Abstract
BACKGROUND AND OBJECTIVE:
Some studies show a decline of FEV1 only one month after withdrawal of inhaled corticosteroids (ICS), while others show no decline. We speculate that the presence of an asthma phenotype in the Chronic Obstructive Pulmonary Disease (COPD) population, and that its exclusion may result in no spirometric deterioration.
METHODS:
We performed a prospective clinical observation study on 32 patients who fulfilled the Global Initiative for Chronic Obstructive lung disease definition of COPD (Grade II-IV). They were divided into two phenotypic groups. 1. Irreversible asthma (A and B) (n = 13): A. Asthma: Bronchial biopsy shows diffuse thickening of basement membrane (≥ 6.6 μm). B. Airflow limitation (AFL) likely to be asthma: KCO > 80% predicted if the patient refused biopsy. 2. COPD (A and B) (n = 19): A. COPD: hypercapneic respiratory failure with raised bicarbonate, panlobular emphysema with multiple bullas, or bronchial biopsy showing squamous metaplasia and epithelial/subepithelial inflammation without thickening of the basement membrane. B. AFL likely to be COPD: KCO < 80% predicted.
RESULTS:
The asthma phenotype was significantly younger, had a strong association with hypertrophy of nasal turbinates, and registered a significant improvement of FEV1 (350 ml) vs a decline of - 26.5 ml in the COPD phenotype following therapy with budesonide/formoterol for one year. Withdrawal of budesonide for 4 weeks in the COPD phenotype resulted in FEV1 + 1.33% (SD ± 5.71) and FVC + 1.24% (SD ± 5.32); a change of <12% in all patients.
CONCLUSIONS:
We recorded no spirometric deterioration after exclusion of the asthma phenotype from a COPD group.
Keywords: Asthma, COPD, radiology and other imaging, respiratory function tests
The role of inhaled corticosteroids (ICS) in Chronic Obstructive Pulmonary Disease (COPD) management is still controversial. Although ICS are universally accepted to reduce COPD exacerbations by about 25%, their efficacy in other areas is widely debated.[1–7] The balance seem to be tipping in their favor; many studies show a significant rise of FEV 1 with ICS, compared with placebo.[8–11] On the other hand, a decline in FEV1 with ICS withdrawal (after only one month) has also been reported.[12] This has deterred many of our colleagues from withdrawing ICS, even in patients who do not qualify for such treatment according to the Global Initiative for Chronic Lung Disease (GOLD) criteria (frequent exacerbations and FEV1 < 50% predicted).[13] Studies on the effects of withdrawing ICS on spirometric deterioration yield conflicting results.[12,14–16]
We hypothesize that ICS withdrawal would not produce significant spirometric deterioration provided that by careful selection, patients with irreversible asthma are removed from the “COPD” group. Studies show that 20-48% of “COPD” patients have features characteristic of irreversible asthma.[17–19] Smoking is an important risk factor for both irreversible asthma and COPD.[20] Moreover, the two conditions have been known to be concomitantly, or alternately, ascribed to the same patient.[21] Phenotyping is documented to predict the response to corticosteroids in COPD.[17,19,22–24] Several authors have recommended targeting treatment to the COPD phenotype.[25,26]
In our practice, ICS are routinely withdrawn in the COPD phenotype in the spring and summer months when the risk of exacerbations is at its lowest. We decided to study the effect of the withdrawal on spirometry.
Methods
Patient selection and diagnostic criteria
This is a prospective clinical observation study conducted between April and May 2009 at King Khalid University Hospital, King Saud University, Riyadh, Saudi Arabia, and approved by the King Saud University's Internal Review Board for ethics approval. All patients attending the Respiratory Medicine clinics in the six months prior to the study were screened. Subjects were smokers (≥ 10 pack/year) with airflow limitation (AFL) (FEV1 < 80% predicted, FEV1/FVC < 70%) that was persistent despite budesonide/formoterol treatment (320 μcg budesonide/9 μcg formoterol bid for at least 3 weeks). Patients in this group who fulfilled the Global Initiative for Chronic Obstructive lung disease diagnostic criteria for COPD were labeled as undiagnosed AFL, and further subdivided into irreversible asthma or COPD phenotypes. Irreversible asthma was diagnosed if the bronchial biopsy showed uniform thickening of the basement membrane of 6.6 μm or more.[27] The diagnosis was registered as AFL likely to be irreversible asthma, in any patient who refused biopsy and had normal diffusing capacity for carbon monoxide coefficient (KCO3 80 % predicted).
