea (Pisum sativum L, cv Alaska) were grown for 4, 6, and 5 d, respectively, in the dark between two layers of wetted paper towel held upright in a round wire cage. Light-grown pea seedlings were grown in vermiculite for 6 to 8 d under constant fluorescent light (cool-white lamps) at 25°C.
Two samples of synthetic GACC were kindly provided by Dr. S.T. Chen (Institute of Biological Chemistry, Academia Sinica, Taipei, Taiwan) and Drs. T. Ogawa and S. Sano (Tokushima University, Japan). Structure of GACC was confirmed by proton NMR and MS and its purity was confirmed by paper chromatography. [2,3-14C]ACC was obtained by special purchase from Commissariat à l'Energie Atomique (sur-Yvette, France). [14C]MACC was synthesized enzymatically from [2,3-14C]ACC, malonyl-CoA, and partially purified N-malonyltransferase from tomato fruit (Liu et al., 1985). Purity of [2,3-14C]ACC and [14C]MACC were confirmed by paper radiochromatography.
In Vivo Feeding of [2,3-14C]ACC
Two discs, 1.2 cm in diameter and about 0.4 g fresh weight, were cut with a no. 8 cork borer from each tomato fruit. The inner portion of the discs were cut off to give a thickness of 3 to 4 mm. The discs were placed individually, inner side up, in the bottom of 16- × 100-mm test tubes. To the surface of each disc 24 μL of [2,3-14C]ACC (3.7 × 104 Bq, 0.63 mm) in 25 mm Mes-KOH at pH 6.7 was added. The tube was sealed with a serum stopper. A center well containing 100 μL of 0.5 m KOH with a filter paper wick and another filter paper strip wetted with about 25 μL of 0.25 m mercuric perchlorate were attached to the serum stopper to absorb CO2 and C2H4, respectively. The discs were incubated for 8 h at 25°C. At the end of the incubation period the discs were homogenized in 80% (v/v) ethanol and the radioactivities in the KOH wick from the center well and the mercuric perchlorate-wetted paper strip were counted. The tomato disc homogenate was centrifuged, the supernatant was removed, and the pellet was re-extracted with 80% (v/v) ethanol. The ethanol extracts were combined and dried using a vortex evaporator (Buchler, Kansas City, MO). The residue was taken up in a small amount of water and the radioactive products were separated using high-voltage paper electrophoresis (Savant Instruments, Farmingdale, NY) at pH 7.0. The radioactivity was located using a radiochromatogram scanner (Packard Instruments, Downers Grove, IL), and the radioactive areas were cut out and counted in a scintillation counter for quantitation of radioactivity (Adams and Yang, 1979; Peiser et al., 1984). These experiments were conducted twice using fruit (one to two) of each developmental stage.
Tissue from mung bean, common vetch, and pea were also fed with [2,3-14C]ACC. Approximately 1-cm segments with a total fresh weight of about 0.4 g of etiolated mung bean hypocotyls, etiolated vetch and pea epicotyls, and green pea stems just below the tip were placed in 16- × 100-mm test tubes with 75 μL of [2,3-14C]ACC (3.7 × 104 Bq, 0.20 mm) in 10 mm Mes-KOH, pH 6.7. Also, 1-cm segments of pea root tips with a fresh weight of about 0.15 g were similarly fed with [2,3-14C]ACC. CO2 and C2H4 were absorbed as with the tomato discs. All segments were incubated for 7 h at 25°C. Radiolabeled product separation was the same as for the tomato discs. Duplicate samples for each tissue were used and these experiments were conducted twice.
