Abstract
During physiological or psychological stress, catecholamines produced by the sympathetic nervous system (SNS) regulate the immune system. Previous studies report that the activation of β-adrenergic receptors (βARs) mediates the actions of catecholamines and increases pro-inflammatory cytokine production in a number of different cell types. The impact of the SNS on the immune modulation of social defeat has not been examined. The following studies were designed to determine whether SNS activation during social disruption stress (SDR) influences anxiety-like behavior as well as the activation, priming, and glucocorticoid resistance of splenocytes after social stress. CD-1 mice were exposed to one, three, or six cycles of SDR and HPLC analysis of the plasma and spleen revealed an increase in catecholamines. After six cycles of SDR the open field test was used to measure behaviors characteristic of anxiety and indicated that the social defeat induced increase in anxiety-like behavior was blocked by pre-treatment with the β-adrenergic antagonist propranolol. Pre-treatment with the β-adrenergic antagonist propranolol did not significantly alter corticosterone levels indicating no difference in activation of the hypothalamic–pituitary–adrenal axis. In addition to anxiety-like behavior the SDR induced splenomegaly and increase in plasma IL-6, TNFα, and MCP-1 were each reversed by pre-treatment with propranolol. Furthermore, flow cytometric analysis of cells from propranolol pretreated mice reduced the SDR-induced increase in the percentage of CD11b+ splenic macrophages and significantly decreased the expression of TLR2, TLR4, and CD86 on the surface of these cells. In addition, supernatants from 18 h LPS-stimulated ex vivo cultures of splenocytes from propranolol-treated SDR mice contained less IL-6. Likewise propranolol pre-treatment abrogated the glucocorticoid insensitivity of CD11b+ cells ex vivo when compared to splenocytes from SDR vehicle-treated mice. Together, this study demonstrates that the immune activation and priming effects of SDR result, in part, as a consequence of SNS activation.
Keywords: Stress, Anxiety-like behavior, β-Adrenergic antagonism, Glucocorticoids, Toll-like receptors
1. Introduction
Organisms survive by maintaining the complex dynamic equilibrium of homeostasis. When homeostasis is disturbed by endogenous or exogenous stressors, the sympathetic nervous system (SNS) and the hypothalamic–pituitary-adrenal axis (HPA) axis are activated. Under physiologic conditions, catecholamines and glucocorticoids (GCs) act as major regulators of fuel metabolism, heart rate, blood vessel tone, and thermogensis (Elenkov et al., 2000). The SNS is the largest component of the autonomic nervous system; it innervates all parts of the body including primary and secondary lymphoid organs. The splenic nerves are comprised of approximately 98% sympathetic nerve fibers and lack major cholinergic innervations (Klein et al., 1982; Madden et al., 1995). Noradrenergic innervation of lymphoid tissue is also regionally specific; for example, within the spleen, innervation occurs in dense zones of macrophages, T cells, and plasma cells. Products of the endocrine and nervous systems alter immune cell functioning in response to a stressor (Ader, 2006). The SNS contributes to the stress response through the release of catecholamines by sympathetic nerves in lymphoid tissue; catecholamines then mediate their effects on immune cells through the G-protein coupled adrenergic receptors. Moreover, norepinephrine and epinephrine are released from the adrenal medulla after SNS is activation during a stress response, in parallel to the release of glucocorticoids from the adrenal cortex. Norepinephrine is also released from sympathetic nerve fibers that heavily innervate lymphoid tissues. Norepinephrine and epinephrine mediate their effects on immune cells via stimulation of two major receptor subclasses: the alpha(α)- and the beta(β)-adrenergic receptors (ARs). When activated, this catecholaminergic system can provide the body with a needed ‘boost’ to deal with the immediate threat of the stressor. Hormones and neurotransmitters induced under stress bind specific receptors on immune cells and subsequently influence their activation and function. Recent studies indicate that activation of β2ARs in murine macrophages with the specific agonist, salmeterol, up-regulated IL-1β and IL-6 mRNA and protein levels resulting in an increased pro-inflammatory immune response (Tan et al., 2007). In addition, propranolol treatment 30 min prior to tailshock exposure attenuated plasma IL-1β and IL-6 levels (Johnson et al., 2005). Collectively, this indicates that stressor-induced activation of the HPA and SNS, which results in the release of glucocorticoids and catecholamines respectively, provides a significant link among the nervous, endocrine, and immune systems during the stress response in order to restore homeostasis (Chrousos, 1992; Madden, 2003).
Studies from our laboratory and others have demonstrated that social stress impacts peripheral physiologic responses as the host responds to new environmental demands caused by agonistic interactions (Bailey et al., 2006; Engler et al., 2005; Kinsey et al., 2007, 2008; Merlot et al., 2004; Quan et al., 2001). Social disruption stress (SDR), a model of repeated social defeat in mice, is associated with splenomegaly and the development of steroid insensitivity in splenic, CD11b+ and CD11c+ myeloid derived cell populations (Powell et al., 2009; Stark et al., 2001). Two hours of SDR induces a threefold increase in circulating corticosterone, yet the level of corticosterone returns to baseline by morning after each stress cycle (Sheridan et al., 2004). Six cycles of SDR results in the development of glucocorticoid (GC) resistance, where CD11b+ cells in the spleen become insensitive to GC-induced cell death (Stark et al., 2002). In addition, after LPS stimulation CD11b+ cells from SDR mice secrete more IL-6 than control cells (Stark et al., 2002), and CD11b+ macrophages from SDR mice have enhanced microbicidal activity when challenged with Escherichia coli (Bailey et al., 2007). Along with significant alterations in immune function, social defeat has been shown to cause lasting behavioral changes in rodents. For example, mice that observed a partner mouse as it received a series of electrical shocks displayed increased freezing during the shocks and also froze when placed back into the observing chamber the following day (Jeon et al., 2010). Also, mice that received one session of social defeat demonstrated increased immobility in the Porsolt forced swim test, a behavior associated with depression (Hebert et al., 1998). Similarly, repeated social defeat during SDR in mice increased anxiety-like behavior in the open field test, light/dark preference test, and the novel object test of neophobia, but had no effects on depressive-like behavior in the Porsolt forced swim test or the tail suspension test (Bailey et al., 2009b; Kinsey et al., 2007, 2008).
