Abstract
BACKGROUND:
Noninvasive diagnosis of pleural tuberculosis (TB) remains a challenge due to the paucibacillary nature of the disease. As Mycobacterium tuberculosis (MTB)-specific T cells are recruited into pleural space in TB effusion; their indirect detection may provide useful clinical information.
OBJECTIVES:
Evaluation of pleural fluid interferon (INF)-γ levels vs Quantiferon–TB Gold In tube assay (QFT- IT) in blood and its adapted variants, using pleural fluid or isolated pleural fluid cells in the diagnosis of pleural TB.
METHODS:
Thirty-eight patients with pleural effusion of unknown etiology presented at Assiut University Hospital, Egypt, were recruited. Blood and pleural fluid were collected at presentation for INF-γ assays. Ex vivo pleural fluid INF-γ levels, QFT-IT in blood and its adapted variants were compared with final diagnosis as confirmed by other tools including blind and/or thoracoscopic pleural biopsy.
RESULTS:
The final clinical diagnosis was TB in 20 (53%), malignancy in 10 (26%), and effusion due to other causes in eight patients (21%). Ex vivo pleural fluid INF-γ levels accurately identified TB in all patients and were superior to the QFT-IT assays using blood or pleural fluid (70 and 78% sensitivity, with 60 and 83% specificity, respectively). QFT-IT assay applied to isolated pleural fluid cells had 100% sensitivity and 72% specificity. The optimal cut-off obtained with ROC analysis was 0.73 for TB Gold assay in blood assay, 0.82 IU/ml for the cultured pleural fluid assay, and 0.94 for isolated pleural cells assay.
CONCLUSION:
The ex vivo pleural fluid INF-γ level is an accurate marker for the diagnosis of pleural TB. QFT- IT assay in peripheral blood or its adapted versions of the assay using pleural fluid and/or washed pleural fluid cells had no diagnostic advantage over pleural fluid INF-γ in the diagnosis of pleural TB.
Keywords: Diagnosis, interferon-γ, interferon-γ release assays, quantiferon–TB gold in tube assay, tuberculous effusion
Tuberculosis (TB) is the most important single infectious cause of death worldwide; WHO 2006[1] reported approximately 2 million deaths annually. Rapid diagnosis and treatment for active TB patients is crucial for the effective and efficient control of TB in developing countries not only for patients, but also for TB control in the community.[2]
The diagnosis of pleural TB is hampered by its paucibacillary nature. Invasive interventions such as closed pleural biopsy or thoracoscopy are often needed to confirm the diagnosis.[3] While repeating invasive diagnostic procedures may yield positive results, such an approach places patients at increased risk of complications and also increases costs.[4]
Different biological markers have been proposed to facilitate the diagnosis of pleural TB, including increased pleural fluid concentrations of adenosine deaminase (ADA),[5] interferon (INF)- γ,[6,7] interleukin (IL)-12p40, IL-18, immunosuppressive acidic protein (IAP),[8] and soluble IL-2 receptors (sIL-2Rs).[9] These biological markers are useful for the diagnosis of tuberculous pleuritis.[4] However, those methods are not widely practiced in routine clinical laboratories, and which of these markers is most useful for the diagnosis of tuberculous pleuritis has not been determined.[10]
Effector cells specific to Mycobacterium Tuberculosis (MTB) produce INF-γ in response to TB-specific antigens, and this can be measured by whole-blood INF-γ enzyme-linked immunosorbent assay (ELISA) or enzyme-linked immunospot assay (ELISPOT),[11–13] and are called INF-γ release assays (IGRA).
Blood-based INF-g assays are fast, highly specific, and sensitive for MTB infection with no interobserver variation and unaffected by BCG vaccination status.[14,15] These assays are based on in vitro culture of either whole-blood or isolated peripheral blood mononuclear cells (PBMCs) in the presence of MTB-specific antigens;[3] a commercial whole-blood INF-γ ELISA assay (QuantiFERON-TB Gold [QFT-G] and the QuantiFERON-TB Gold In-Tube assay (QFT-IT) measures the level of INF-γ in the supernatant of the stimulated whole blood by MTB-specific antigens.[16] ,[17]
A reliable surrogate pleural fluid markers permitting rapid and accurate diagnosis of tuberculous pleuritis is greatly needed.[18] Hence, the aim of this study was to estimate the usefulness of different INF-γ-based assays as diagnostic markers in patients with pleural TB in comparison with baseline ex vivo pleural fluid INF-γ levels to help deciding accurate diagnostic and therapeutic information.