Assay of in Vitro GACC-Forming Activity
The assay procedure used was very similar to that of Martin et al. (1995). A homogenate from the same tomatoes used in the in vivo feeding experiment was prepared by grinding pericarp tissue in a buffer containing 100 mm Tris-Cl (pH 8.0), 2 mm EDTA, and 5 mm DTT. Two milliliters of buffer per gram of tissue was used. The homogenate was centrifuged for 3 min at 14,000g to remove cellular debris. The assay was performed in 50 μL containing 30 μL of enzyme, 100 mm Tris-Cl (pH 8.0), 1 mm DTT, 1 mm EDTA, 2 mm GSH, and 2 mm [2,3-14C]ACC (1 × 104 Bq/assay). The assay was incubated for 1 h at 30°C and stopped by adding 10 μL of concentrated glacial acetic acid. This reaction solution was taken to dryness in a SpeedVac (Savant Instruments, Farmingdale, NY) and the residue was taken up in 25 μL of water. An aliquot of this solution was separated on high-voltage paper electrophoresis at pH 7.0 and the radioactivity was located using a radiochromatogram scanner. The radioactive spots were cut from the paper and counted in a scintillation counter. This experiment was conducted twice.
Ethylene Production from Tomato Discs
Discs were cut as described for [2,3-14C]ACC-feeding experiments (above) from fruit of various developmental stages. No ACC or nonradioactive ACC, equivalent to that applied as [2,3-14C]ACC (24 μL of 0.63 mm ACC), was applied to the discs, and the tubes were sealed with serum stoppers and incubated at 25°C. CO2 was absorbed in each tube with 100 μL of 0.5 m KOH in a center well attached to the serum stopper. After 8 h, ethylene concentration in the tubes was measured by GC with an alumina column and flame-ionization detector. This experiment was conducted twice.
RESULTS
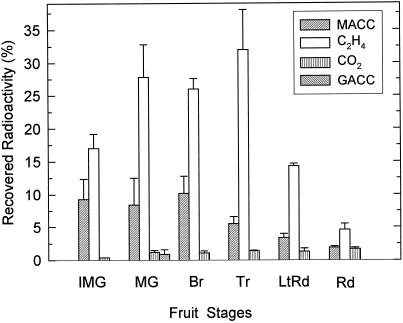
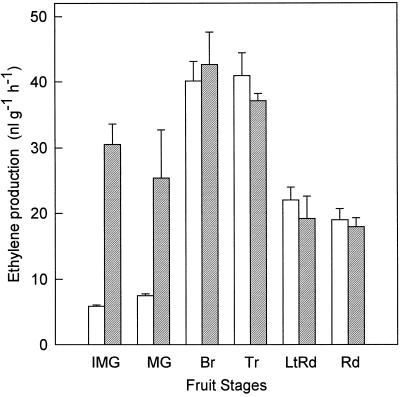
MACC was the sole or main conjugate formed when discs prepared from tomato fruit from immature-green through the red developmental stages were fed with [2,3-14C]ACC (Fig. 1). MACC was formed in all of the developmental stages examined. Of the radioactivity recovered from the discs, including unreacted [14C]ACC, [14C]ethylene, and 14CO2, [14C]MACC made up about 10% in the immature-green, mature-green, and breaker stages, but was less in discs from the more ripe stages of fruit. In the red-stage fruit, only about 2% of the recovered radioactivity was in [14C]MACC. [14C]Ethylene was the main product of [2,3-14C]ACC in all fruit stages. Ethylene production was measured from discs of fruit of these same developmental stages without and with added ACC (equivalent to that added as [2, 3-14C]ACC) to determine the influence of the added ACC (Fig. 2). The added ACC did not affect ethylene production from the discs from breaker through the red-stage fruit, but it did increase the ethylene formation in the discs from the immature and mature-green fruit.
Figure 1.
Metabolites of [2,3-14C]ACC fed to tomato discs. Results are expressed as percentages of the total radioactivity recovered from the discs. Results are the averages ± sd of two to three fruits for each developmental stage, and two discs from each fruit were fed separately. The fruit stages are: IMG, immature green; MG, mature green; Br, breaker; Tr, turning; LtRd, light red; and Rd, red (an explanation of fruit stages is given in Methods).
Figure 2.
