Abstract
CONTEXT:
Asthma is a chronic airway disorder which is associated to the inflammatory cells. Inflammatory and immune cells generate more reactive oxygen species in patients suffering from asthma which leads to tissue injury.
AIMS:
To investigate the role of oxidant-antioxidant imbalance in disease progression of asthmatic patients.
SETTINGS AND DESIGN:
In this study, 130 asthmatic patients and 70 healthy controls were documented.
METHODS:
For this malondialdehyde level, total protein carbonyls, sulfhydryls, activity of superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), total blood glutathione, and total antioxidant capacity (FRAP) were measured.
STATISTICAL ANALYSIS USED:
Analysis of the data was done using unpaired student t test and one-way ANOVA analysis. P < 0.05 was considered significant.
RESULTS:
The present work showed that the systemic levels of MDA (4.19 ± 0.10 nmol/ml, P < 0.001) and protein carbonyls (1.13 ± 0.02 nmol/mg, P < 0.001) were found to be remarkably higher in asthmatic patients while protein sulfhydryls (0.55 ± 0.01 mmol/l, P < 0.05) decreased as compared to controls (2.84 ± 0.12 nmol/ml, 0.79 ± 0.02 nmol/mg and 0.60 ± 0.02 mmol/l, respectively). We also observed decrease in activities of SOD (2047 ± 50.34 U/g Hb, P < 0.05), catalase (4374 ± 67.98 U/g Hb, P < 0.01), and GPx (40.97 ± 1.05 U/g Hb, P < 0.01) in erythrocytes compared to control (2217 ± 60.11 U/g Hb, 4746 ± 89.94 U/g Hb, and 48.37 ± 2.47 U/g Hb, respectively). FRAP level (750.90 ± 21.22 μmol/l, P < 0.05) in plasma was decreased, whereas total blood glutathione increased (0.94 ± 0.02 mmol/l, P < 0.05) as seen in control (840.40 ± 28.39 μmol/l and 0.84 ± 0.04 mmol/l).
CONCLUSIONS:
This work supports and describes the hypothesis that an imbalance between oxidant-antioxidant is associated to the oxidative stress which plays a significant role in severity of the disease.
Keywords: Antioxidants, asthma, oxidants, oxidative stress, reactive oxygen species
Asthma is the most frequent chronic airway disease and is one of the major global health problems[1] which is characterized by chronic inflammation of the airways involving variable and recurrent airflow obstruction and increased airway responsiveness to a variety of stimuli.[2,3] Recent research suggests that airway inflammation is the most proximate cause of the recurrent episodes of airflow limitation in asthma and is associated with increased oxidative stress.[4] Thus, oxidative stress (oxidant-antioxidant imbalance) has been increasingly recognized as a major factor contributing to the chronic inflammation. When oxidative stress overwhelms antioxidant mechanisms, reactive oxygen species (ROS) interact with proteins, lipids, and DNA leading to pathological consequences.[5] Oxidative stress is thought to initiate airway inflammation; therefore, it is an important component of asthma pathogenesis.[6] Many evidences suggest that ROS produced both intracellularly by lung parenchymal cells and extracellularly by lung macrophages. It is present in all forms of asthma including mild and asymptomatic cases and involves central as well as peripheral airways.[7,8]
Increased generation of oxidants have been reported in asthma patients than in healthy individuals,[9,10] which aggravated airway inflammation by inducing diverse pro-inflammatory mediators including macrophages, neutrophils and eosinophils.[11,12] Numerous studies have suggested that oxidative stress is caused by overproduction of various free radicals or by an insufficient antioxidant defense system in asthma and thus it contributes to the tissue damage which is induced by inflammatory cells.[9,13–16] These include a range of toxic ROS such as superoxide radical (O2·), hydrogen peroxide (H2O2), hypochlorous acid (HOCl), and hydroxyl radical (OH·).[11,14] In asthmatic patients, ROS play a vital role in pathophysiologic changes such as increased lipid peroxidation, airway responsiveness, production of chemoattractants, and vascular permeability.[17] To counter the oxidant-mediated toxicity, lung and blood cells are endowed with several antioxidants including glutathione peroxidase (GPx), superoxide dismutase (SOD), catalase, glutathione, α-tocopherol, and ascorbic acid.[18,19] The increased oxidative stress in asthma is reflected by plasma level of malondialdehyde (MDA) and protein carbonyls in both bronchoalveolar lavage (BAL) fluid and peripheral blood samples;[12,20] together with increased oxidized glutathione in BAL fluid.[21] It has also been reported that markers of oxidative stress such as H2O2, 8-isoprostane, nitric oxide, and carbon monoxide were found in higher concentration in exhaled air of asthmatic patients.[10,16,22] Changes in antioxidant defenses have noticed due to oxidative stress in asthmatic patients as decreased in GPx activity in whole blood, plasma, and platelets.[23,24] Furthermore, protein sulfhydryls and total antioxidant capacity were also found to be decreased in plasma concomitant with increased SOD activity in BAL cells.[25]
In the present study, the imbalance between oxidant and antioxidant level was determined in asthmatic patients which plays a key role in severity of the disease. Moreover, we have also correlated it with the degree of airflow obstruction (FEV1 % predicted) in patients with asthma.