While the overall immune and behavioral outcomes of SDR have been reported (Avitsur et al., 2001; Kinsey et al., 2007; Meagher et al., 2007; Stark et al., 2001), the contribution of social stress-induced SNS activation on the development of anxiety-like behavior and altered monocyte/macrophage function is unknown. Therefore, the purpose of this study was to determine the extent of SNS activation induced by SDR and determine how this relates to the social stress-induced activation, priming and glucocorticoid resistance of CD11b+ cells. Here we showed that the SNS and HPA axis were activated by social defeat. In addition, blockade of the SNS response through β-adrenergic receptor antagonism did not significantly alter the HPA axis response, but did attenuate the SDR-induced splenomegaly, anxiety-like behavior and plasma IL-6 responses. Moreover, blockade of the β-adrenergic receptor abrogated the SDR enhanced expression of TLR2+, TLR4+, and CD86+ on the surface of splenocytes from socially defeated mice. β-adrenergic receptor antagonism reduced SDR macrophage activation and decreased the ex vivo response to LPS stimulation. It also restored the sensitivity of splenocytes to glucocorticoids ex vivo. Taken together, these data showed that activation of the SNS, and release of catecholamines, was responsible for the behavioral and peripheral physiological changes induced by exposure to repeated social stress.
2. Materials and methods
2.1. Animals
Male CD1 mice at 6–8 weeks of age were obtained from Charles River Breeding Laboratories, Inc. (Hollister, CA) and allowed to acclimate to their surroundings for 7–10 days before initiation of any experimental procedures. Mice were housed three to five animals per cage and maintained at 21 °C under a 12 h light:12 h dark cycle with ad libitum access to water and rodent chow in an American Association of Accreditation of Laboratory Animal Care-accredited facility in Postle Hall at the Ohio State University. The health status of the mice was carefully examined throughout all experiments by veterinary assistants assigned to the Postle Hall animal facility. All procedures were in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee.
2.2. Pharmacological treatments and experimental protocol
In the first study, CD-1 mice received a subcutaneous (s.c.) injection of vehicle or propranolol (Sigma, St. Louis, MO) at 10 mg/kg, 30 min prior to each of the 6 cycles of SDR. The dose of propranolol was based on previous studies and for its capacity to block all β-AR receptors (Kohut et al., 2005; Miura et al., 2007). Mice were humanely euthanized by decapitation immediately after the 1st, 3rd and 6th cycle of SDR or 20 min, 3, 12 or 24 h after termination of the 6th cycle of SDR to facilitate maximal collection of blood from the trunk and to diminish any handling effect on the level of catecholamines. Plasma and spleens were subsequently collected and the levels of norepinephrine and epinephrine were determined (n = 6). Plasma and tissues for catecholamine analysis were taken from undisturbed home cage control (HCC) mice.
In a second study, CD-1 mice were treated with the β-adrenergic antagonist or vehicle and subjected to SDR as described above. Mice were injected s.c., with propranolol (10 mg/kg, as described above) or vehicle 30 min prior to SDR. Evening and morning serum was collected by orbital sinus blood collection to determine corticosterone levels immediately following the last cycle of SDR. Fourteen hours after that last cycle of SDR, anxiety-like behavior was determined (n = 9). After the completion of behavioral testing, mice were sacrificed by CO2 asphyxiation and spleen weight was determined (n = 9). Blood was collected by cardiac puncture with EDTA lined 1 ml syringes, centrifuged and plasma was collected to determine cytokine levels (n = 9). While we did obtain blood samples from the same mouse over the course of the experiment, it is important to note that no animals were bled twice on the same day.
In a third set of studies, CD-1 mice received a subcutaneous (s.c.) injection of vehicle or propranolol (10 mg/kg, as described above) prior to each of the 6 cycles of SDR. Fourteen hours after the 6th cycle of SDR, mice were sacrificed by CO2 asphyxiation and the spleens were homogenized as described above. Cell surface expression of CD11b, Gr-1, TLR4, TLR2, MHC I, MHCII, CD80 and CD86 expression was determined by flow cytometric analyses (n = 9). Splenocytes were also stimulated ex vivo with LPS and LPS plus increasing doses of corticosterone to assess cytokine production and glucocorticoid insensitivity (n = 9). In all the experiments, the subjects in the SDR group were defeated residents.
2.3. Social disruption stress paradigm (SDR)
SDR was performed as previously described (Avitsur et al., 2001). In brief, an aggressive intruder male CD-1 mouse was introduced into cages of established cohorts for 2 h between 17:00 and 19:00 for 6 consecutive nights. Behavior was observed to ensure that the intruder was aggressive. If the intruder did not initiate an attack within 5–10 min or was attacked by any of the resident mice, then a new intruder was introduced; different intruders were used on consecutive nights. At the end of the 2 h period, the intruder was removed and the residents were left undisturbed in their home cages until the following day when the protocol was repeated. During SDR, resident mice displayed submissive behaviors including upright submissive posture, fleeing, and crouching (Avitsur et al., 2001; Stark et al., 2001).
2.4. Plasma and tissue catecholamine measurement
Plasma and tissue were removed and immediately flash frozen in liquid nitrogen to halt degradation of catecholamines. Samples were stored at –80 °C until extracted. Tissue was processed by homogenizing in a 0.2 N acetic acid buffer (1000 μl glacial acetic acid, 0.05% EDTA, 0.1% sodium bisulfite in 99 ml of HPLC grade H2O) using glass homogenizers, and supernatants from homogenized tissue and plasma were further processed for catecholamine extraction using ESA pcat extraction kit per manufacturer protocol (ESA Magellan Biosciences; Chelmsford MA), and then quantified by HPLC electrochemical detection by comparing against external standards.
2.5. Preparation of Cells
Spleens were collected in ice-cold Hanks’ balanced salt solution (HBSS) and mechanically disrupted to obtain single cell suspensions. Red blood cells were lysed by adding 2 ml of room temperature lysis buffer (0.16 M NH4Cl, 10 mM KHCO3, and 0.13 mM EDTA) for 2 min, followed by one wash with HBSS/10% heat-inactivated fetal bovine serum (FBS). Each cell pellet was resuspended in HBSS, filtered and washed a final time in HBSS. Cells were counted and samples were resuspended (2.5 × 106 cells/ml) in supplemented RPMI medium (10% heat-inactivated FBS, 0.075% sodium bicarbonate, 10 mM HEPES buffer, 100 U/ml penicillin G, 100 mg/ml streptomycin sulfate, 1.5 mM l-glutamine, and 0.0035% 2-mercaptoethanol).