Methods
Study design
Cross-sectional.
Setting
This study was conducted in Assiut University Hospital (AUH), Upper Egypt.
Participants and samples
Purposive sampling technique based on high clinical probability of TB effusion was used. Thirty-eight inpatients who presented with clinically suspected TB pleural effusions were included in this study. The studied patients included 26 men and 12 women, with a mean age of 48 years.
The clinical workup included history, physical examination, radiological examination, sputum microbiological examination, blood examination, pleural fluid analysis, and blind pleural biopsy using Cope's needle. Thoracoscopic pleural biopsy using Storz semi rigid thoracoscope was reserved for cases with non-conclusive blind pleural biopsy results. The study was approved by the Ethical Committee of Assiut Faculty of Medicine and informed consents were obtained from all patients or their next of keen.
Standard laboratory procedures
Pleural fluid was examined for pH, lactate dehydrogenase (LDH), total protein and albumin concentration, glucose, Gram and Ziehl-Neelsen stain, as well as bacterial and TB culture. The total and differential white cell count of pleural fluids was determined. Needle and/or thoracoscopic biopsy of pleura was performed as appropriate.
Cytological analysis was done with cytospin preparations using hematoxylin-eosin or Papanicolaou stains.
Specimen collection and processing
At least 40 ml of pleural fluid was collected from each patient in a syringe during thoracentesis, which was performed with oral informed consent.
A portion of the sample was submitted for acid-fast staining, bacteriologic examination, cytology examination, and measurement of protein, albumin, LDH, and glucose.
INF-γ assays
Pleural fluid was centrifuged and the supernatant collected for determination of baseline (ex vivo) pleural fluid INF-γ levels for all subjects using ELISA.
INF-g assays were performed using blood, pleural fluid, and isolated pleural fluid cells with the QTF Gold In-Tube assay kit (Cellestis, Ltd., Carnegie, Victoria, Australia), and the test results were evaluated according to the guidelines of CDC.[19]
The assays based on blood and pleural fluids were performed according to the manufacturer's instructions for whole blood as follow: during sample collection for diagnostic analysis, 1 ml of blood or pleural fluid was collected directly in the supplied tubes containing either the MTB-specific antigen cocktail [ESAT-6, CFP-10 and TB7.7 (peptide 4)] or the antigen-free control (nil) control, and cultured within 2 hours of collection for 20 to 22 hours at 37°C. Then, after overnight incubation, 200 μl of plasma was removed from each well and the concentration of INF-γ was determined using the assay kit according to the instructions of the manufacturer. The cutoff value for a positive response was 0.35 IU/ml of INF-γ.[14,20] The INF-γ concentration in the nil-control well was considered background and subtracted from the measurements made in the sample wells.[21]
Pleural fluid cells were isolated, washed, and cultured at a fixed concentration to correct for the influence of different lymphocyte numbers and for the effect of baseline INF-γ concentrations in the pleural fluid on INF-g levels after antigenic stimulation as described by Chegou et al., 2008.[3] Briefly, pleural fluid was centrifuged at 1 600 γ for 10 minutes and the supernatants harvested for ex vivo INF-g determination.
The pelleted cells were washed twice with phosphate-buffered saline and resuspended in RPMI-1640 medium supplemented with glutamine, 10% human AB serum, and penicillin/streptomycin (100 units penicillin/ml and 100 μg of streptomycin/ml) at a final concentration of 2 × 106 lymphocytes/ml with cells viability >95% assessed with phase contrast microscopy. One-milliliter aliquots of the washed and resuspended cells were cultured in 24-well tissue culture plates in analogy to the QTF Gold method.[22]
Statistical analysis
Differences in pleural fluid characteristics markers between tuberculous and non-tuberculous pleural effusions were examined. Statistical analysis including mean values and their standard deviations were calculated for each variable under study using the Statistical Package for Social Sciences for windows (SPSS) version 11.0. Statistical comparison of demographic data and pleural fluid characteristics and markers in tuberculous and non-tuberculous pleural effusions was performed through t-test or Mann-Whitney U-test as appropriate. P values of <0.05 were considered significant, and highly significant at P <0.01. Receiver operating characteristic (ROC) curves were constructed to compare performances of different assays used in the study.[23]
Results
Demographic data and clinical diagnosis
Demographic data of the 38 studied patients are shown in Table 1. Twenty patients (53%) had tuberculous pleuritis and 18 patients (47%) had an effusion due to a non-tuberculous etiology; ten of them (56%) had malignant effusions, the other eight effusions were diagnosed as empyema (n = 3), or as due to congestive heart failure (n = 2), liver cirrhosis (n = 2), and connective tissue disease (n = 1), pleural fluid characteristics were shown in Table 2.