COPD was diagnosed in the presence of any of the following: 1) Chronic hypercapneic respiratory failure at least one month outside exacerbations; defined as PaO2 ≤ 60 mm Hg, PaCO2 > 45 mm Hg, and serum bicarbonate > 28 m mol/l; 2) Panlobular emphysema with bullas larger than 2 cm observed with high-resolution computerized tomography (HRCT). Panlobular emphysema was defined as areas of low attenuation without a well-defined wall and associated with vascular and septal disruption; 3) Squamous metaplasia with epithelial/subepithelial inflammation and a reticular basement membrane of 6.0 μm or less, observed with bronchial biopsy.[27] AFL likely to be COPD was diagnosed if the KCO was less than 80% predicted. The rationale for this classification is described in more detail in the discussion, and elsewhere.[19]
It was routine practice to use HRCT, arterial blood gases, fiberoptic biopsy of the 3rd order bronchus, and diffusion studies, as part of the diagnostic work-up of AFL in smokers of more than 10 pack/year and who fulfilled the GOLD definition of COPD.[19] We had four diagnostic slots: irreversible asthma or AFL likely to be asthma (grouped as the irreversible asthma phenotype), and COPD or AFL likely to be COPD (grouped as the COPD phenotype). ICS are routinely withdrawn in the COPD phenotype in the spring and summer months (April-September) when the risk of exacerbations is at its lowest. The data from this study was published as an abstract in the Gulf Thoracic 2011, the annual meeting of the Saudi Thoracic Society.[28]
Statistical analysis
The data were analyzed using the statistical Software Package (SPSS pc + version 13.0; SPSS Inc, USA). The Mann-Whitney U-Test was used to compare the median values of skewed continuous variables. The Chi-square and Fisher's exact test were used to observe an association between categorical study and outcome variables. A P value < 0.05 was considered statistically significant.
Results
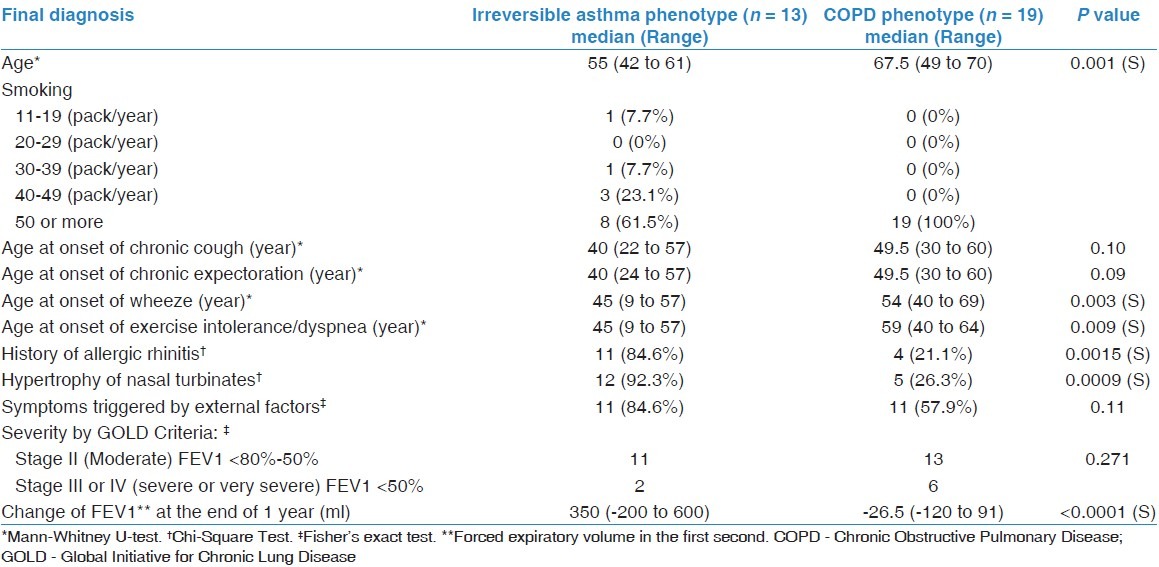
There were a total of 32 patients with undiagnosed AFL. Of these, 13 were of the irreversible asthma phenotype: eight with asthma and five with AFL likely to be asthma. The remaining 19 were of the COPD phenotype based on the following: six had panlobular emphysema with bullas, four patients had hypercapneic respiratory failure, one patient had both, and a positive bronchial biopsy in five. Three patients had AFL likely to be COPD. All were male patients. Table 1 gives the characteristics of both phenotypes and shows significant differences. Patients in the irreversible asthma phenotype were younger, had earlier onset of wheeze and dyspnea (45 vs 59 years), and were more likely to suffer from allergic rhinitis and hypertrophy of nasal turbinates. They also achieved a mean rise of FEV1 of 350 ml with budesonide/formoterol, vs a decline of -26.5 ml in the COPD phenotype.
Table 1.
Characteristics of irreversible asthma and COPD phenotypes
Four weeks after budesonide withdrawal in the COPD phenotype group, changes in FEV1 and FVC were 1.33% (±5.71) and 1.24% (±5.32), respectively. The change was <12% and 200 ml in all patients [Figure 1].