2.6. Anxiety-like behavior testing
Behavior was assessed the morning following the last cycle of social defeat. Anxiety-like behavior was measured in the open field test, consisting of a 40 × 40 × 25 cm square plexiglass box with a solid floor and a 6 × 6 grid drawn on the floor separating the open field into 36 identical squares. This test takes advantage of a rodent's natural tendencies to explore the environment while avoiding open spaces. For this experiment, the dependent variables were: time spent moving, rearing activity, total time spent in the center or perimeter of the open field, latency to entering the center and the number of entries into the open field in a 5 min period. The test apparatus was cleaned with water between subjects. Behavior was taped, digitized and coded using the Observer program (Noldus Information Technologies, Netherlands).
2.7. Serum corticosterone determination
Serum corticosterone was measured with a ImmuChem Double Antibody Corticosterone I125 Radioimmunoassay (RIA) kit (MP Biomedicals; Orangeburg, NY), per the manufacturer's protocol. Blood was collected from the retroorbital plexus within 3 min of opening the cage, immediately following the last cycle of SDR (i.e., at 1900 EST) and at 18 h after the last cycle of SDR (i.e., at 0900 EST). Blood samples were immediately put on ice and allowed to clot for 15 h at 4 °C; afterwards, the clots were removed and the samples were centrifuged at 4000g at 4 °C for 20 min. Serum was stored at –70 °C until analysis. Blood samples were collected from each animal in this study and were assayed in duplicate.
2.8. Plasma cytokine measurement by cytometric bead array (CBA)
The interleukin-6 (IL-6), monocyte chemotactic protein-1 (MCP-1) and tumor necrosis factor-alpha (TNF-α) plasma response was detected using the mouse Inflammatory Cytokine CBA kit according to the manufacturer's instructions (BD PharMingen; San Diego, CA). Briefly, 50 ll of each plasma sample was mixed with 50 μl of mixed capture beads and 50 μl of the Inflammation PE detection reagent. The samples were incubated at room temperature for 3 h in the dark. After incubation with the PE detection reagent, the samples were washed once and resuspended in 300 μl of wash buffer before analysis on a FACSCalibur flow cytometer (BD Biosciences; San Jose, CA). Data were analyzed using CBA software (BD PharMingen). Using the mixed cytokine standard provided in each kit, standard curves were generated for each respective cytokine. The concentration for each cytokine in cell supernatants was determined by extrapolation from the corresponding standard curve by applying a 4-parameter curve fit option. The limit of detection for IL-6, MCP-1 and TNF-α was 5, 52.7 and 7.3 pg/ml, respectively.
2.9. Flow cytometric analysis of splenocytes
Single cell suspensions derived from HCC vehicle, HCC propranolol, SDR vehicle and SDR propranolol spleen tissue (1 × 106 cells per sample) were incubated with 1 μg each of fluorescently-labeled monoclonal antibodies (or the appropriate isotype controls). Antibody labeling was performed at 4 °C for 45 min. The cells were then washed twice in PBS containing 1% FBS and 0.09% NaN3. All antibodies were obtained from BD PharMingen (San Jose, CA), including APC-labeled anti-CD11b, PerCP-labeled anti-Gr-1/Ly-6C, PE-labeled anti-TLR2, anti-TLR4, and anti-CD86, FITC-labeled anti-MHCI, anti-MHCII, and anti-CD80. Leukocytes were gated based on forward versus side scatter and a total of 10,000–100,000 events were analyzed on a FACSCalibur flow cytometer using Cell Quest and Cell Quest Pro analysis software (Becton-Dickenson; San Jose, CA).
2.10. Cell culture supernatant cytokine measurement by ELISA
Single cell suspensions derived from HCC vehicle, HCC propranolol, SDR vehicle and SDR propranolol spleen tissue were plated in triplicate in a 96-well plate (2 × 105 cells per well), and stimulated with RPMI media or 1 mg/ml LPS (Sigma) and increasing concentrations of corticosterone (0.005–5 μM) to determine cell cytokine secretion. Corticosterone was obtained commercially from Sigma and dissolved in ethanol; the final concentration of ethanol in the culture medium was 0.2%. Supernatants were harvested at 18 h post culture and quantified for pro-inflammatory cytokines (IL-6) by standard sandwich ELISA, as described previously (Avitsur et al., 2001; Stark et al., 2001). For IL-6 determination, rat anti-mouse antibody was used and preformed per manufacturer's instructions (BD Pharmingen; San Diego, CA).
2.11. Cell viability assay
The Cell Titer 96 aqueous nonradioactive proliferation assay (Promega; Madison, WI) was used to determine cell viability in companion cultures prepared as above and incubated at 37 °C in 5% CO2 for 48 h in culture. The tetrazolium substrate solution (20 μl) was added to each well of the 96-well plates. The plates were further incubated at 37 °C in 5% CO2 for 3 h, and the resulting color changes were quantified by obtaining optical density (OD) readings at 490 nm on an ELISA plate reader. To account for differences in background activity of cells, the mean OD of three control wells (culture fluid only) were subtracted for a given treatment from each of the corresponding LPS-stimulated values.
2.12. Statistical analysis
All data are expressed as means ± standard error of mean (SEM). All data were analyzed using SPSS Statistics v.17.0 General Linear Model procedures. Differences between stressed and non-stressed groups were determined using analysis of variance (ANOVA). Data were subjected to either one (HCC vs. SDR) or two-(HCC vs. SDR and Vehicle vs. Propranolol) factor ANOVAs. A mixed factor ANOVA with two between subjects factors (SDR × Propranolol) and one within subjects factor (Corticosterone dose) was used to test the effects of stress and corticosterone on cell viability and cytokine production. Post hoc testing was accomplished with t tests utilizing Bonferonni correction. In all cases, the level of significance was set at an α = .05.