Ethylene production rate from discs of tomato fruit at different developmental stages. Discs were incubated without (open bars) or with (hatched bars) ACC (24 μL of 0.63 mm ACC) and after 8 h of ethylene accumulation in the reaction tubes was determined as described in Methods. Results are the averages ± sd for two discs from two fruit. Explanation of fruit-stage abbreviations are given in Figure 1.
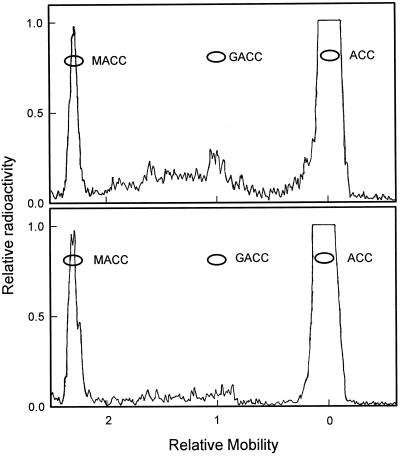
In discs from the mature-green fruit a small amount of another labeled product in addition to MACC was observed. It coelectrophoresed with authentic GACC (Fig. 3, top). This minor 14C-metabolite was not found in discs from any of the other fruit stages. In discs from the mature-green fruit, it made up about 1% of the recovered radioactivity (Fig. 1) and was about 10% of that of the MACC. The detectability limit for this minor 14C-metabolite was about 0.2% of the recovered radioactivity. Discs from four mature-green fruit were examined and had each formed some of this product. The radioelectrophoretogram of the products from the mature-green fruit that formed the greatest amount of this minor 14C-metabolite is shown in Figure 3 (top). The radioelectrophoretogram of the products from a breaker fruit is shown in Figure 3 (bottom). There was a slight amount of radioactivity in the area where GACC would move in this electrophoretogram, suggesting that a small amount of this minor 14C-metabolite was formed. However, when the area of the electrophoretogram where GACC would move was eluted and all of the sample developed on paper chromatography with the n-butanol:acetic acid:water (4:1:1, v/v) solvent system, no radioactivity was found at the RF where authentic GACC ran (data not shown). This indicates that the breaker fruit did not form this minor 14C-metabolite.
Figure 3.
Radioactivity scans of electrophoretograms run at pH 7.0 showing radiolabeled products from mature-green (top) and breaker (bottom) tomato fruit fed with [2,3-14C]ACC. Mobility is relative to N-2,4-dinitrophenyl-l-Ala that moves as an anion with a charge of −1 at this pH.
The minor 14C-metabolite was identified as GACC using paper electrophoresis and chromatography. Extracts of the discs from mature-green fruit were separated using electrophoresis at pH 7.0. Paper electrophoresis at pH 7.0 clearly resolves MACC, GACC, and ACC, since they have overall charges of −2, −1, and 0, respectively, at pH 7.0. The minor 14C-metabolite that coelectrophoresed with authentic GACC at pH 7.0 (Fig. 3, top) was eluted and separated again on paper electrophoresis at pH 11.0, as well as in two paper chromatography solvent systems (Table I). In each case this minor 14C-metabolite comigrated with authentic GACC. The major 14C-metabolite (Fig. 3) comigrated with authentic MACC in both paper chromatography and electrophoresis (Table I) and was therefore concluded to be MACC.
Table I.
Paper chromatographic and paper electrophoretic behavior of authentic ACC, GACC, and MACC, the minor radiolabeled ACC metabolite isolated from mature-green tomato fruit fed with [2,3-14C]ACC, and the major radiolabeled ACC metabolite isolated from all the fruit stages fed with [2,3-14C]ACC
| Compounds | RF in
PC
|
Relative Mobilities in PE
|
||
|---|---|---|---|---|
| BAW | BPW | pH 7.0 | pH 11.0 | |
| ACC | 0.40 | 0.40 | 0 | 1.90 |
| GACC | 0.24 | 0.24 | 1.04 | 2.12 |
| Minor 14C-metabolite | 0.24 | 0.24 | 1.04 | 2.11 |
| MACC | 0.67 | 0.35 | 2.28 | 2.81 |
| Major 14C-metabolite | 0.66 | 0.35 | 2.28 | 2.80 |
Mobilities for paper electrophoresis (PE) at pH 7.0 and 11.0 are relative to N-2,4-dinitrophenyl-l-Ala. The solvent systems used for paper chromatography (PC) were n-butanol:acetic acid:water (4:1:1, v/v; BAW) and butanol:pyridine:water (1:1:1, v/v; BPW).