Figure 1.
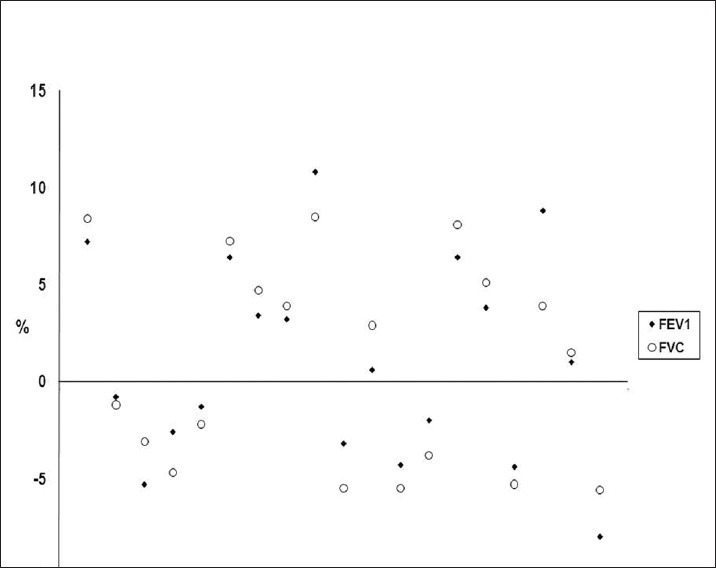
Change of FEV1 and FVC after withdrawal of inhaled corticosteroids
Discussion
We showed no spirometric deterioration following budesonide withdrawal in a small group of COPD patients where the asthma phenotype was excluded (based on bronchial histology and normal coefficient of carbon monoxide diffusion, KCO). We also show that phenotyping based on CT scanning and bronchial biopsy can be applied in routine clinical practice to predict responses to withdrawal of ICS. Phenotyping has been described as the “future of COPD.”[29] There is mounting evidence that COPD phenotypes differ in their physiology and response to B2 -agonists and corticosteroids. Using HRCT scanning, Fujimoto et al. classified COPD into the following three phenotypes: 1) absent emphysema regardless of bronchial wall thickening (phenotype A), 2) emphysema with bronchial wall thickening (phenotype M), and 3) predominantly emphysema (phenotype E).[30] Phenotype A is associated with normal diffusing capacity for carbon monoxide (DLCO) and is significantly reversible with β2 agonists. Phenotype M is also reversible but has low DLCO.[30] Phenotype E has a low DLCO and no significant reversibility.[30] Fujimoto et al. speculated that some patients in phenotype A are asthmatic.[30] Chanez et al. showed that 12 of 25 “COPD” patients had significant reversibility with oral prednisolone and bronchial biopsy revealed uniform thickening of the basement membrane in those 12 patients, compatible with irreversible asthma.[17] In 2010, Lee et al. measured the CT emphysema score in the lung (areas of attenuation below -950 Hounsfield units at full inspiration) and found a correlation with low DLCO and lack of bronchodilator response, or improvement of dyspnea score following three months of combined LABA/ICS therapy.[24] Other studies report improved FEV1 and shuttle distance in response to ICS or prednisolone only in COPD patients with a high sputum eosinophil count.[22,23] Barnes and others speculate that eosinophilic cases represent an asthmatic component of the COPD syndrome, or concomitant asthma and COPD.[7] If phenotyping with HRCT, bronchial biopsy, or sputum cytology had not been applied in these studies, the patients would have been labeled as “COPD” and prednisolone and ICS would have shown a bronchodilator effect. A recently released Consensus Document in Spain recommends major and minor criteria for diagnosing the asthma phenotype in the COPD population.[31]
We found that a physiological and anatomical phenotyping based on arterial blood gases, HRCT, and bronchial biopsy in addition to KCO is a simple and reliable means of differentiating asthma and COPD and predicting the response to ICS and yearly decline of FEV1.[19] We currently use this phenotyping approach in smokers who remain obstructive despite treatment with formoterol (9 μcg) and budesonide (360 μg bid), administered for at least three weeks. In this study, COPD was diagnosed by the presence of chronic hypercapneic respiratory failure, panlobular emphysema with bullas, or bronchial biopsy showing COPD changes (as described in the methods section). Large studies on patients receiving long-term oxygen for chronic respiratory failure included no asthmatics.