3. Results
3.1. SDR increased circulating and tissue catecholamines
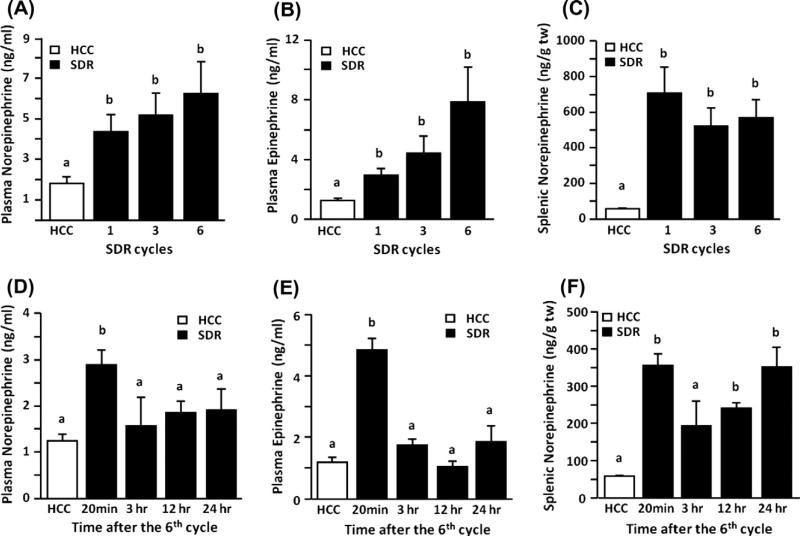
Repeated social defeat increased the plasma levels of norepinephrine (Fig. 1A) and epinephrine (Fig. 1B) immediately after 1, 3 or 6 cycles of SDR. In addition, SDR also increased the concentration of norepinephrine in the spleen compared to controls (HCC) (Fig. 1C) while the levels of epinephrine in the spleen were not increased following SDR (data not shown). Plasma levels of both norepinephrine and epinephrine were increased immediately after 6 cycles of SDR (20 min) and returned to control levels by 3 h after termination of the stressor (Fig. 1D and E). While splenic norepinephrine levels were also elevated at 20 min after the 6th cycle of SDR and were decreased within 3 h, the levels of norepinephrine started to increase by 12 h and were similar to levels seen immediately after the 6th cycle 24 h after termination of the stressor, indicating a possible conditioning effect (Fig. 1F).
Fig. 1.
Repeated social defeat significantly increased plasma and splenic catecholamines. Separate groups of mice were subjected to one, three or six cycles of repeated social defeat (SDR; filled bars) or left undisturbed in their home cage (HCC; open bars). Immediately following the first, third or sixth stress cycle plasma and spleens were collected to determine catecholamine levels. Mice subjected to SDR had increased levels of (A) plasma norepinephrine and (B) plasma epinephrine as well as (C) increased splenic norepinephrine regardless of the number of SDR cycles experienced. In a follow up study separate groups of mice were subjected to six cycles of SDR in order to collect plasma and spleens at 20 min, 3, 12 or 24 h post termination of the stressor in order to assess catecholamine levels. (D and E) Plasma norepinephrine and epinephrine were elevated 20 min following termination of the stressor but returned to control levels at later time points. (F) Defeated mice had elevated splenic norepinephrine except at 3 h post termination of the stressor. Bars represent the mean ± SEM (n = 6). Means with different letters (a or b) are significantly different (p < 0.05) from each other. (tw = total tissue weight).
3.2. βAR blockade decreased the anxiety-like behavior seen in SDR mice
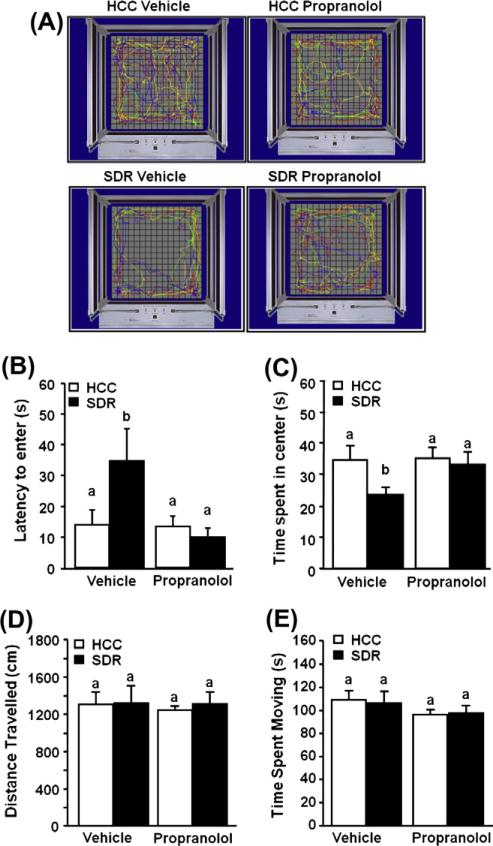
Consistent with previous data from our laboratory, SDR promoted anxiety-like behavior. Individual mice were placed in the corner of open field box and allowed to freely explore the apparatus. Fig. 2A shows representative motion plots of the experimental mice in the open field testing apparatus. Mice subjected to SDR took longer to first enter the center of the open field compared to control (HCC) mice. For example, mice subjected to SDR took 34.6 ± 10.6 s to first enter the center of the open field compared to 14.1 ± 5.1 s for the controls (main effect of stress, F(1,34) = 4.83, p < 0.05; Fig. 2B). Furthermore, Fig. 2C as well as Fig. 2A shows that mice subjected to 6 cycles of SDR spent less time in the open field (main effect of stress, F(1,34) = 3.13, p < 0.02). In addition, we demonstrated that SDR-induced SNS activation influenced the anxiety-like behavior induced by social defeat. Pre-treatment of mice with the β-adrenergic antagonist propranolol attenuated the SDR-induced increased latency to enter the open field (stress × propranolol interaction, F(1,34) = 3.94; p < 0.05; Fig. 2B) and reversed the SDR induced decrease in the total time spent in the open field (stress × propranolol interaction, F(1,45) = 2.10; p < 0.05; Fig. 2C). Neither SDR nor propranolol treatment of SDR mice, affected the distance traveled or the time spent moving in the open field (Fig. 2D and E, respectively), indicating that differences were not due to changes in locomotion or activity.
Fig. 2.
β-adrenergic antagonism reduced anxiety-like behavior in socially defeated mice. CD-1 male mice were subjected to six days of repeated social defeat (SDR; filled bars) or left undisturbed in their home cage (HCC; open bars) after a s.c. injection of vehicle or propranolol prior to each cycle of defeat. Mice were assessed in the open field test the day after the last stress cycle. (A) Defeated mice spent less time in the center of the open field, propranolol prevents this behavior. (B) Defeated mice took longer to enter the center of the field than controls, propranolol blocks this behavior. (C) Distance traveled and (D) Time spent moving were not significantly different between any groups. Bars represent the mean ± SEM (n = 9). Means with different letters (a or b) are significantly different (p < 0.05) from each other.