Table 1.
Demographic and clinical data of the studied 38 patients with pleural effusion
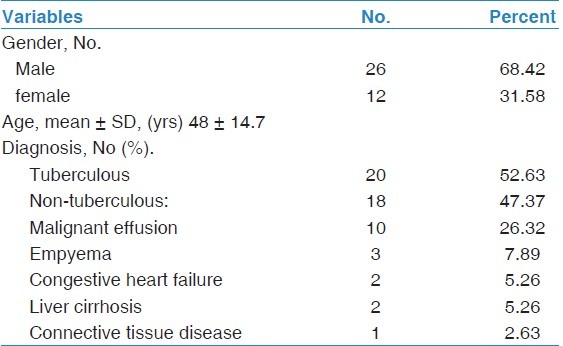
Table 2.
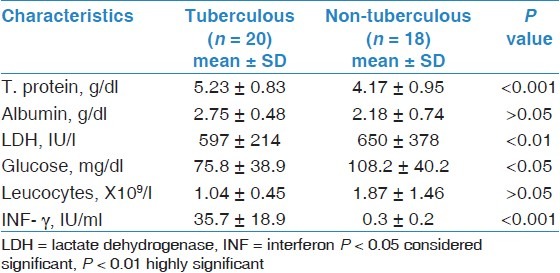
Pleural fluid characteristics in the studied 38 patients with tuberculous and non-tuberculous pleural effusions
The diagnosis of pleural TB was made on the basis of pleural effusion on chest radiograph and TB isolated from pleural fluid or tissue showing staining for acid-fast bacilli; or a sputum culture finding positive for TB in the presence of a pleural effusion, blind and/or thoracoscopic pleural biopsy results showing the presence of pathological changes consistent with pleural TB. Malignant pleural effusions were diagnosed either by pleural fluid cytology or biopsy specimen. Miscellaneous pleural effusions were those not linked to TB or malignant disease as defined above.
Blood and pleural fluid interferon-g
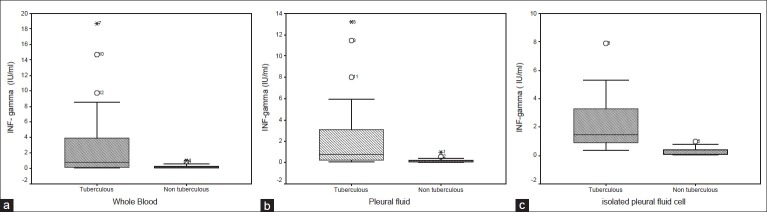
As shown in Table 2 and Figure 1A–C, ex vivo quantitative measurements of pleural fluid INF-γ levels and INF-γ production using the QTF TB Gold In-Tube assays in whole blood [Figure 1a], pleural fluid [Figure 1b] or pleural fluid cells [Figure 1c] accurately differentiated TB from non-TB effusions. TB patients exhibited statistically significant higher pleural fluid concentrations of these markers. The mean ± SD of ex vivo pleural fluid INF-γ level from patients with TB effusion was 35.7 ± 18.9 IU/ml and 0.3 ± 0.2 for non-tuberculous effusions (P = <0.001).
Figure 1.
INF-γproduction using the QTF TB Gold In-Tube assays in the 38 studied patients with tuberculous and non-tuberculous pleural effusion (a) INF-γproduction using the QTF TB Gold In-Tube assay in whole blood (b) INF-γproduction using the QTF TB Gold In-Tube assay in pleural fluid, (c) INF-ãproduction using the QTF TB Gold In-Tube assay in isolated pleural fluid cell
To determine the most sensitive and specific indicator for diagnosis of tuberculous pleuritis using TB gold and adapted variants of the assay, we performed receiver operating curve (ROC) analysis [Figure 2] that clearly demonstrated that pleural fluid INF-γ was the most useful diagnostic indicator for tuberculous pleurisy. The Quantiferon TB Gold assay performed with blood or pleural fluid or pleural fluid cells does not add diagnostic value. The optimal cut-off obtained with ROC analysis was 0.73 for peripheral blood TB Gold assay, 0.82 IU/ml for the cultured pleural fluid assay, and 0.94 for isolated pleural cells assay [Figure 2].