[32,33] We found no reports of chronic hypercapnia with raised bicarbonate in stable asthma outside exacerbations. We found a report where some elderly subjects with irreversible asthma (average FEV1 55 ± 0.5% predicted) required home oxygen therapy.[34] Even in that extreme group with marked obstruction, the alveolar-arterial gradient for oxygen was only 22.1 ± 1.6 mm Hg, and PaO2 was 76.9 ± 1.7 mm Hg. It is not mentioned whether home oxygen was used intermittently or asthma exacerbations or continuously.[34] Although mild centrilobular and paraseptal emphysematous changes are occasionally seen in asthma, panlobular emphysema has never been described.[35] Irreversible asthma is diagnosed exclusively by bronchial biopsy where diffuse thickening of the basement membrane to 6.6 μm or more is observed. Early studies stated that thickening of the basement membrane could not differentiate asthma and COPD,[36,37] but these studies were conducted on patients with reversible asthma and normal FEV1.[37] In Fabbri's landmark study on the histological differentiation of asthma and COPD, they used patients with FEV1 of about 60% predicted in both groups and showed no overlap in the thickness of the basement membrane between the groups (6.6-9.7 μm vs 4.2-6.2 μm).[27] In Fabbri's study, and others, the thickness of the basement membrane was a predictor of response to oral prednisolone and ICS.[17,19,27] In addition, the thickness of the basement membrane (and other histological features) was strongly associated with atopic features such as a history of allergic rhinitis and hypertrophy of nasal turbinates.[19] Patients with a KCO <80% predicted and who refused bronchial biopsy were given the diagnostic label of undiagnosed AFL likely to be COPD. Normal or high diffusion capacity is consistently observed in asthma, even when it is severe or irreversible, unless there is concomitant bronchiectasis where DLCO may be reduced.[38,39] In Fabbri's study, the mean carbon monoxide diffusion capacity was 65.4 and 85% predicted in irreversible asthma and COPD, respectively, and the mean KCO was 60% and 82.9%, respectively.[27] Al-Kassimi et al. recently reported total concurrence between bronchial histology and KCO in both irreversible asthma and COPD.[19] Earlier studies produced conflicting results on the sensitivity and specificity of diffusion capacity for distinguishing asthma and COPD but no phenotyping was done. In Fujimoto's stratification by CT scan, the DLCO of E and M phenotypes were 49.3 ± 2.1 and 61.6 ± 2.8% of predicted, respectively.[30]