3.3. Propranolol treatment decreased SDR induced splenomegaly
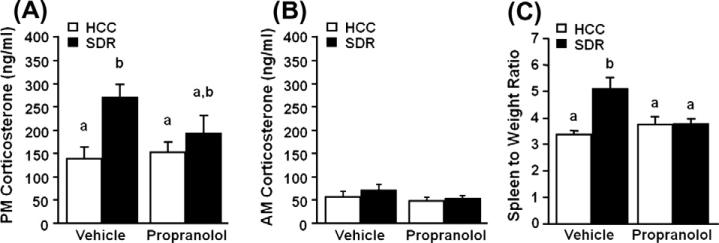
In addition to the activation of the SNS, we demonstrated that mice had significantly elevated levels of serum corticosterone immediately after the last cycle of SDR compared to control mice (main effect of stress, F(1,35) = 8.82, p = 0.005; Fig. 3A), consistent with previous data from our laboratory and others (Avitsur et al., 2001; Johnson et al., 2004). In addition, there was no significant difference between SDR propranolol-treated mice and SDR vehicle treated mice (stress × propranolol interaction, F(1,35) = 2.44, p = 0.13; Fig. 3A). Along with anxiety-like behavior and increases in serum corticosterone, SDR has been shown to increase spleen size and weight (i.e., splenomegaly) that is associated with an increased number of monocytes/macrophages (Avitsur et al., 2003). Therefore, we next aimed to determine if β-adrenergic antagonism would attenuate the SDR-induced splenomegaly. SDR increased the mean spleen weight from 115.12 mg ± 5.27 in HCC mice to 170.89 mg ± 14.99 in SDR mice (main effect of stress, F(1,35) = 5.945, p = 0.02) and this effect persisted when corrected for individual body mass (main effect of stress, F(1,35) = 8.983, p = 0.005; Fig. 3C). Furthermore, propranolol pre-treatment significantly reduced the SDR induced splenomegaly with a mean spleen mass of 120.12 mg ± 7.73 in SDR propranolol mice (stress × propranolol interaction, F(1,35) = 10.09, p = 0.003) and this effect persisted when corrected for individual body mass (stress × propranolol interaction, F(1,35) = 8.664, p = 0.006; Fig. 3C).
Fig. 3.
β-adrenergic antagonism treatment of socially defeated mice significantly altered spleen weight but not serum corticosterone. Male CD-1 mice were injected s.c. with vehicle or propranolol prior to defeat each day for 6 days. Corticosterone was measured via radioimmune assay from defeated (SDR; filled bars) and home cage control (HCC; open bars) mice in the serum obtained via retro orbital plexus (A) immediately following the last cycle of SDR (i.e., at 1900 EST) and at 18 h after the last cycle of SDR (i.e. at 0900 EST). (C) Spleen and body weight were measured the day after the last cycle of defeat and a relative spleen mass (spleen mass/body mass) was calculated. Bars represent the mean ± SEM (n = 9). Means with different letters (a or b) are significantly different (p < 0.05) from each other.
3.4. SDR induced cell activation was decreased by propranolol
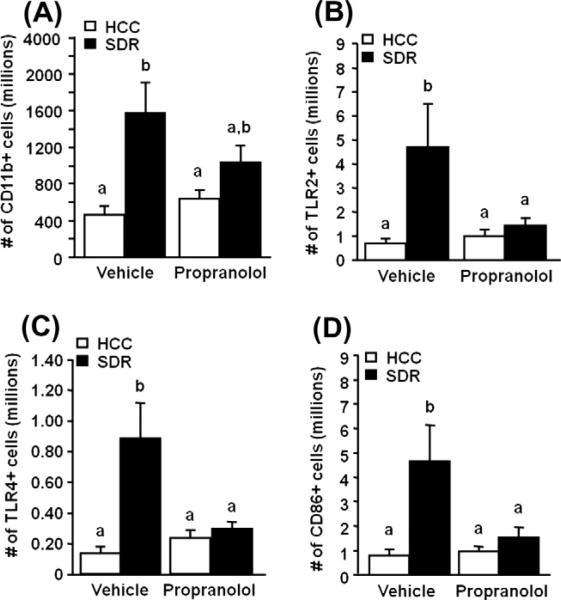
Flow cytometric analysis of total splenocytes indicated that the enlarged spleens of SDR mice had more CD11b+ macrophages compared to HCC (main effect of stress, F(1,47) = 13.23, p = 0.0007; Fig. 4A). And similar to spleen mass, propranolol treatment trended towards decreasing the stress induced increase in CD11b+ macrophages (stress × propranolol interaction, F(1,47) = 2.96, p = 0.09; Fig. 4A). The number of TLR2+ splenic CD11b+ macrophages was significantly higher from mice exposed to SDR, 4.68 × 106 ± 1.82 × 106, than in HCC mice, 6.74 × 105 ± 1.96105 (main effect of stress, F(1,47) = 6.34, p = 0.02; Fig. 4B). A similar increase was also seen in the total number of TLR4+ splenic CD11b+ macrophages (main effect of stress, F(1,47) = 9.53, p = 0.004; Fig. 4C). Furthermore, propranolol pre-treated mice showed reduced numbers of TLR2+ and TLR4+ CD11b+ cells, 1.43 × 106 (stress × propranolol interaction, F(1,47) = 4.089, p = 0.05; Fig. 4B) and 2.89 × 105 (stress × propranolol interaction, F(1,47) = 6.847, p = 0.01; Fig. 4C) respectively, when compared to SDR mice. Flow cytometric analysis also revealed that SDR significantly increased the number of CD11b+/CD86+ cells as reported previously (Bailey et al., 2007) (main effect of stress, F(1,47) = 8.22, p = 0.006; Fig. 4D) and that propranolol treatment of SDR mice significantly reduced this increase in CD86 expression (stress × propranolol interaction, F(1,47) = 4.58, p = 0.04; Fig 4D). Conversely, other markers of macrophage activation were not significantly affected by SDR. For example, the activation markers major histocompatibility complex I and II (MHCI, MHCII) and CD80 were not significantly different between CD11b+ cells from HCC or SDR-propranolol and SDR mice (data not shown).
Fig. 4.
β-adrenergic antagonism treatment of socially defeated mice significantly alters cell activation. Male CD-1 mice were injected s.c. with vehicle or propranolol prior to defeat each day for 6 days. (A) The day after the last cycle of defeat there was a significant increase in CD11b+ cells within the spleen of socially defeat (SDR; filled bars) mice compared to home cage control (HCC; open bars) mice as assessed via flow cytometry. (B, C, and D) The expression of Toll-like receptor (TLRs) 2 and 4 (TLR2 and TLR4) and CD86 on splenic CD11b+ monocytes/macrophages was increased as assessed via flow cytometry using fluorescent anti-TLR and anti-CD86 antibody staining. Bars represent the mean ± SEM (n = 9). Means with different letters (a or b) are significantly different (p < 0.05) from each other.