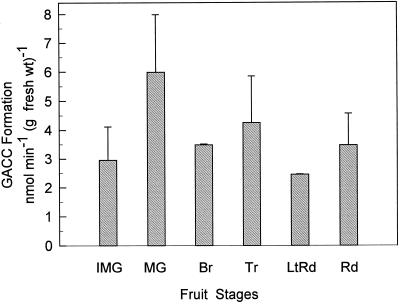
In contrast to the results for the in vivo [2,3-14C]ACC feeding, in which [14C]GACC was formed to only a small extent in discs from mature-green fruit but not from fruits at other stages, it was formed in vitro in extracts from fruit at all developmental stages (Fig. 4). The extracts examined for in vitro GACC formation (Fig. 4) were from the same fruit as those used for the in vivo feeding (Fig. 1). Our results for the in vitro formation of GACC (Fig. 4) are similar to those of Martin et al. (1995). They found the GACC-formation activity to be 3 to 4 nmol min−1 g−1 fresh weight for extracts from pericarp tissue for mature-green through ripe fruit.
Figure 4.
Formation of GACC in vitro using a homogenate of tomato pericarp tissue and [2,314C]ACC as the substrate. The homogenate was from the same fruit that discs were taken from for the in vivo feeding in Figure 1. Results are the averages from two to three fruits, and assays were performed in duplicate. Vertical bars indicate the sd. Assay conditions are given in Methods. Explanation of fruit-stage abbreviations are given in Figure 1. fresh wt, Fresh weight.
The assay for in vitro GACC formation was conducted at pH 8.0, since this was the pH used by Martin et al. (1995). Using an extract from a red tomato fruit we also conducted the assay at pH 7.5 and 7.15. The GACC-formation activities at pH 7.5 and 7.15 were 47 and 3%, respectively, of the activity at pH 8.0, which was 2.2 nmol min−1 g−1 fresh weight. Using GGT purified from tomato fruit, Martin and Slovin (1997) report that the pH optimum for GACC formation was between 8.0 and 9.5, and our results are consistent with this.
There are no other reports of in vivo GACC formation from plants fed with [14C]ACC. In previous work from our laboratory using mung bean and common vetch (Peiser et al., 1984), although we did not examine for GACC, we did not observe any major radioactive product that had chromatographic mobility properties different from that of MACC. We reexamined this by feeding [2,3-14C]ACC to mung bean etiolated hypocotyls and vetch etiolated epicotyls. Again, we were unable to detect any nonvolatile radioactive product other than MACC (data not shown). In common vetch β-cyanoalanine is radiolabeled using [1-14C]ACC, not [2,3-14C]ACC, as the substrate (Peiser et al., 1984). We also fed [2,3-14C]ACC to pea root tips and etiolated epicotyl and green stem tissue and found no [14C]GACC in any of these tissues, although [14C]MACC was formed in each tissue (data not shown).
DISCUSSION
Our results indicate that the major conjugate formed in vivo when tomato fruit discs were fed with [2,3-14C]ACC was [14C]MACC. It was formed in all of the developmental stages from immature-green through the red stage (Fig. 1). Only in the mature-green-stage fruit was a small amount of [14C]GACC formed, only one-tenth that of the [14C]MACC formed.