Methods
Study population
This study was conducted in the Department of TB and Respiratory Diseases, Jawaharlal Nehru Medical College Hospital, Aligarh Muslim University, Aligarh, India, on patients attending outpatient department (OPD) and inpatient departments (IPD). We documented 130 (12-83 years of age) patients with bronchial asthma. Clinical severity of asthma was determined using criteria (appropriate clinical and respiratory function tests) defined in global initiative for asthma guidelines (GINA).[26] The diagnosis was established on the basis of recurrent symptoms of breathlessness, chest tightness, and wheezing with an improvement of greater than 12% in forced expiratory volume in 1 second (FEV1) after inhalation of 200 μg of salbutamol from a nebulizer.
None of the patients were taking any antioxidant supplements and did not have any symptoms of lower or upper respiratory tract infection. Those patients who had pulmonary or systemic disease or an acute exacerbation of asthma within the past 4 weeks were in exclusion criterion. No drug was allowed on the day of testing. Seventy control subjects (10-75 years of age) were selected without respiratory symptoms and normal lung functions. All recruited subjects were nonsmokers and majority of the patients included in the present study were on treatment with inhaled corticosteroids. Written informed consent was obtained from each recruited subjects and the Institutional Ethics Committee of the Medical College approved the study.
Each patient's demographic profile, clinical, radiological findings, associated disease, pulmonary function measurement, history of allergy, and treatment details were recorded.
Evaluation of lung function
Pulmonary function tests were performed in each patient as well as in controls. Lung function parameters were measured by Easy on PC spirometer (ndd Medizintechnik AG, Zurich, Switzerland) and the best value from 3 manoeuvres was recorded as an absolute value. FEV1 and FVC were expressed as a percentage of the predicted values for age, sex, and height.
Anthropometry
All anthropometric measurements were taken according to a standardized protocol. Body weight was measured with participants wearing light clothes without shoes by using a balance beam scale. Height was measured at the same time. Body mass index (BMI) was calculated as body weight divided by height squared (kg/m2).
Collection of blood samples and storage
After a detailed history and pulmonary function test, 5.0 ml fasting venous blood was drawn into heparinized vials and tubes without anticoagulants. Tubes were centrifuged at 1500 g for 15 minutes to obtain plasma. Samples were divided into 0.5 ml aliquots. Erythrocytes and plasma were separated and stored at -80°C until biochemical measurements were taken.
Study measurements
Lipid peroxide assay
Plasma level of MDA was measured by measuring thiobarbituric acid reactive substances (TBARS) according to the method described by Buege and Aust.[27] For assay, 0.5 ml of plasma sample was mixed with equal volume of 0.67% TBA and 30% of TCA in tubes. The mixture was heated in a boiling water bath for 20 minutes. The tubes were centrifuged at 4320 g for 15 minutes. The absorbance of the pink supernatant was taken at 530 nm on the UV-visible spectrophotometer. Results were expressed in nmol of MDA/ml of plasma.
Plasma protein carbonyl content assay
The measurement of protein oxidation was carried out by the estimation of total carbonyl content in the sample using 2,4-dinitrophenylhdrazine[28] and its absorbance was recorded at 360 and 280 nm on the UV-visible spectrophotometer. The carbonyl formation was expressed as the ratio of absorbance at 360 nm to 280 nm and the units were expressed in nmol/mg protein of plasma.
Plasma protein sulfhydryl assay
The estimation of plasma protein sulfhydryl level was performed at 412 nm on the UV-visible spectrophotometer according to the method described by Sedalk and Lindsay.[29] Results were expressed in mmol/l of plasma.
Superoxide dismutase assay in erythrocytes
SOD activity was assayed according to the method of Marklund and Marklund on the basis of autoxidation of pyrogallol.[30] The change in absorbance was recorded at 412 nm on the UV-visible spectrophotometer for 3 minutes. Results were expressed in U/g Hb.