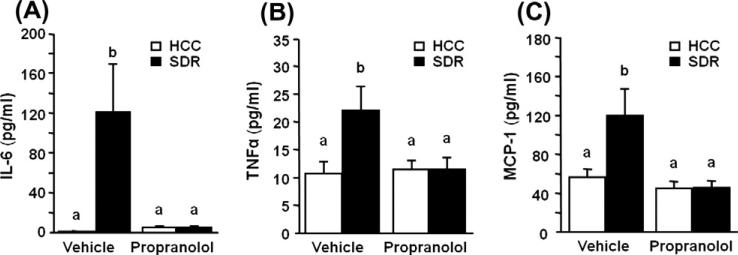
3.5. SDR induced plasma cytokines were decreased by propranolol
As seen previously, SDR altered circulating levels of IL-6 15 h after the last cycle of SDR (Meagher et al., 2007; Stark et al., 2001), with SDR mice having significantly elevated levels of plasma IL-6 (main effect of stress, F(1,35) = 6.197, p = 0.018; Fig. 5A). Blockade of the β-adrenergic receptor by propranolol pre-treatment during stress prevented this increase in circulating IL-6 (stress × propranolol interaction, F(1,35) = 6.178, p = 0.018; Fig. 5A). Similar effects were found with circulating levels of TNFα (main effect of stress, F(1,35) = 4.421, p < 0.05; Fig. 5B) and MCP-1 (main effect of stress, F(1,35) = 4.407, p < 0.05; Fig. 5C). Moreover, in propranolol pre-treated mice, TNFα levels (stress × propranolol interaction, F(1,35) = 4.421, p < 0.05; Fig. 5B) and MCP-1 levels(stress × propranolol interaction, F(1,35) = 4.243, p < 0.05; Fig. 5C) were significantly different than levels found in vehicle treated SDR mice.
Fig. 5.
β-adrenergic antagonism treatment prevented stress induced cytokines after social defeat in plasma. Male CD-1 mice were subjected to six cycles of repeated social defeat (SDR; filled bars) or left undisturbed in their home cage (HCC; open bars). Mice were injected s.c. with vehicle or propranolol prior to each cycle of defeat. The day after the last cycle of defeat (A) IL-6, (B) MCP-1, and (C) TNF-α protein levels were determined in plasma. Bars represent the mean ± SEM (n = 9). Means with different letters (a or b) are significantly different (p < 0.05) from each other.
3.6. Propranolol-treated splenocytes were less sensitive to stimulation by the mitogen LPS
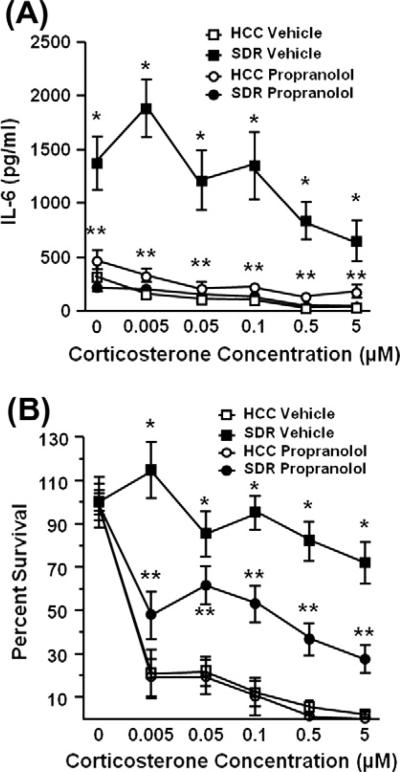
In addition to increasing the accumulation of CD11b+ cells in the spleen, SDR also increased cytokine production by total splenocytes. Cytokine secretion was significantly elevated in spleen cells from socially defeated vehicle-treated mice in comparison to cytokine production of splenocytes from non-stressed vehicle-treated control mice 18 h after LPS-stimulation (main effect of stress, F(1,29) = 11.54, p = 0.002; Fig. 6A). In cells from both non-stressed HCC mice and SDR mice, cytokine production was significantly reduced by ex vivo treatment with corticosterone but supernatants from SDR splenocytes stimulated with LPS and corticosterone contained significantly increased levels of interleukin-6, even when higher pharmacological doses of corticosterone were added to the cultures (main effect of stress × corticosterone, F(1,158) = 2.90, p = 0.016; Fig. 6A). Furthermore, splenocytes from propranolol-treated mice produced less IL-6 than splenocytes from SDR vehicle treated mice (stress × propranolol, F(1,158) = 156.16, p < 0.0001; Fig. 6A) and this effect persisted when treated with corticosterone (stress × propranolol × corticosterone, F(1,158) = 3.53, p = 0.005; Fig. 6A).
Fig. 6.
β-adrenergic antagonism prevented social defeat induced IL-6 production and attenuated social defeat-induced glucocorticoid insensitivity. Spleen cells were harvested from socially defeated (SDR) or home cage control (HCC) mice treated with vehicle or propranolol, stimulated with LPS, and 18 h later IL-6 was quantified from collected supernatants by ELISA. 48 h later GC insensitivity, defined as high cell viability in the presence of increased concentrations of corticosterone, was determined. SDR mice (closed square) demonstrate GC insensitivity compared to control groups (open square & circle). Cell viability was decreased in socially defeated mice treated with propranolol (closed circle). Lines represent the mean ± SEM (n = 9). *p < 0.05 vs. HCC vehicle treated mice; **p < 0.05 vs. SDR vehicle treated mice.
3.7. βAR blockade decreased glucocorticoid insensitivity of splenocytes from SDR mice
To determine the extent of glucocorticoid sensitivity, cell viability of spleen cells from vehicle and propranolol pre-treated mice exposed to SDR was evaluated 48 h after stimulation with LPS and increasing concentrations of corticosterone. Cell viability was significantly higher in spleen cells from mice exposed to SDR compared to the viability of splenocytes from non-stressed control mice 48 h after LPS and corticosterone stimulation (main effect of stress, F(1,14) = 25.65, p = 0.0004; Fig. 6B). Furthermore, splenocytes from propranolol-treated mice did not survive as well when compared to splenocytes to SDR-vehicle treated mice (stress × propranolol, F(1,79) = 28.16, p < 0.0001; Fig. 6B) and this effect persisted when treated with corticosterone (stress × propranolol × cort, F(1,158) = 3.53, p = 0.005; Fig. 6B).