Although we measured a small amount of GACC in the discs of mature-green fruit fed ACC, it is not known whether GACC is formed in fruit not fed with ACC. Concentrations of ACC and activity of ACC synthase are very low in fruit at this mature-green stage (Su et al., 1984). ACC synthase and ACC concentrations begin a sharp increase at the breaker stage and continue to increase through the turning stage (Su et al., 1984). ACC-conjugate formation, presumed to be MACC although not characterized as such, follows a pattern similar to that of ACC in these stages. MACC formation has been considered a means of regulating ethylene production in addition to regulation by changes in ACC synthase activity (Yang, 1987). If GACC was also formed and was important for regulation of ethylene production, we would anticipate that it would be formed during this climacteric stage of ripening. However, MACC was the only conjugate formed in the climacteric fruit stages. Thus, the physiological significance of the minute formation of GACC following the exogenous application of ACC cannot be assigned.
When comparing results from Figure 1 for ethylene and MACC formation with that of intact fruit, it is important to note that these results (Fig. 1) do not take into account pool sizes of the metabolites (specific radioactivity of the products was not determined) or the effect of the applied ACC. Although the amount of [14C]MACC in discs from immature-green-, mature-green-, and breaker-stage fruit was higher than that from the latter-stage fruit, it is known that the endogenous level of ACC and ACC conjugate, presumed to be MACC, are very low in the immature-green and mature-green fruit (Su et al., 1984). Thus, the specific radioactivities of ACC in discs from ripe fruits are expected to be lower than that in discs from immature-green and mature-green fruits. Hence, the low amount of [14C]MACC in discs from the red fruits (Fig. 1) could be a reflection of the low specific radioactivity of [14C]ACC in these discs from which [14C]MACC was formed.
Likewise, the pattern of [14C]ethylene production could be different than ethylene formation in intact fruit. In intact immature and mature-green tomato fruit the ethylene production rate is very low, between 0.03 and 0.3 μL g−1 h−1, compared with breaker and turning fruit, 2 to 8 μL g−1 h−1 (Su et al., 1984). When ethylene formation from discs with and without added ACC was measured (Fig. 2), the added ACC had little effect on the ethylene formation in discs from breaker- through the red-stage fruit, indicating that ACC was not the limiting factor for ethylene production. However, in the immature and mature-green fruit, the added ACC did increase ethylene formation, indicating that in these discs ACC was limiting. Therefore, the applied ACC led to higher [14C]ethylene production in discs from the immature and mature-green fruit. Also, the wounding caused by cutting the discs would induce ACC synthase and ACC oxidase activities, resulting in more ACC and ethylene formation (Yang and Hoffman, 1984). This would account for the higher ethylene production in the discs of immature- and mature-green fruit without added ACC, 5 to 8 μL g−1 h−1 (Fig. 2), compared with intact fruit, 0.03 to 0.3 μL g−1 h−1 (Su et al., 1984).
Many plants, especially the Leguminosae (Southon, 1994), contain various γ-glutamyl peptides and much work has been conducted to elucidate their synthesis. The enzyme suspected to be responsible for their synthesis is GGT, which is also the enzyme that Martin et al. (1995) concluded was responsible for GACC formation in their studies. Martin and Slovin (1997) have since purified GGT from tomato fruit and observed that hydrolase and transpeptidase activities copurified and had pH optima between 8.0 and 9.5. Goore and Thompson (1967) purified this enzyme from kidney bean fruit and found that transferase activity occurred above pH 7.5, with a pH optimum of about 9.5. Hydolase activity occurred over the range from pH 6.0 to 11.0, with an optimum at 6.5 and 9.5. The γ-glutamyl conjugate of 2-amino-3-(methylenecyclopropyl)propanoic acid (hypoglycin A) is found in the ackee plant (Blighia sapida), and Kean and Hare (1980) purified GGT from its seed. Using the purified enzyme they observed hydrolase activity at pH 6.5 and transferase activity at pH 9.5. γ-Glutamyl conjugates are formed in onion (Allium cepa) and other Allium species. Examining GGT activity in onion, Lancaster and Shaw (1994) concluded that the physiological function of GGT was as a hydrolase and not as a transferase. Kasai et al. (1982) examined the activity and specificity of GGT, as well as the appearance and loss of γ-glutamyl peptides in soybean, broad bean, mung bean, adzuki bean, pea, and asparagus. They were unable to positively correlate activity and/or specificity of GGT with the increase in γ-glutamyl peptides in these plants and concluded that GGT functions in vivo as a hydrolase and not as a transferase. All of this information indicates that the physiological function of GGT is primarily or only as a hydrolase and not as a transferase. Thus, the in vivo formation of GACC via GGT is not established.