Figure 2.
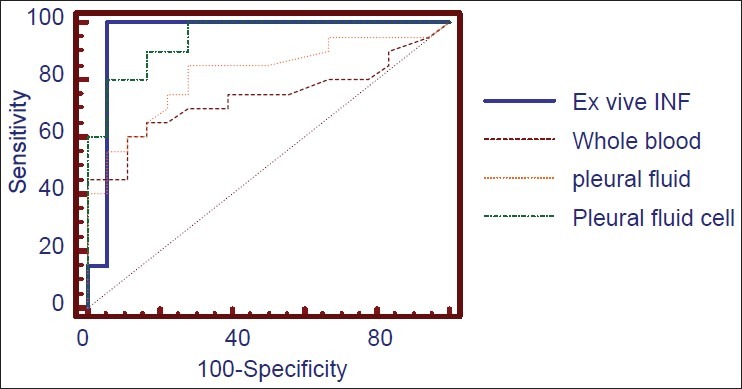
ROC analysis for the studied markers of tuberculous pleural effusion; ex vivo pleural fluid INF-g concentration, adapted QTF TB Gold In-Tube assays in whole blood, pleural fluid, and pleural fluid cells
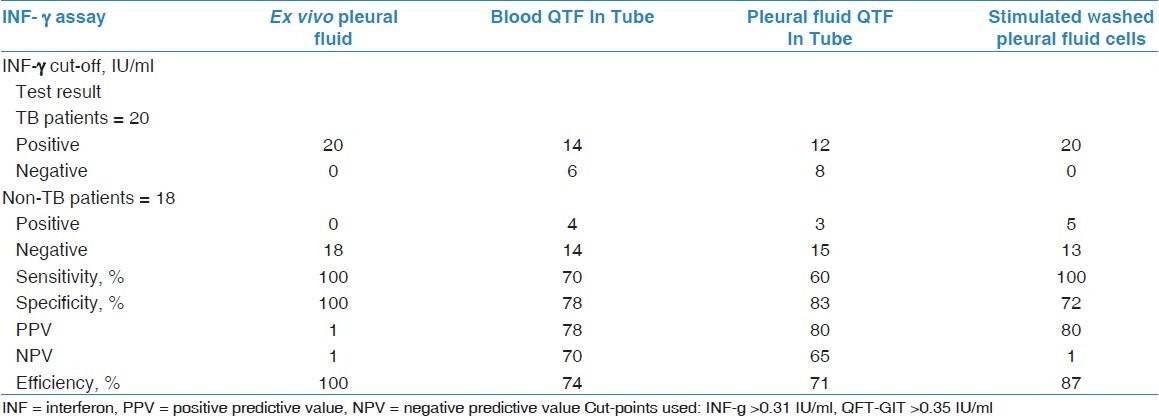
The isolated pleural cells from patients with TB effusions produced significantly more INF-γ. Culture of washed pleural fluid cells stimulated with MTB-specific antigens did not lead to increased INF-γ levels compared with non-stimulated washed pleural fluid cells. INF-γ produced by the non-stimulated isolated pleural fluid cells (cultured with the nil control) distinguished between TB and non-TB with the same sensitivity (100%) and specificity (72%) as the cells stimulated with the MTB-specific antigens [Table 3].
Table 3.
Results of the INF-γ assays among the 38 studied patients with pleural effusion
Discussion
Making a differential diagnosis between tuberculous and non-tuberculous pleural effusions represents a critical clinical problem. Conventional methods for diagnosis of pleural TB have proven to be inadequate.[24]
This study confirms that ex vivo pleural fluid INF-γ level is an accurate marker for the diagnosis of pleural TB. Also, it showed that QFT-IT assay in peripheral blood or its adapted versions using pleural fluid and/or washed pleural fluid cells had no diagnostic advantage over pleural fluid INF-γ in the diagnosis of pleural TB.