Catalase activity in erythrocytes
The quantitation of catalase activity in erythrocyte hemolysates was based on the oxidation of H2O2. The activity of catalase was measured by taking 1.95 ml of potassium phosphate buffer (50 mM, pH 7.0), 50 μl of the sample to which 1 ml of H2O2 (30 mM) was pipetted in dark conditions.[31] The solution was immediately read at 240 nm for 3 minutes by UV-visible spectrophotometer. The results were expressed in U/g Hb.
GPx activity in erythrocytes
GPx activity was determined spectrophotometrically in erythrocyte hemolysates using the technique adapted by Paglia and Valentine.[32] GPx catalyses the oxidation of glutathione (GSH) by cumene hydroperoxide. In the presence of GSH reductase and NADPH, the oxidized GSH is reduced and NADPH is oxidized to NADP. GPx activity was measured by the decrease in NADPH absorbance at 340 nm on UV-visible spectrophotometer. Its activity was expressed in U/g Hb.
Total blood glutathione assay
The assay was carried out according to the method of Griffith,[33] in which the reaction was initiated by addition of DTNB and the rate of reduction was measured at 412 nm on UV-visible spectrophotometer. Results were expressed in mmol/l.
Total antioxidant capacity (Ferric reducing antioxidant power of plasma assay)
The total antioxidant power in the plasma was estimated by measuring the ferric reducing ability by using the method of Benzie and Strain.[34] The absorbance was taken at 593 nm by UV-visible spectrophotometer and total antioxidant status was calculated using ferrous sulfate as standard. The results were expressed in μmol/l.
Statistical analysis of data
Statistical analyses were performed with statistical package for the social sciences for Windows (Version 16.0; SPSS Inc) and Graph Pad Prism 5.01. Data were expressed as Mean ± SEM. The unpaired t test was used to compare the healthy controls with patients. The biochemical parameters were compared among patients with different asthma severity group by using one-way ANOVA. The relationship between different study parameters and the degree of airways obstruction was evaluated by computing the Pearson's correlation coefficient. P < 0.05 was considered significant.
Results
Table 1 shows the clinical and demographic characteristics of the study subjects. Patients with asthma had a significantly lower FEV1 (% predicted) and other spirometry indices than controls. The post changes in FEV1, FVC, and FEV1/FVC (% predicted) after nebulization with 200 μg of salbutamol were found remarkably at higher level than controls. Relative percent of the measured anthropometric and spirometric parameters have also been demonstrated in Figure 1.
Table 1.
Clinical and demographic characteristics of the study groups
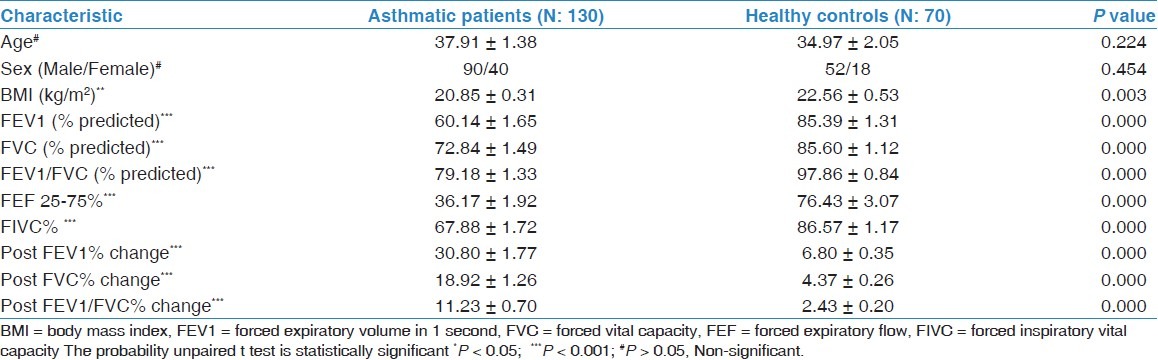
Figure 1.

Relative percent of the measured anthropometric and spirometric parameters of asthmatic patients comparative to controls #P > 0.05, Non-significant; **P < 0.01; ***P < 0.001
It was shown that oxidative stress indicator measured as TBARS in plasma (4.19 ± 0.10 nmol/ml, P < 0.001; Figure 2a) and total protein carbonyls (1.13 ± 0.02 nmol/mg, P < 0.001; Figure 2b) were markedly enhanced in asthmatic patients as compared to control (2.84 ± 0.12 nmol/ml and 0.79 ± 0.02 nmol/mg, respectively). It was also observed that the level of total protein sulfhydryls (0.55 ± 0.01 mmol/l, P < 0.05; Figure 2c) was decreased in patients with asthma as compared to healthy controls (0.60 ± 0.02 mmol/l).