Aside from tomato fruit, the other tissues we examined were etiolated mung bean, etiolated vetch, pea root, and etiolated pea epicotyls and green stem tissues. None of these tissues formed [14C]GACC when fed with [2,3-14C]ACC. In these tissues [14C]MACC was the sole nonvolatile product identified. It is noteworthy that we found no [14C]GACC in the mung bean tissue, in which 11 different γ-glutamyl peptides have been found in the seed (Otoul et al., 1975; Kasai et al., 1986). However, to verify that mung bean tissue does not form GACC, a more appropriate tissue to examine would be the developing seed or fruit tissue, since presumably this is where these γ-glutamyl peptides are synthesized. Considering the mobility of GACC in paper chromatography with the solvent system n-butanol:acetic acid:water (4:1:1, v/v) (Table I), where GACC clearly separates from ACC and MACC, we can also conclude that GACC was not present in wheat leaves (Hoffman et al., 1982), peanut seeds (Hoffman et al., 1983), or cotton plants (Morris and Larcombe, 1995) that were fed with [14C]ACC. In each of these reports this solvent system was used, and it is clear from the data that [14C]MACC was the major product and there was little or no radioactivity near the RF that GACC would run. In conclusion, the present in vivo study supports the previous conclusion that MACC is the major, if not the sole, conjugate of ACC in plant tissues, and does not support the proposal of Martin et al. (1995) that GACC formation could be more important than MACC formation based on in vitro studies.
ACKNOWLEDGMENTS
We greatly appreciate the gifts of synthetic GACC from Dr. S.T. Chen (Institute of Biological Chemistry, Academia Sinica, Taipei, Taiwan) and Drs. T. Ogawa and Shigeki Sano (Tokushima University Medical School, Tokushima, Japan).
Abbreviations:
- GACC
1-(γ-glutamyl)-ACC
- GGT
γ-glutamyl transpeptidase
- MACC
1-(malonyl)-ACC
Footnotes
This work was supported by the National Science Foundation (grant no. MCB-9303801) and the Republic of China National Research Council (grant no. NSC 85–2321-B-01).
LITERATURE CITED
- Adams DO, Yang SF. Ethylene biosynthesis: identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci USA. 1979;76:170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrhein N, Breuing F, Eberle J, Skorupka H, Tophof S. The metabolism of 1-aminocyclopropane-1-carboxylic acid. In: Wareing PF, editor. Plant Growth Substances 1982. London: Academic Press; 1982. pp. 249–258. [Google Scholar]
- Amrhein N, Schneebeck D, Skoripka H, Tophof S, Stockigt J. Identification of a major metabolite of the ethylene precursor 1-aminocyclopropane-1-carboxylic acid in higher plants. Naturwissenschaften. 1981;68:619–620. [Google Scholar]
- Goore MY, Thompson JF. γ-Glutamyl transpeptidase from kidney bean fruit. I. Purification and mechanism of action. Biochim Biophys Acta. 1967;132:15–26. doi: 10.1016/0005-2744(67)90187-8. [DOI] [PubMed] [Google Scholar]
- Hoffman NE, Fu J, Yang SF. Identification and metabolism of 1-(malonylamino)cyclopropane-1-carboxylic acid in germinating peanut seeds. Plant Physiol. 1983;71:197–199. doi: 10.1104/pp.71.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman NE, Yang SF, McKeon T. Identification of 1-(malonylamino)cyclopropane-1-carboxylic acid as a major conjugate of 1-aminocyclopropane-1-carboxylic acid, an ethylene precursor in higher plants. Biochem Biophys Res Commun. 1982;104:765–770. doi: 10.1016/0006-291x(82)90703-3. [DOI] [PubMed] [Google Scholar]