The 19 patients in this study, who were strictly selected from a total of 32 “COPD” patients where irreversible asthma phenotypes were excluded, displayed no decline of FEV1 or FVC, four weeks after withdrawal of ICS. Previous studies on the effect of withdrawal of ICS on FEV1 yielded conflicting results. In a small crossover study on 24 patients (only 15 completed the study), FEV1 declined significantly on withdrawal of beclomethasone, but the six-minute walk test was unchanged.[16] Their results support the possibility that asthmatic patients were inadvertently included: some patients had normal diffusion capacity, and the decline of FEV1 occurred in some but not all patients.[16] Another study reported a significant decline of FEV1 one month after withdrawal of fluticasone.[12] However, the decline of FEV1 was modest at 4.1% (95% CI, 1.6-6.6) even at twelve months.[12] The study also used 10% reversibility upon salbutamol inhalation as a criterion for excluding asthma and single reversibility tests have been shown to be unable to distinguish irreversible asthma and COPD.[40] On the other hand, the COPE study failed to detect any significant decline of FEV1 , 6-minute walk test, or Borg Scale of breathlessness following withdrawal of ICS.[15] Similarly, a study conducted in primary care in the U.K. found no significant deterioration of FEV1 or the St. George's respiratory questionnaire.[14]
The claims that ICS, either after induction or withdrawal, have a large effect on FEV1 raise the following objections:
In COPD, there is reduced activity of histone deacetylation, which is the main mechanism of suppression of inflammatory genes by corticosteroids.[41,42] This persists even after cessation of smoking.[41] ICS were found to have no effect on inflammatory cells or markers in patients with COPD, or in vitro at a cellular level.[43–47]
Some of the large trials of ICS in COPD used two sets of patients: 1) a “reversible,” group who improved their average FEV1 by 29% post 400 mcg salbutamol, and 2) an “irreversible” group, with only 8% salbutamol reversibility.[10] El-Kassimi suggested in 2004 that these studies on "COPD" had inadvertently included patients with asthma, which explained the bronchodilator effect of ICS.[48] The suggestion was based, not only on the above figures of salbutamol reversibility, but also on the fact that patients with a previous history of asthma were included.[10]
Our study has the limitation of a small number of patients. This is related to the fact that the large-scale epidemic of smoking is a recent phenomenon in Saudi Arabia. A further limitation is the short duration of ICS withdrawal. This limitation may be mitigated, however, by the fact that previous studies reported spirometric deterioration only one month after ICS withdrawal.[12] Lastly, this was not a drug trial where we could also have withdrawn budesonide in the irreversible asthma phenotype patients. As seen from Table 1, the irreversible asthma phenotype achieved a 350 ml rise of FEV1 after one year of budesonide/formoterol therapy and probably would have experienced significant deterioration upon ICS withdrawal. The previous studies on ICS withdrawal did not also use an asthmatic control group. The Spanish Consensus Document advises caution with abrupt withdrawal of ICS in the asthma phenotype.[31]
In conclusion, in a small population of COPD sufferers, where the irreversible asthma phenotype (+ve bronchial biopsy or normal KCO) was excluded, there was no significant drop of FEV1 or FVC four weeks after withdrawal of budesonide.