Figure 2.
(a-c) Levels of lipid peroxidation products measured as TBARS expressed as nmol of MDA/ml of plasma, ***P < 0.001; total protein carbonyls in plasma expressed as nmol/mg of protein, ***P < 0.001; and total protein sulfhydryls in plasma expressed as mmol/l, *P < 0.05. Data are expressed in Mean ± SEM
Figure 3a–c illustrates that the activity of SOD (2047 ± 50.34 U/g Hb, P < 0.05), catalase (4374 ± 67.98 U/g Hb, P < 0.01), and GPx (40.97 ± 1.05 U/g Hb, P < 0.01) in erythrocytes were remarkably lower in asthmatic patients than the healthy controls (2217 ± 60.11 U/g Hb; 4746 ± 89.94 U/g Hb, and 48.37 ± 2.47 U/g Hb, respectively) while the levels of total blood glutathione (0.94 ± 0.02 mmol/l, P < 0.05; Figure 4a) were significantly increased in asthmatic patients as seen in controls (0.84 ± 0.04 mmol/l). It was observed that total antioxidant status in plasma, which is measured as FRAP (750.90 ± 21.22 μmol/l, P < 0.05) was remarkably decreased in asthmatic patients as compared to controls (840.40 ± 28.39 μmol/l; Figure 4b).
Figure 3.
(a-c) Superoxide dismutase, catalase, and glutathione peroxidase activity in erythrocytes expressed as U/g Hb; *P < 0.05 and **P < 0.01. Data are expressed in Mean ± SEM
Figure 4.
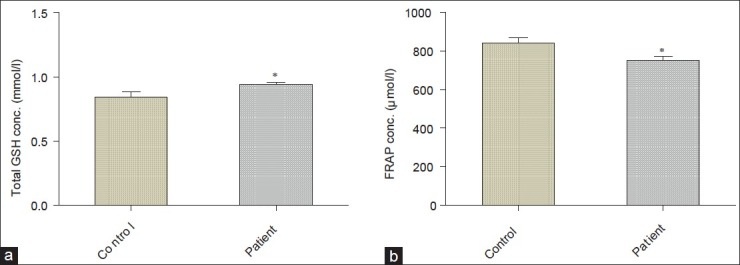
(a and b) Total blood glutathione and ferric reducing antioxidant power in plasma expressed as mmol/l and μmol/l, respectively, *P < 0.05.
There were marked differences in biochemical parameters and other demographic variables among patients with different severities of asthma except age, sex, and SOD activity [Table 2]. Pearson's correlation coefficients of different studied oxidative variables with FEV1 (% predicted) showed remarkable negative correlation between plasma TBARS and total blood glutathione with FEV1 (% predicted) in patients with asthma as well as in controls, but there was no statistically significant difference found between total protein carbonyls and FEV1 (% predicted) in controls (r = -0.18, P > 0.05) [Table 3]. A remarkable positive correlation was found between total protein sulfhydryls, erythrocyte GPx, and total antioxidant status with FEV1 (% predicted) in asthmatic patients as well as in controls. Conversely, erythrocyte SOD (r = -0.07, P > 0.05) exhibited a slight negative correlation, whereas catalase showed an insignificant positive correlation in asthmatic patients (r =0.01, P > 0.05).
Table 2.
Comparison of indices of oxidant-antioxidant status and other demographic variables among patients with asthma severity
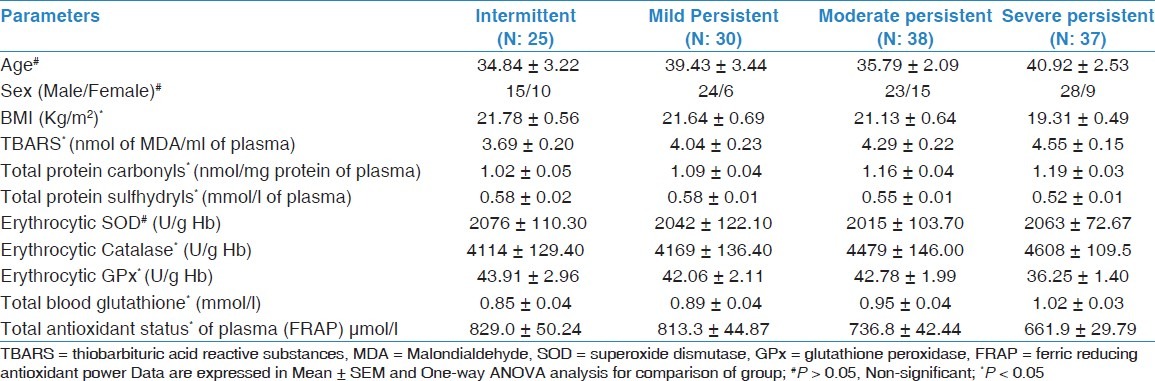
Table 3.