4. Discussion
The results of the current study indicate that activation of the SNS and catecholamine release during repeated social defeat are important mediators of the stress-induced anxiety-like behavior and modulation of splenocyte responses. First, the data showed there was a significant increase in circulating and tissue catecholamines in socially-defeated mice compared to controls. Second, β-adrenergic receptor antagonism blocked the SDR induced anxiety-like behavior. Third, pretreatment with the β-adrenergic receptor antagonist propranolol attenuated the stress-induced splenomegaly, macrophage priming (number of TLR2+, TLR4+ and CD86+ cells) and plasma cytokine responses without significantly altering activation of the HPA axis by SDR. Finally, the data indicate β-adrenergic receptor blockade reduced glucocorticoid resistance and hyper-proinflammatory cytokine responses by splenocytes from SDR mice. This is the first time that adrenergic receptors have been demonstrated to mediate the glucocorticoid insensitivity and exaggerated splenic macrophage response to repeated social defeat.
In the present study, we demonstrated that SNS activation by SDR modulates behavioral changes and peripheral inflammatory and immune responses. SDR activation of the SNS results in the release of catecholamines from the adrenal medulla (plasma epinephrine and norepinephrine) as well as from sympathetic nerves within the spleen (splenic norepinephrine) as observed by the increase in circulating norepinephrine and epinephrine and the increased splenic norepinephrine. Pharmacological pre-treatment with the β-adrenergic receptor antagonist propranolol attenuated these stress-induced immune and behavioral changes characteristic of SDR. In addition to the increased sympathetic activity of mice subjected to social defeat, we also demonstrated that vehicle- and propranolol-treated mice do not exhibit significant differences in serum corticosterone immediately after the last cycle of SDR. These data suggest that the SNS response, signaling through the β-adrenergic receptor, is not significantly influencing activation of the HPA axis, indicating that the attenuation of the observed stress-induced immune and behavioral changes is in fact due to SNS activity and not due to altered HPA axis activation. Likewise, it is important to note that in all groups the level of corticosterone was significantly lower in the morning after the last cycle of SDR compared with the level of corticosterone observed immediately after the last cycle of SDR, indicating that the natural circadian rhythm of corticosterone was preserved in socially defeated mice and was not influenced by propranolol or vehicle treatment.
In addition to inducing endocrine responses, HPA axis and SNS activation, it has been described previously that exposure to SDR induces long lasting behavioral changes (Kinsey et al., 2007, 2008). During SDR resident male mice are repeatedly subjected to an aversive anxiety-inducing stimulus, inescapable attack by an aggressive intruder mouse. In order to better understand the mechanism of anxiety-like behavior during social stress, we examined the effect of SNS blockade during SDR on anxiety-like behavior. SDR resulted in an increase in anxiety-like behavior (increased latency to entering the open field, and decreased time spent in the open field) but β-adrenergic receptor blockade by propranolol inhibited the stress-induced increase in anxiety-like behavior. While other forms of social defeat or pharmacological treatment have been associated with decreased locomotion (Avgustinovich et al., 1997; Kudryavtseva et al., 2004), and if this were true for SDR or propranolol treatment, such reduced activity could bias the interpretation of the open field test for anxiety. However, data from the open field test indicated that neither SDR nor propranolol treatment had an effect on locomotion (distance traveled or time spent moving) suggesting that the effect of propranolol on SDR-induced anxiety-like behavior did not impact overall activity levels.
Previous evidence also indicates that increase in circulating levels of the proinflammatory cytokine IL-6 is associated with alterations in behavior (Zorrilla et al., 2001). Likewise, increases in circulating levels of the proinflammatory cytokines TNF-α and IL-1β have also been reported in depressed patients (Anisman et al., 1999; Maes et al., 1993). In animal models, proinflammatory cytokines induce a decrease in appetite, weight loss, sleep disturbances, and reduced interest in the physical and social environment (Kelley et al., 2003). In healthy human volunteers, endotoxin-induced endogenous cytokine production is associated with the transient development of depressed mood, anxiety, and memory impairments. Similarly the therapeutic administration of cytokines (e.g., in antiviral or cancer therapy), such as IFN-α administration results in significant neuropsychiatric side effects such as anxiety, anhedonia, fatigue, and cognitive and psychomotor slowing, which persist unless treatment is terminated (Capuron et al., 2002; Reichenberg et al., 2001). Thus it is plausible that the well-described increased inflammatory response of SDR is reinforcing the anxiety stimulus and exacerbating the anxiety-like behaviors observed during repeated social defeat. As β-AR antagonism mediated a reduction in plasma IL-6 and TNF-α, this is likely another mechanism by which propranolol treatment is attenuating anxiety-like behavior during SDR in addition to its blockade of epinephrine and norepinephrine on β-adrenergic receptors. Yet β-adrenergic receptor antagonists, such as propranolol, have previously been shown to act as biased agonist resulting in the activation of the β-arrestin-dependent p38 pathway (Samama et al., 1993; Violin and Lefkowitz, 2007). Such revelations, that propranolol may influence p38 dependent pathways which in turn can negatively or positively affect cytokine signaling by several different mechanisms (Ashwell, 2006), need to be considered when interpreting the results of this current study as well as the design of any future studies.
In addition to decreased plasma levels of IL-6 and TNF-α β-adrenergic receptor antagonism also reduced the SDR-induced plasma levels of MCP-1, a important chemokine that influences the trafficking of monocytes from the bone marrow to peripheral immune sites (Serbina et al., 2008). Correspondingly, β-adrenergic receptor antagonism also inhibited the SDR-induced splenomegaly (Avitsur et al., 2002, 2001). This effect is most likely the result of decreased myelopoiesis and trafficking of cells from the bone marrow, because studies have shown that β-adrenergic receptor signaling mediates the egress of hematopoietic stem and myeloid derived progenitor populations from the bone marrow into peripheral circulation (Katayama et al., 2006). These data taken together with previous results demonstrating that SDR results in the egress of glucocorticoid-insensitive myeloid progenitor cells from the bone marrow, indicate that the SDR induced splenomegaly results as a re-distribution of CD11b+ monocytes likely through catecholaminergic induced pathways (Curry et al., 2010; Engler et al., 2004; Wohleb et al., 2011).