Acknowledgments
The College of Medicine Research Center of King Saud University has offered technical support for the research.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–89. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 2.Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: A randomized controlled trial. Lancet. 2003;361:449–56. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- 3.Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: The ISOLDE trial. BMJ. 2000;320:1297–303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kardos P, Wencker M, Glaab T, Vogelmeier C. Impact of salmeterol/fluticasone proprionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:144–9. doi: 10.1164/rccm.200602-244OC. [DOI] [PubMed] [Google Scholar]
- 5.Glaab T, Taube C. Effect of inhaled corticosteroids in stable chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2011;24:15–22. doi: 10.1016/j.pupt.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigo GJ, Castro-Rodriguez JA, Plaza V. Safety and efficacy of combined long-acting beta-agonists and inhaled corticosteroids vs long-acting beta-agonists monotherapy for stable COPD: A systematic review. Chest. 2009;136:1029–38. doi: 10.1378/chest.09-0821. [DOI] [PubMed] [Google Scholar]
- 7.Barnes PJ. Inhaled corticosteroids in COPD: A controversy. Respiration. 2010;80:89–95. doi: 10.1159/000315416. [DOI] [PubMed] [Google Scholar]
- 8.Tashkin DP, Rennard SI, Martin P, Ramachandran S, Martin UJ, Silkoff PE, et al. Efficacy and safety of budesonide and formoterol in one pressurized metered-dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease: Results of a 6-month randomized clinical trial. Drugs. 2008;68:1975–2000. doi: 10.2165/00003495-200868140-00004. [DOI] [PubMed] [Google Scholar]
- 9.Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus devise in the treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:1084–91. doi: 10.1164/rccm.2112055. [DOI] [PubMed] [Google Scholar]
- 10.Hanania NA, Darken P, Horstman D, Reisner C, Lee B, Davis S, et al. The efficacy and safety of fluticasone propionate (250 μg)/salmeterol (50 μg) combined in the Diskus Inhaler for the treatment of COPD. Chest. 2003;124:834–43. doi: 10.1378/chest.124.3.834. [DOI] [PubMed] [Google Scholar]
- 11.Calverley PM, Kuna P, Monsó E, Costantini M, Petruzzelli S, Sergio F. Beclomethasone/formoterol in the management of COPD: A randomized controlled trial. Respir Med. 2010;104:1858–68. doi: 10.1016/j.rmed.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Wouters EFM, Postma DS, Fokkenst B, Hop WC, Prins J, Kuipers AF, et al. For the COSMIC group. Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: A randomized controlled trial. Thorax. 2005;60:480–7. doi: 10.1136/thx.2004.034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Global Initiative for chronic obstructive pulmonary disease. Updated 2010. [28th June 2011]. Accessed at http://www.goldcopd.org .
- 14.Choudhury AB, Dawson CM, Kilvington HE, Eldridge S, James WY, Wedzicha JA. Withdrawal of inhaled corticosteroids in people with COPD in primary care: A randomized controlled trial. Respir Res. 2007;8:93. doi: 10.1186/1465-9921-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Valk P, Monninkhof E, van der Palen J, Zielhuis G, van Herwaarden C. Effect of discontinuation of inhaled corticosteroids in patients with Chronic Obstructive Pulmonary Disease. The COPE study. Am J Respir Crit Care Med. 2002;166:1358–63. doi: 10.1164/rccm.200206-512OC. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien A, Russo-Magno P, Karki A, Hiranniramol S, Hardin M, Kaszuba M, et al. Effects of withdrawal of inhaled steroids in men with severe irreversible airflow obstruction. Am J Respir Crit Care Med. 2001;164:365–71. doi: 10.1164/ajrccm.164.3.2002052. [DOI] [PubMed] [Google Scholar]