Coefficients of correlation between oxidation variables and FEV1 (% predicted)

Discussion
Several studies have revealed that oxidants-antioxidants balance is an essential factor for the normal physiological function of the lungs. An increased oxidant and/or decreased antioxidant may reverse the physiologic oxidant-antioxidant balance in favor of oxidants. Thus, oxidative stress plays an important role in the pathogenesis of asthma and oxidants caused lipid peroxidation by oxidizing fatty acids and polyunsaturated lipids of cell membranes.
The oxidant–antioxidant status was investigated in blood (red blood cell and plasma) because it is an easily available source and also considered as an important pool of antioxidant defenses in the body rather than BAL fluid which is obtained through an invasive bronchoscope technique.[35] Furthermore, asthma is an inflammatory disease in which cells are recruited from the peripheral blood.
In this work, it has been observed that the asthmatic patients have increased oxidative stress. It was shown by remarkable increase in lipid peroxidation product (MDA) and protein carbonyls as well as decrease in protein sulfhydryls in plasma [Figure 2a–c]. These are accompanied by alterations in several endogenous enzymatic antioxidants in blood including decreased GPx and SOD activities. The distinguished changes were noticed in catalase activity of erythrocytes and total antioxidant power of plasma of asthmatic patients. Rahman et al. reported that the plasma MDA level was higher in asthmatic patients than controls as well as in patients with asthma exacerbation as compared to stable asthma.[12] Similarly, another study entails that MDA level in BAL fluid was higher in mild to moderate asthmatic patients.[20] Moreover, protein carbonyl content was also significantly higher in asthmatic patients because most of the amino acids can be oxidized by ROS. Peroxynitrite anion is a potent oxidant that mediates not only the oxidation of both non-protein and protein sulfhydryls but also induces lipid peroxidation.[36]
Several studies have demonstrated alterations in different endogenous antioxidants in patients with asthma. The alterations in antioxidant defenses may involve either an increase or a decrease depending on the changes occurring due to a defense response. SOD is an intracellular antioxidant enzyme, helping in removing superoxide anion. In some studies, it has been reported that SOD activity in airway epithelium and erythrocytes of asthmatic patients was lower than healthy controls.[17,37] We have also noticed significantly lower activities of SOD and catalase in red blood cells [Figure 3a,b] and these findings were very much similar to the earlier reported by Rai and Phadke (2006).[38]
In a previous study, it has been reported that GPx play a significant role in peroxyl scavenging mechanism and maintaining functional integration of the cell membrane. Here, we observed a significant decrease in GPx activity in asthmatic patients as compared to controls [Figure 3c], which led to the high elevation of lipid peroxidation products as reported in the same investigated samples. Lower GPx level in asthmatic patients can be related to the clinical presentation of the disease and it indicates the presence of H2O2 in breath condensate of exhaled air which is elevated in asthmatics patients.[37] Decreased activity of GPx has been well documented in literature[39] and it is in favor of our findings. Selenium is an essential component of GPx and it indirectly helps in protecting cells against damage caused by free radicals. This might arise as a result of deficiency of selenium or inactivation caused by OH· and O2·.[25,40,41] A number of studies have been done to measure the selenium deficiency in asthma through the antioxidant effects of GPx and it has been found that plasma levels of selenium was significantly lower in asthmatic patients.[42–44] GPx is essential for removing toxic lipid oxidation products and H2O2, which are continuously generated as a result of sequestration and infiltration of inflammatory leukocytes in the lung. Low SOD activity together with low GPx activity in asthmatic patients proved the contribution of oxidative stress in the etiology of asthma. It was reported that the glutathione system is altered in lung inflammatory conditions such as asthma and many reports have shown that alterations in glutathione level have been found in asthmatic airways.[45]