Several previous studies demonstrated that ADA, INF-γ, IL-12p40, IL-18, IAP, and sIL-2R levels were significantly higher in tuberculous than in non-tuberculous pleural effusions;[10] INF- γ is the most sensitive and specific indicator of tuberculous pleuritis among these six biological markers.[18] The high specificity of INF-γ is based on the activity of MTB-specific effector T cells at the inflammatory site; INF-γ is produced by T lymphocytes in response to stimulation by specific antigens or nonspecific antigens and is capable of modifying the response of other cells to the immune system.[25] INF-γ is known to activate macrophages so that they increase their bactericidal capacity against MTB. Therefore, INF-γ levels in pleural fluid may reflect the stimulation of T lymphocytes by tuberculous antigens.[26] Intriguingly, in TB patients, these T cells seem to keep on producing high levels of INF-γ in the absence of added TB antigen.[27] Presumably, this is due to a highly activated state that does not require ongoing antigenic stimulation. Alternatively, the accessory cells present in the pleural fluid may already be fully loaded with TB antigen.[28]
Previous studies of the value of INF-γ in diagnosing pleural TB revealed a mean sensitivity of 89% and a specificity of 97%.[29] Another study by Chegou et al., 2008[3] denoted that ex vivo INF-γ has 100% accuracy in diagnosing TB pleuritis. The same promising result was found in this study, where pleural fluid INF-γ levels accurately differentiated between all TB and non-TB effusions with 100% sensitivity and specificity.
The clinical value of all surrogate markers for active TB depends on the pre-test probability.[6] In high clinical probability settings, the use of ADA ≥ 50 U/l makes additional tests unnecessary in a high proportion of patients.[3] However, ADA as a single marker performed relatively poorly as mentioned in Chegou et al.'s[3] study, probably due to the high proportion of non-TB pleural infections, which are characterized by neutrophil-predominant effusions and are a well-known source of false-positive ADA results. In contrast, in the same study[3] and that of Jiang et al.'s,[29] a similar high diagnostic accuracy for ex vivo pleural fluid INF-γ levels in pleural TB was obtained. Also, Hiraki et al.[18] directly compared six markers including those of ADA, IL-12, p40, IL-18, IAP, sIL-2R, and INF-γ where INF-γ is the most sensitive and specific indicator of tuberculous pleuritis among these six biological markers.
Although determination of INF-γ at the onset of pleural effusion is informative for the diagnosis of tuberculous pleuritis, INF-γ levels have not been used as a routine diagnostic test in suspected TB pleuritis, which may be due to the fact that INF-γ ELISA tests are relatively expensive.[30]
In place of the TST test in vivo, the QFT-TB test in vitro, an IGRA using ESAT-6 and CFP-10 antigens for the detection of MTB infection was first used commercially for patients with TB infection in Japan in April, 2005.[31]
In particular, quantitative results can be obtained within 1 day of blood sampling, and the booster effect caused by sequential skin tests is not an issue, as the assay is performed in vitro. However, it also has certain limitations. As the assay is based on cellular immunity, its results might depend on the status of the latter. Earlier reports have confirmed low accuracy of the QFT-G in immune-compromised patients.[4] In addition, the amount of INF-γ produced in this assay was determined by multiple factors, including the number of PBMCs responsible for its production, the time interval between blood sampling and co-incubation with antigens, and the duration of incubation systemic immunosuppression or anergy could cause the inability of TB-specific antigens as well as mitogen to induce INF-γ release[32] and often leads to the occurrence of the so-called indeterminate results in the QTF Gold assay due to an inability of cells to mount an INF-γ response against the mitogen.[30] The identification of an appropriate cut-off concentration to fit the objectives is therefore essential.
The usefulness of standardized IGRA in diagnosing active TB disease, especially in high TB incidence areas, is not clear as they do not distinguish latent from active TB disease. The sensitivity of the test in previous studies varied from 55 to 88%, with a mean of 75%.[32] In this study, the sensitivity obtained with the In Tube version of the blood assay was within this range (70%).
Previous report suggested that the use of pleural effusion mononuclear cells may diagnose pleural TB with a high accuracy (95% sensitivity and 76% specificity). Jafari et al., 2006[33] used bronchoalveolar lavage-derived mononuclear cells in an INF-γ ELISPOT assay and diagnosed smear-negative pulmonary TB in 37 patients with 100% accuracy, and the technique was superior to the standard PBMC-based ELISPOT assay. Another study by Chegou et al.[3] reported a similar high diagnostic accuracy (100% sensitivity and 67% specificity) even without MTB-specific antigen stimulation. Our data suggest that pleural cell-based INF-γ assays give 100% sensitivity and 87% specificity which were superior to the measurement of the peripheral blood or pleural fluid, and inferior to the direct measurement of ex vivo pleural fluid INF-γ in pleural TB. Anatomical factors like the confined pleural space which allows the accumulation of cytokines in the fluid may explain why ex vivo pleural fluid INF-g performs better than antigen-stimulated cells, as well as an end-stage state of activation of immune cells present in the pleural space.