- 17.Chanez P, Vignola AM, O’Shaugnessy T, Enander I, Li D, Jeffery PK, et al. Corticosteroid reversibility in COPD is related to features of asthma. Am J Respir Crit Care Med. 1997;155:1529–34. doi: 10.1164/ajrccm.155.5.9154853. [DOI] [PubMed] [Google Scholar]
- 18.Bellia V, Battaglia S, Catalano F, Scichilone N, Incalzi RA, Imperiale C, et al. Aging and disability affect misdiagnosis of COPD in elderly asthmatics: The SARA study. Chest. 2003;123:1066–72. doi: 10.1378/chest.123.4.1066. [DOI] [PubMed] [Google Scholar]
- 19.Al-Kassimi FA, Abba AA, Al-Hajjaj MS, Alhamad EH, Raddaoui E, Shaikh SH. Asthma Masquerading as Chronic Obstructive Pulmonary Disease: A Study of Smokers Fulfilling the GOLD definition of Chronic Obstructive Pulmonary Disease. Respiration. 2011;82:19–27. doi: 10.1159/000323075. [DOI] [PubMed] [Google Scholar]
- 20.Ten Brinke A. Risk factors associated with irreversible airflow limitation in asthma. Curr Opin Allergy Clin Immunol. 2008;8:63–9. doi: 10.1097/ACI.0b013e3282f3b5b5. [DOI] [PubMed] [Google Scholar]
- 21.Shaya FT, Dongyi D, Okazawa M, Blanchette CM, Wang J, Mapel DW, et al. Burden of concomitant asthma and COPD in Medicaid population. Chest. 2008;134:14–9. doi: 10.1378/chest.07-2317. [DOI] [PubMed] [Google Scholar]
- 22.Brightling CE, McKenna S, Hargadon B, Birring S, Green R, Siva R, et al. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax. 2005;60:193–8. doi: 10.1136/thx.2004.032516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brightling CE, Monteiro W, Ward R, Parker D, Morgan MD, Wardlow AJ, et al. Sputum eosinophila and short-term response to prednisolone in chronic obstructive pulmonary disease: A randomized controlled trial. Lancet. 2000;356:1480–5. doi: 10.1016/S0140-6736(00)02872-5. [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, Lee YK, Kim EK, Kim TH, Huh JW, Kim WJ, et al. Responses to inhaled long-acting beta-agonist and corticosteroid according to COPD subtype. Respir Med. 2010;104:542–9. doi: 10.1016/j.rmed.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 25.Fingleton J, Weatherall M, Beasley R. Towards individualized treatment in COPD. Thorax. 2011;66:363–4. doi: 10.1136/thx.2010.155564. [DOI] [PubMed] [Google Scholar]
- 26.Shirtcliffe P, Weatherall M, Travers J, Beasley R. The multiple dimensions of airways disease: Targeting treatment to clinical phenotypes. Curr Opin Pulm Med. 2011;17:72–8. doi: 10.1097/MCP.0b013e328341f181. [DOI] [PubMed] [Google Scholar]
- 27.Fabbri LM, Romagnoli M, Corbetta, Casoni G, Busljetic K, Turato G, et al. Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:418–24. doi: 10.1164/rccm.200203-183OC. [DOI] [PubMed] [Google Scholar]
- 28.Al-Kassimi FA, Al-Hajjaj MS, Alhamad EH, Abba AA, Raddaoui E, Shaikh SA. Abrupt withdrawal of inhaled corticosteroids in COPD does not produce spirometric deterioration: Role of phenotyping. Ann Thorac Med. 2011;6:174. doi: 10.4103/1817-1737.102185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, et al. Chronic obstructive pulmonary disease phenotypes: The future of COPD. Am J Respir Crit Care Med. 2010;182:598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujimoto K, Kitaguchi Y, Kubo K, Honda T. Clinical analysis of chronic obstructive pulmonary disease phenotypes classified using high-resolution computed tomography. Respirology. 2006;11:731–40. doi: 10.1111/j.1440-1843.2006.00930.x. [DOI] [PubMed] [Google Scholar]
- 31.Soler-Catluña JJ, Cosio B, Izquierdo JL, López-Campos JL, Marin JM, Agüero R, et al. Consensus Document on the mixed asthma-COPD phenotype in COPD. Arch Bronconeumol. 2012 doi: 10.1016/j.arbres.2011.12.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Caramori G, Fabbri M, Paioli D, Falcone F, Severino C, Felisatti G, et al. Asthma is not a common cause of severe chronic respiratory failure in non-smokers: ALOT study. Monaldi Arch Chest Dis. 2005;63:84–7. doi: 10.4081/monaldi.2005.643. [DOI] [PubMed] [Google Scholar]