The present finding of our study also shows that the asthmatic patients have significantly higher level of total blood glutathione as compared to control subjects [Figure 4a]. Mak et al. have also reported that the total glutathione level increased in erythrocytes of asthmatic individuals.[46] There have been several reports on the decreased antioxidant capacity in asthmatic patients with intense oxidative load. In this study, we found very low total antioxidant status [Figure 4b] which is correlated to the findings reported by Rahman et al.[12]
The present study also demonstrates that the measures of oxidative stress and antioxidants differ significantly between patients grouped according to severity of asthma except SOD [Table 2]. It revealed that severe persistent asthmatics have highest levels of plasma lipid peroxidation products and simultaneously lowest level of plasma antioxidants. An increasing trend was observed in the level of MDA, total protein carbonyls, and total blood glutathione among patients with intermittent to severe asthma while total protein sulfhydryls, enzymatic antioxidants, and total antioxidant capacity shows a decreasing pattern in these respect (P < 0.05). We have also observed a dramatic enhancement in catalase activity among asthmatic patients (intermittent to severe). Al-Abdulla et al. also reported that the mean serum level of MDA was significantly raised with increasing severity of asthmatic attack among patients grouped according to degree of severity.[47] Previously, Kanazawa et al. have also found differences related to severity in patients with acute exacerbations.[48] The association between asthma severity and anthropometric measurement demonstrates that age and gender was unrelated to disease severity similar to our findings.[49] Our results are also of interest in the context of increased oxidative burden remarkably associated to the pulmonary airways obstruction [Table 3]. Markers of lipid peroxidation (TBARS), total protein carbonyls, and total blood glutathione level showed negative associations with FEV1 (% predicted). Furthermore, it was also noticed that total antioxidant status, erythrocyte GPx, and total protein sulfhydryls were positively associated with FEV1 (% predicted). Schunemann et al. found a remarkable negative correlation between plasma TBARS and red cell GSH, but they did not find any significant correlation with GPx and total antioxidant status.[50] A negative association in erythrocyte SOD activity was also observed, but this was not significant. Wood and co-workers also reported that SOD activity was negatively associated with the severity of asthma.[30] Some workers also demonstrated that the superoxide anion release was greater in patients with exacerbation of their disease and it was also inversely correlated with FEV1.[48,51] It suggested that the lower antioxidant defense cause greater degree of airway obstruction (decreased FEV1 % predicted).
In conclusion, this work describes the role of oxidative stress and antioxidants in severity of disease which provides a strong evidence for oxidant-antioxidant imbalance in disease progression. The findings of this study further strengthen the evidence that the altered oxidant-antioxidant balance is associated with airways obstruction. In further studies, we realized the need to elucidate pathophysiological mechanism involved in lung injury related to oxidant-antioxidant imbalance.
Acknowledgments
The authors wish to thank all the subjects who participated in this study and Dr. Mohammad Faisal Siddiqui for work in his Interdisciplinary Brain Research Centre lab and critically reading manuscript.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: Executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–78. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Chung KF. Role of inflammation in the hyper reactivity of the airways in asthma. Thorax. 1986;41:657–62. doi: 10.1136/thx.41.9.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes PJ. Reactive oxygen species and airway inflammation. Free Radic Biol Med. 1990;9:235–43. doi: 10.1016/0891-5849(90)90034-g. [DOI] [PubMed] [Google Scholar]
- 4.Vignola AM, Chanez P, Campbell AM, Souques F, Lebel B, Enander I, et al. Airway inflammation in mild intermittent and in persistent asthma. Am J Respir Crit Care Med. 1998;157:403–9. doi: 10.1164/ajrccm.157.2.96-08040. [DOI] [PubMed] [Google Scholar]