- Kader AA, Morris LL (1976) Correlating subjective and objective measurements of maturation and ripeness of tomatoes. Proceedings of the Second Tomato Quality Workshop, University of Californis, Davis, p 57–62
- Kasai T, Ohmiya A, Sakamura S. γ-Glutamyltranspeptidases in the metabolism of γ-glutamyl peptides in plants. Phytochemistry. 1982;21:1233–1239. [Google Scholar]
- Kasai T, Shiroshita Y, Sakamura S. γ-Glutamyl peptides of Vigna radiata seeds. Phytochemistry. 1986;25:679–682. [Google Scholar]
- Kean EA, Hare ER. γ-Glutamyl transpeptidase of the ackee plant. Phytochemistry. 1980;19:199–203. [Google Scholar]
- Lancaster JE, Shaw ML. Characterization of purified γ-glutamyl transpeptidase in onions: evidence for in vivo role as a peptidase. Phytochemistry. 1994;36:1351–1358. [Google Scholar]
- Liu Y, Su L-Y, Yang SF. Ethylene promotes the capability to malonylate 1-aminocyclopropane-1-carboxylic acid and d-amino acids in preclimacteric tomato fruits. Plant Physiol. 1985;77:891–895. doi: 10.1104/pp.77.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MN, Cohen JD, Saftner RA. A new 1-aminocyclopropane-1-carboxylic acid-conjugating activity in tomato fruit. Plant Physiol. 1995;109:917–926. doi: 10.1104/pp.109.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MN, Saftner RA. Purification and characterization of 1-aminocyclopropane-1-carboxylic acid N-malonyltransferase from tomato fruit. Plant Physiol. 1995;108:1241–1249. doi: 10.1104/pp.108.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MN, Slovin JP. Purification and characterization of a gamma-glutamyl hydrolase/transpeptidase from tomato fruit (abstract no. 807) Plant Physiol. 1997;114:S-166. [Google Scholar]
- Morris DA, Larcombe NJ. Phloem transport and conjugation of foliar-applied 1-aminocyclopropane-1-carboxylic acid in cotton (Gossypium hirsutum L.) J Plant Physiol. 1995;146:429–436. [Google Scholar]
- Otoul E, Marechal R, Dardenne G, Desmedt F. Des dipeptides soufres differencient nettement Vigna radiata de Vigna mungo. Phytochemistry. 1975;14:173–179. [Google Scholar]
- Peiser GD, Wang T-T, Hoffman NE, Yang SF, Hung-Wen L, Walsh CT. Formation of cyanide from carbon 1 of 1-aminocyclopropane-1-carboxylic acid during its conversion to ethylene. Proc Natl Acad Sci USA. 1984;81:3059–3063. doi: 10.1073/pnas.81.10.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southon IW, compiler (1994) Phytochemical Dictionary of the Leguminosae. Chapman & Hall, London
- Su L-Y, McKeon T, Grierson D, Cantwell M, Yang SF. Development of 1-aminocyclopropane-1-carboxylic acid synthase and polygalacturonase activities during the maturation and ripening of tomato fruit. Hortscience. 1984;19:576–578. [Google Scholar]
- Yang SF (1987) The biosynthesis and metabolism of 1-(malonylamino)cyclopropane-1-carboxylic acid in relation to ethylene production. In K Schreiber, HR Schütte, G Sembdner, eds, Conjugated Plant Hormones: Structure, Metabolism, and Function. VEB Deutscher Verlag der Wissenschaaften, Berlin, pp 92–101
- Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol. 1984;35:155–189. [Google Scholar]