- 33.Szafranski W, Polsce JZ. Long-term domiciliary oxygen therapy (LTOT) in Poland in the years 1986-2005 (in Polish) Pneumonol Alergol Pol. 2007;75:331–42. [PubMed] [Google Scholar]
- 34.Tsuda Y, Nogochi T, Mochizuki H, Makino F, Nanjo Y, Sawabe M, et al. Patients with mild-to-moderate asthma may develop clinically significant chronic obstructive pulmonary disease. Respirology. 2009;14:529–36. doi: 10.1111/j.1440-1843.2009.01533.x. [DOI] [PubMed] [Google Scholar]
- 35.Harmanci E, Kebapci M, Metintas M, Ozkan R. High-resolution computed tomography findings are correlated with disease severity in asthma. Respiration. 2002;69:420–6. doi: 10.1159/000064018. [DOI] [PubMed] [Google Scholar]
- 36.Bourdin A, Serre I, Flamme A, Vic P, Neveu D, Aubas B, et al. Can endobronchial biopsy analysis be recommended to discriminate between asthma and COPD in routine practice? Thorax. 2004;59:488–93. doi: 10.1136/thx.2003.016899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liesker JJ, Ten Hacken NH, Zeinstra-Smith M, Rutgers SR, Postma DS, Timens W. Reticular basement membrane in asthma and COPD: Similar thickness, yet different composition. Int J Chron Obstruct Pulmon Dis. 2009;4:127–35. doi: 10.2147/copd.s4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitsunobu F, Mifune T, Ashida K, Hosaki Y, Tsugeno H, Okamoto M, et al. Influence of age and disease severity on high resolution CT lung densitometry in asthma. Thorax. 2001;56:851–6. doi: 10.1136/thorax.56.11.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boulet LP, Turcotte H, Hudon C, Carrier G, Maltais F. Clinical, physiological and radiological features of asthma with incomplete reversibility compared with those of COPD. Can Respir J. 1998;5:270–7. doi: 10.1155/1998/780739. [DOI] [PubMed] [Google Scholar]
- 40.Chhabra SK. Acute bronchodilator response has limited value in differentiating bronchial asthma from COPD. J Asthma. 2005;42:367–72. doi: 10.1081/JAS-62992. [DOI] [PubMed] [Google Scholar]
- 41.Barnes PJ. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br J Pharmacol. 2006;148:245–54. doi: 10.1038/sj.bjp.0706736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnes PJ, Ito K, Adcock IM. Corticosteroid resistance in chronic obstructive pulmonary disease: Inactivation of histone deacetylase. Lancet. 2004;363:731–3. doi: 10.1016/S0140-6736(04)15650-X. [DOI] [PubMed] [Google Scholar]
- 43.Keatings VM, Jatakanon A, Worsdell YM, Barnes PJ. Effects of inhaled and oral glucocorticoids on inflammatory indices in asthma and COPD. Am J Respir Crit Care Med. 1997;155:542–8. doi: 10.1164/ajrccm.155.2.9032192. [DOI] [PubMed] [Google Scholar]
- 44.Culpitt SV, Maziak W, Loukidis S, Nightingale JA, Matthews JL, Barnes PJ. Effect of high dose inhaled steroid on cells, cytokines, and proteases in induced sputum in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1635–9. doi: 10.1164/ajrccm.160.5.9811058. [DOI] [PubMed] [Google Scholar]
- 45.Loppow D, Schleiss MB, Kanniess F, Taube C, Jörres RA, Magnussen H. In patients with chronic bronchitis a four week trial with inhaled steroids does not attenuate airway inflammation. Respir Med. 2001;95:115–21. doi: 10.1053/rmed.2000.0960. [DOI] [PubMed] [Google Scholar]
- 46.Gizycki MJ, Hattotuwa KL, Barnes N, Jeffery PK. Effects of fluticasone propionate on inflammatory cells in COPD: An ultrastructural examination of endobronchial biopsy tissue. Thorax. 2002;57:799–803. doi: 10.1136/thorax.57.9.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saha S, Brightling CE. Eosinophilic airway inflammation in COPD. Int J Chron Obstruct Pulmon Dis. 2006;1:39–47. doi: 10.2147/copd.2006.1.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Kassimi FA. Differentiating asthma and COPD patients (Letter to the editor) Chest. 2004;126:653–9. doi: 10.1378/chest.126.2.653. author reply 654-5. [DOI] [PubMed] [Google Scholar]