- 5.Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol. 2008;122:456–68. doi: 10.1016/j.jaci.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riedl MA, Nel AE. Importance of oxidative stress in the pathogenesis and treatment of asthma. Curr Opin Allergy Clin Immunol. 2008;8:49–56. doi: 10.1097/ACI.0b013e3282f3d913. [DOI] [PubMed] [Google Scholar]
- 7.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–45. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 8.Hamid Q, Song Y, Kotsimbos TC, Minshall E, Bai TR, Hegele RG, et al. Inflammation of small airways in asthma. J Allergy Clin Immunol. 1997;100:44–51. doi: 10.1016/s0091-6749(97)70193-3. [DOI] [PubMed] [Google Scholar]
- 9.Henricks PA, Nijkamp FP. Reactive oxygen species as mediators in asthma. Pulm Pharmacol Ther. 2001;14:409–20. doi: 10.1006/pupt.2001.0319. [DOI] [PubMed] [Google Scholar]
- 10.Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol. 2006;533:222–39. doi: 10.1016/j.ejphar.2005.12.087. [DOI] [PubMed] [Google Scholar]
- 11.Terada LS. Specificity in reactive oxidant signaling: Think globally, act locally. J Cell Biol. 2006;174:615–23. doi: 10.1083/jcb.200605036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD and smokers. Am J Respir Crit Care Med. 1996;154:1055–60. doi: 10.1164/ajrccm.154.4.8887607. [DOI] [PubMed] [Google Scholar]
- 13.Fujisawa T. Role of oxygen radicals on bronchial asthma. Current Drug Targets Inflamm Allergy. 2005;4:505–9. doi: 10.2174/1568010054526304. [DOI] [PubMed] [Google Scholar]
- 14.Caramori G, Papi A. Oxidants and asthma. Thorax. 2004;59:170–3. doi: 10.1136/thorax.2002.002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirkham P, Rahman I. Oxidative stress in asthma and COPD: Antioxidants as a therapeutic strategy. Pharmacol Ther. 2006;111:476–94. doi: 10.1016/j.pharmthera.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Comhair SA, Erzurum SC. Antioxidant responses to oxidant-mediated lung diseases. Am J Physiol Lung Cell Mol Physiol. 2002;283:L246–L55. doi: 10.1152/ajplung.00491.2001. [DOI] [PubMed] [Google Scholar]
- 17.De Raeve HR, Thunnissen FB, Kaneko FT, Guo FH, Lewis M, Kavuru MS, et al. Decreased Cu, Zn-SOD activity in asthmatic airway epithelium: Correction by inhaled corticosteroid in vivo. Am J Physiol. 1997;272:L148–54. doi: 10.1152/ajplung.1997.272.1.L148. [DOI] [PubMed] [Google Scholar]
- 18.Psarras S, Caramori G, Contoli M, Papadopoulos N, Papi A. Oxidants in asthma and in chronic obstructive pulmonary disease (COPD) Curr Pharm Des. 2005;11:2053–62. doi: 10.2174/1381612054065774. [DOI] [PubMed] [Google Scholar]
- 19.Bowler RP. Oxidative stress in the pathogenesis of asthma. Curr Allergy Asthma Rep. 2004;4:116–22. doi: 10.1007/s11882-004-0056-7. [DOI] [PubMed] [Google Scholar]
- 20.Ozaras R, Tahan V, Turkmen S, Talay F, Besirli K, Aydin S, et al. Changes in malondialdehyde levels in bronchoalveolar fluid and serum by the treatment of asthma with inhaled steroid and beta2-agonist. Respirology. 2000;5:289–92. doi: 10.1046/j.1440-1843.2000.00260.x. [DOI] [PubMed] [Google Scholar]
- 21.Wood LJ, Fitzgerald DA, Gibson PG, Cooper DM, Garg ML. Lipid peroxidation as determined by plasma isoprostanes is related to disease severity in mild asthma. Lipids. 2000;35:967–74. doi: 10.1007/s11745-000-0607-x. [DOI] [PubMed] [Google Scholar]
- 22.Chanez P, Dent G, Yukawa T, Barnes PJ, Chang KF. Generation of oxygen free radicals from blood eosinophils from asthma patients after stimulation with PAF or phorbol ester. Eur Respir J. 1990;3:1002–7. [PubMed] [Google Scholar]
- 23.Kelly FJ, Mudway L, Blomberg A, Frew A, Sandstrom T. Altered lung antioxidant status in patients with mild asthma. Lancet. 1999;354:482–3. doi: 10.1016/S0140-6736(99)01812-7. [DOI] [PubMed] [Google Scholar]
- 24.Kharitonov SA, Yates D, Robbins RA, Logan-Sinclair R, Shinebourne EA, Barnes PJ. Increased nitric oxide in exhaled air of asthmatic patients. Lancet. 1994;343:133–5. doi: 10.1016/s0140-6736(94)90931-8. [DOI] [PubMed] [Google Scholar]
- 25.Pearson DJ, Saurez-Mendez VJ, Day JP, Miller PF. Selenium status in relation to reduced glutathione peroxidase activity in aspirin-sensitive asthma. Clin Exp Allergy. 1991;21:203–8. doi: 10.1111/j.1365-2222.1991.tb00831.x. [DOI] [PubMed] [Google Scholar]
- 26.Global Initiative for Asthma (GINA) Report [Internet] Global Strategy for Asthma Management and Prevention. Updated 2007. [Accessed November 10, 2008]. Available at: http://www.ginasthma.com .