Beside ADA, other tools have been used to diagnose TB effusion in different studies including fluid lymphocyte/neutrophil ratio, histology and culture of pleural biopsy taken blindly or via thoracoscopy. In this study, we did not measure ADA and not all patients were subjected to pleural biopsy; however, in the study done by Diacon et al.,[34] they concluded that the combination of ADA with the differential pleural fluid WBC count increases specificity. Medical thoracoscopy had the highest diagnostic accuracy with 100% on histology and 76% positive cultures. Of note was the very good result of the combined approach with pleural fluid ADA and lymphocytes: neutrophils plus closed needle biopsy histology and culture, which came very close to thoracoscopy.
The 100% accuracy obtained in the present study is in keeping with other studies.[3,4,10] However, INF-γ levels have not gained widespread acceptance as a routine diagnostic test in suspected TB pleuritis, which may be due to the fact that INF-γ ELISA tests are relatively expensive and that there is a discrepancy between high prevalence of TB pleuritis and available healthcare resources. The cost is nearly tripled if QTF-IT-based tests are used; the most expensive test of them is QTF-IT in pleural fluid cells. However, based on the results of this study, we recommend use of INF-γ ELISA with ex vivo pleural fluid. It would be both sufficient and more cost effective than pleural fluid or peripheral blood QTF tests and can be performed with small numbers of samples or even single samples by using ELISA.
The strength of this study is the prospective comparison of INF-γ ELISA and three different QTF-based diagnostic tests all applied to the same subjects, who had clinically suspected TB pleural effusion. However, the weak points include the limited number of patients and lack of comparison of these tests with other biomarkers due to limited resources.
In conclusion, INF-γ ELISA with ex vivo pleural fluid would be both sufficient and more cost effective than pleural fluid or peripheral blood QTF-G In tube tests as the use of the QTF test based on either blood or pleural fluid offers no advantage over ex vivo pleural fluid INF-γ measurement in the diagnosis of pleural TB, which should be considered as the test of choice. We have adapted the commercial QTF test to a promising in vitro application using inflammatory cells from the site of disease instead of blood. Such stimulated IGRA using cells from the site of disease are therefore feasible and suggest that new applications should be investigated further in forms of TB that are difficult to diagnose, including smear and culture-negative pulmonary TB, peritonitis, and pericarditis, where aspirated cells could be cultured in the presence of mycobacterial antigens.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Geneva, Switzerland: World Health Organization; 2006. WHO report 2006: Global tuberculosis control, surveillance, planning, financing. [Google Scholar]
- 2.Kang YA, Lee HW, Hwang SS, Um SW, Han SK, Shim YS, et al. Usefulness of whole-blood interferon-g assay and interferon- g enzyme-linked immunospot assay in the diagnosis of active pulmonary tuberculosis. Chest. 2007;132:959–65. doi: 10.1378/chest.06-2805. [DOI] [PubMed] [Google Scholar]
- 3.Chegou NN, Walzl G, Bolliger CT, Diacon AH, van den Heuvel MM. Evaluation of adapted whole-blood interferon- -g release assays for the diagnosis of pleural tuberculosis. Respiration. 2008;76:131–8. doi: 10.1159/000128575. [DOI] [PubMed] [Google Scholar]
- 4.Aoe K, Hiraki A, Murakami T, Eda R, Maeda T, Sugi K, et al. Diagnostic significance of interferon-g in tuberculous pleural effusions. Chest. 2003;123:740–4. doi: 10.1378/chest.123.3.740. [DOI] [PubMed] [Google Scholar]
- 5.Tahhan M, Ugurman F, Gozu A, Akkalyoncu B, Samurkasoglu B. Tumour necrosis factor- alpha in comparison to adenosine deaminase in tuberculous pleuritis. Respiration. 2003;70:270–4. doi: 10.1159/000072008. [DOI] [PubMed] [Google Scholar]
- 6.Villegas MV, Labrada LA, Saravia NG. Evaluation of polymerase chain reaction, adenosine deaminase, and interferon-in pleural fluid for the differential diagnosis of pleural tuberculosis. Chest. 2000;118:1355–64. doi: 10.1378/chest.118.5.1355. [DOI] [PubMed] [Google Scholar]
- 7.Chen YM, Yang WK, Whang-Peng J, Tsai CM, Perng RP. An analysis of cytokine status in the srum and effusions of patients with tuberculous and lung cancer. Lung Cancer. 2001;31:25–30. doi: 10.1016/s0169-5002(00)00165-3. [DOI] [PubMed] [Google Scholar]
- 8.Tamura S, Nishigaki T, Moriwaki Y, Fujioka H, Nakano T, Fujii J, et al. Tumor markers in pleural effusion diagnosis. Cancer. 1988;61:298–302. doi: 10.1002/1097-0142(19880115)61:2<298::aid-cncr2820610219>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.Porcel JM, Gázquez I, Vives M, Pérez B, Rubio M, Rivas MC. Diagnosis of tuberculous pleuritis by the measurement of soluble interleukin 2 receptor in pleural fluid. Int J Tuberc Lung Dis. 2000;4:975–9. [PubMed] [Google Scholar]
- 10.Hiraki A, Aoe K, Matsuo K, Murakami K, Murakami T, Onoda T, et al. Simultaneous measurement of T-helper 1 cytokines in tuberculous pleural effusion. Int J Tuberc Lung Dis. 2003;7:1172–7. [PubMed] [Google Scholar]
- 11.Pai M, Riley LW, Colford JM., Jr Interferon gamma assays in the immunodiagnosis of tuberculosis: A systematic review. Lancet Infect Dis. 2004;4:761–76. doi: 10.1016/S1473-3099(04)01206-X. [DOI] [PubMed] [Google Scholar]
- 12.Lee JY, Choi HJ, Park IN, Hong SB, Oh YM, Lim CM, et al. Comparison of two commercial interferon-gamma assays for diagnosing Mycobacterium tuberculosis infection. Eur Respir J. 2006;28:24–30. doi: 10.1183/09031936.06.00016906. [DOI] [PubMed] [Google Scholar]
- 13.Pai M, Kalantri S, Dheda K. New tools and emerging technologies for the diagnosis of tuberculosis: Part I. Latent tuberculosis. Expert Rev Mol Diagn. 2006;6:413–22. doi: 10.1586/14737159.6.3.413. [DOI] [PubMed] [Google Scholar]
- 14.Mori T, Sakatani M, Yamagishi F, Takashima T, Kawabe Y, Nagao K, et al. Specific detection of tuberculosis infection: An interferon-gamma-based assay using new antigens. Am J Respir Crit Care Med. 2004;170:59–64. doi: 10.1164/rccm.200402-179OC. [DOI] [PubMed] [Google Scholar]
- 15.Kobashi Y, Mouri K, Obase Y, Fukuda M, Miyashita N, Oka M. Clinical evaluation of QuantiFERON TB-2G test for immunocompromised patients. Eur Respir J. 2007;30:945–50. doi: 10.1183/09031936.00040007. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura T, Hasegawa N, Mori M, Takebayashi T, Harada N, Higuchi K, et al. Accuracy of an interferon-g release assay to detect active pulmonary and extra-pulmonary tuberculosis. Int J Tuberc Lung Dis. 2008;12:269–74. [PubMed] [Google Scholar]
- 17.Losi M, Bocchino M, Matarese A, Bellofiore B, Roversi P, Rumpianesi F, et al. Role of the quantiferon-TB test in ruling out pleural tuberculosis: A multi-centre study. Int J Immunopathol Pharmacol. 2011;24:159–65. doi: 10.1177/039463201102400118. [DOI] [PubMed] [Google Scholar]
- 18.Hiraki A, Aoe K, Eda R, Maeda T, Murakami T, Sugi K, et al. Comparison of six biological markers for the diagnosis of tuberculous pleuritis. Chest. 2004;24:987–9. doi: 10.1378/chest.125.3.987. [DOI] [PubMed] [Google Scholar]
- 19.Mazurek GH, Jereb J, Lobue P, Iademarco MF, Metchock B, Vernon A. Division of tuberculosis elimination, national center for HIV, STD, and TB prevention, centers for disease control and prevention (CDC). Guidelines for using the QuantiFERON-TB gold test for detecting mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54:49–55. Erratum in: MMWR Morb Mortal Wkly Rep 2005;54:1288. [PubMed] [Google Scholar]