- 27.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–10. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 28.Levine RL, Williams JA, Stadtman ER, Schacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994;233:346–57. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 29.Sedalk J, Lindsay RH. Estimation of total, protein-bound and non-protein sulfhydryl groups in tissues with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 30.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 31.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 32.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 33.Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–12. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 34.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 35.Loft S, Poulsen HE. Markers of oxidative damage to DNA: Antioxidants and molecular damage. Methods Enzymol. 1999;300:166–84. doi: 10.1016/s0076-6879(99)00124-x. [DOI] [PubMed] [Google Scholar]
- 36.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydrils.The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266:4244–50. [PubMed] [Google Scholar]
- 37.Fenech AG, Ellul-Micallef R. Selenium, glutathione peroxidase and superoxide dismutase in maltese asthmatic patients: Effect of glucocorticoid administration. Pulm Pharmacol Ther. 1998;11:301–8. doi: 10.1006/pupt.1998.0122. [DOI] [PubMed] [Google Scholar]
- 38.Rai RR, Phadke MS. Plasma oxidant-antioxidant status in different respiratory disorders. Ind J Clin Biochem. 2006;21:161–4. doi: 10.1007/BF02912934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antczak A, Nowak D, Shariati B, Krol M, Piasecka G, Kurmanowska Z. Increased hydrogen peroxide and thiobarbituric acid-reactive products in expired breath condensate of asthmatic patients. Eur Respir J. 1997;10:1235–41. doi: 10.1183/09031936.97.10061235. [DOI] [PubMed] [Google Scholar]
- 40.Blum J, Fridovich I. Inactivation of glutathione peroxidase by superoxide radical. Arch Biochem Biophys. 1985;240:500–8. doi: 10.1016/0003-9861(85)90056-6. [DOI] [PubMed] [Google Scholar]
- 41.Searle AJ, Wilson RL. Glutathione peroxidase: Effect of superoxide, hydroxyl and bromine radicals on enzyme activity. Int J Radiat Biol Relat Stud Phys Chem Med. 1980;37:213–7. doi: 10.1080/09553008014550261. [DOI] [PubMed] [Google Scholar]
- 42.Jahnova E, Horvathova M, Gazdik F, Weissova S. Effects of selenium supplementation on expression of adhesion molecules in corticoid-dependent asthmatics. Bratisl Lek Listy. 2002;103:12–6. [PubMed] [Google Scholar]
- 43.Stone J, Hinks LJ, Beasley R, Holgate ST, Clayton BA. Reduced selenium status of patients with asthma. Clin Sci (Lond) 1989;77:495–500. doi: 10.1042/cs0770495. [DOI] [PubMed] [Google Scholar]
- 44.Kadrabova J, Mad’aric A, Kovaciková Z, Podivínsky F, Ginter E, Gazdík F. Selenium status is decreased in patients with intrinsic asthma. Biol Trace Elem Res. 1996;52:241–8. doi: 10.1007/BF02789165. [DOI] [PubMed] [Google Scholar]
- 45.Smith LJ, Houston M, Anderson J. Increased levels of glutathione in bronchoalveolar lavage fluid from patients with asthma. Am Rev Respir Dis. 1993;147(6 Pt 1):1461–4. doi: 10.1164/ajrccm/147.6_Pt_1.1461. [DOI] [PubMed] [Google Scholar]
- 46.Mak JC, Leung HC, Ho SP, Law BK, Lam WK, Tsang KW, et al. Systemic oxidative and antioxidative status in Chinese patients with asthma. J Allergy Clin Immunol. 2004;114:260–4. doi: 10.1016/j.jaci.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 47.Al-Abdulla NO, Al Naama LM, Hassan MK. Antioxidant status in acute asthmatic attack in children. J Pak Med Assoc. 2010;60:1023–7. [PubMed] [Google Scholar]
- 48.Kanazawa H, Kurihara N, Hirata K, Takeda T. The role of free radicals in airway obstruction in asthmatic patients. Chest. 1991;100:1319–22. doi: 10.1378/chest.100.5.1319. [DOI] [PubMed] [Google Scholar]
- 49.Varraso R, Siroux V, Maccario J, Pin I, Kauffmann F. Asthma severity is associated with body mass index and early menarche in women. Am J Respir Crit Care Med. 2005;171:334–9. doi: 10.1164/rccm.200405-674OC. [DOI] [PubMed] [Google Scholar]
- 50.Schunemann HJ, Muti P, Freudenheim JL, Armstrong D, Browne R, Klocke RA, et al. Oxidative stress and lung function. Am J Epidemiol. 1997;146:939–48. doi: 10.1093/oxfordjournals.aje.a009220. [DOI] [PubMed] [Google Scholar]
- 51.Comhair SA, Ricci KS, Arroliga M, Lara AR, Dweik RA, Song W, et al. Correlation of systemic superoxide dismutase deficiency to airflow obstruction in asthma. Am J Respir Crit Care Med. 2005;72:306–13. doi: 10.1164/rccm.200502-180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]