- 20.Brock I, Weldingh K, Lillebaek T, Follmann F, Andersen Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am J Respir Crit Care Med. 2004;170:65–9. doi: 10.1164/rccm.200402-232OC. [DOI] [PubMed] [Google Scholar]
- 21.Harada N, Higuchi K, Sekiya Y, Rothel J, Kitoh T, Mori T. Basic characteristics of a novel diagnostic method (Quantiferon TB-2G) for latent tuberculosis infection with the use of mycobacterium tuberculosis-specific antigens, ESAT-6 and CFP-10. Kekkaku. 2004;79:725–35. [PubMed] [Google Scholar]
- 22.Todd B. The quantiferon TB gold test. A new blood assay offers a promising alternative in tuberculosis testing. Am J Nurs. 2006;106:33–7. doi: 10.1097/00000446-200606000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8:283–98. doi: 10.1016/s0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Pachon E, Soler MJ, Padilla-Navas I, Romero V, Shum C. C-reactive protein in lymphocytic pleural effusions: A diagnostic aid in tuberculous pleuritis. Respiration. 2005;72:486–9. doi: 10.1159/000087672. [DOI] [PubMed] [Google Scholar]
- 25.Pai M, Dheda K, Cunningham J, Scano F, O’Brien R. T-cell assays for the diagnosis of latent tuberculosis infection: Moving the research agenda forward. Lancet Infect Dis. 2007;7:428–38. doi: 10.1016/S1473-3099(07)70086-5. [DOI] [PubMed] [Google Scholar]
- 26.Ewer K, Deeks J, Alvarez L, Bryant G, Waller S, Andersen P, et al. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet. 2003;361:1168–73. doi: 10.1016/S0140-6736(03)12950-9. [DOI] [PubMed] [Google Scholar]
- 27.Lalvani A, Pathan AA, Durkan H, Wilkinson KA, Whelan A, Deeks JJ, et al. Enhanced contact tracing and spatial tracking of mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet. 2001;357:2017–21. doi: 10.1016/S0140-6736(00)05115-1. [DOI] [PubMed] [Google Scholar]
- 28.Ferrara G, Losi M, D’Amico R, Roversi P, Piro R, Meacci M, et al. Use in routine clinical practice of two commercial blood tests for diagnosis of infection with Mycobacterium tuberculosis: A prospective study. Lancet. 2006;367:1328–34. doi: 10.1016/S0140-6736(06)68579-6. [DOI] [PubMed] [Google Scholar]
- 29.Jiang J, Shi HZ, Liang QL, Qin SM, Qin XJ. Diagnostic value of interferon-gamma in tuberculous pleurisy: A metaanalysis. Chest. 2007;131:1133–41. doi: 10.1378/chest.06-2273. [DOI] [PubMed] [Google Scholar]
- 30.Ferrara G, Losi M, Meacci M, Meccugni B, Piro R, Roversi P, et al. Routine hospital use of a new commercial whole blood interferon-gamma assay for the diagnosis of tuberculosis infection. Am J Respir Crit Care Med. 2005;172:631–5. doi: 10.1164/rccm.200502-196OC. [DOI] [PubMed] [Google Scholar]
- 31.Piana F, Codecasa LR, Cavallerio P, Ferrarese M, Migliori GB, Barbarano L, et al. Use of a T-cell-based test for detection of tuberculosis infection among immunocompromised patients. Eur Respir J. 2006;28:31–4. doi: 10.1183/09031936.06.00110205. [DOI] [PubMed] [Google Scholar]
- 32.Pai M, Menzies D. Interferon-gamma release assays: what is their role in the diagnosis of active tuberculosis? Clin Infect Dis. 2007;44:74–7. doi: 10.1086/509927. [DOI] [PubMed] [Google Scholar]
- 33.Jafari C, Ernst M, Kalsdorf B, Greinert U, Diel R, Kirsten D, et al. Rapid diagnosis of smear-negative tuberculosis by bronchoalveolar lavage enzyme- linked immunospot. Am J Respir Crit Care Med. 2006;174:1048–54. doi: 10.1164/rccm.200604-465OC. [DOI] [PubMed] [Google Scholar]
- 34.Diacon AH, Van de Wal BW, Wyser C, Smedema JP, Bezuidenhout J, Bolliger CT, et al. Diagnostic tools in tuberculous pleurisy: A direct comparative study. Eur Respir J. 2003;22:589–91. doi: 10.1183/09031936.03.00017103a. [DOI] [PubMed] [Google Scholar]