Previous studies demonstrate that social defeat markedly altered the phenotype, activation status, and reactivity of myeloid derived CD11b+ cells and CD11c+ cells (e.g., monocytes, macrophages, and dendritic cells in peripheral tissues (Bailey et al., 2009a; Curry et al., 2010; Powell et al., 2009). Previously Bailey et al. (2007) have demonstrated that repeated social defeat increased the expression TLRs on splenic CD11b+ cells. Increased TLR expression is associated with a primed macrophage phenotype (Bosisio et al., 2002; Schroeder et al., 2001) and in the current study, we demonstrated that splenocytes from mice exposed to repeated social defeat had an increased number of TLR2+/CD11b+ and TLR4+/CD11b+ cells indicating that repeated social defeat primed splenic macrophages. Additionally, repeated social defeat also increased the number of CD86+/CD11b+ cells further indicative of a primed phenotype as macrophages from SDR mice would be strong antigen presenting cells and help drive a robust adaptive immune response to infection. These stressor-induced changes in cell phenotype were not present in mice treated with propranolol. Propranolol treatment also abrogated the priming effects of SDR on macrophage reactivity. A heightened reactivity of cytokines after TLR stimulation indicates priming (Schroeder et al., 2001). Splenocytes from propranolol treated mice exposed to SDR failed to produce higher levels of IL-6 when stimulated with LPS. Thus, it was evident that the stress-induced priming of splenocytes was influenced by stimulation of the β-adrenergic receptor. However, the spleens from SDR mice contained a higher portion of CD11b+ cells with more TLR receptors and therefore it was not surprising that SDR mice showed higher levels of cytokine production upon LPS stimulation of splenocytes. Similarly, one cannot conclude that regulation by βAR stimulation on a per cell basis explains this hyper-responsiveness although it was evident that SNS signaling was essential. Likewise we cannot be certain in this study that the increased cytokine levels were derived from CD11b+ cells, as no cell sorting was done, however, we previously published data showing that SDR increased the LPS-induced production of TNFα on a per cell basis using an enriched population of CD11b + cells from the spleen. Enrichment of CD11b + was accomplished using magnetic bead separation (Avitsur et al., 2005) and equal numbers of CD11b + cells from home cage control and SDR mice were cultured with LPS. Thus, it is likely that the physiologically changes induced by social stress can be explained in part by the re-distribution of leukocytes within the body in addition to alterations in the characteristic and capacity of these re-distributed cells, both which are influenced by sympathetic nervous system activation.
High concentrations of corticosterone induces apoptosis in leukocytes, however, previous studies have demonstrated that the CD11b+ cells from the spleens of SDR mice remain viable even in the presence of high doses of the GC corticosterone ex vivo. This GC insensitivity was only evident when these cells were stimulated with LPS from the Gram-negative pathogen E. coli or the Gram-positive pathogen P. gingivalis (Avitsur et al., 2003; Bailey et al., 2009b; Quan et al., 2003). Repeated exposure to the social stressor caused a significant decrease in the GC sensitivity of splenocytes from control mice whereas socially defeated mice treated with the β-adrenergic antagonist propranolol did not develop GC insensitivity to the same extent. Thus, social defeat results in enhanced cell priming and activation that was insensitive to the innate feedback control mechanisms that are typically engaged through the HPA axis to limit an inflammatory response and this insensitivity is dependent upon activation of the SNS. However, the increase in GC insensitivity might be explained by unequal distribution of immune cell subsets in the spleen, where there is a higher proportion of GC insensitive cells in the spleens of SDR mice which was prevented or altered by βAR blockade, and not necessarily due to alterations in GC sensitivity on a per cell basis. In fact, this is very likely given the ability of SDR to alter the egress and trafficking of cells from the bone marrow to the spleen (Engler et al., 2004). Regardless, the development of glucocorticoid insensitive cells suggests that the hyper-inflammatory effect of social defeat was not dependent on corticosterone signaling. We showed previously that the glucocorticoid receptor (GR) failed to translocate to the nucleus in CD11b+ GC insensitive cells from SDR mice (Quan et al., 2003). This observation suggests that another mechanism was responsible for driving the inflammatory response and that the lack of GC signaling has an indirect effect by removing a repressive influence on pro-inflammatory cytokine gene expression most likely through GR modification of NF–κB function. Ligation of TLRs induces the activation of signaling cascades that culminate in the activation of transcription factors, such as NF-κB. It is likely that exposure to SDR enhanced cytokine production by increasing TLR-driven NF-κB expression and this hypothesis is consistent with previous results that showed that NF-κB levels are higher in nuclear fractions of cells from mice exposed to SDR after stimulation with E. coli LPS (Quan et al., 2003).
The stress response is a well-characterized psychophysiological reflex that affects immunological regulation in most animal species, including humans. These stress induced effects are not inconsequential, as high stress reactivity has been linked to increased immune dysfunction. Indeed, exposure to repeated social stress before allergen inhalation enhances and prolongs airway inflammation and alters corticosterone responsiveness, which would be deleterious to anyone during an allergic airway response (Bailey et al., 2009b). Likewise repeated social defeat results in a proinflammatory state that is exacerbated in older mice, which, given the strong role of inflammation in many age-related diseases may also be deleterious to the patient (Kinsey et al., 2008). In humans, chronic stress is associated with significant changes in mood and behavior, including the development of anxiety and depressive-like symptoms (Anisman and Merali, 2002; Rosen and Schulkin, 1998). In addition to increased anxiety and depression there is also an impaired resiliency in the recovery from illness and infection (Björkqvist, 2001; Gold and Irwin, 2006). These changes are recapitulated in a murine model of social threat where repeated social defeat alters immunity and also promotes anxiety-like behavior (Bailey et al., 2007; Kinsey et al., 2007; Mays et al., 2010). This study confirms previous observations that SDR induced GC insensitivity, primed CD11b+ myeloid cells, and enhanced the development of anxiety-like behavior, and extends these findings to role of catecholamines in regulation of these responses. In conclusion, these studies showed that the activation of the SNS in response to repeated social defeat led to changes in immune cell activation, priming and glucocorticoid sensitivity. These data demonstrate the need to further understand the mechanistic effects of stress-induced immunomodulation. Such an understanding could lead to the development of new therapeutic approaches (stress management and/or pharmacological treatment) that prevents or reinstates appropriate glucocorticoid regulation of the immune response in underlying disease states or during infectious challenges.
Acknowledgments
The authors acknowledge the grateful assistance of Amy Hufnagle, Steve G. Kinsey, Jonathan P. Godbout and Tammy Kielian.
Footnotes
This work was supported by the NIH National Institute for Mental Health RO1MH046801 and the National Institute for Dental and Cranial Research T32DE014320 to J.F.S.
References
- Ader R, editor. Psychoneuroimmunology. Academic Press; San Diego: 2006. [Google Scholar]
- Anisman H, Merali Z. Cytokines, stress, and depressive illness. Brain Behav. Immun. 2002;16:513–524. doi: 10.1016/s0889-1591(02)00009-0. [DOI] [PubMed] [Google Scholar]
- Anisman H, Ravindran AV, Griffiths J, Merali Z. Endocrine and cytokine correlates of major depression and dysthymia with typical or atypical features. Mol. Psychiatry. 1999;4:182–188. doi: 10.1038/sj.mp.4000436. [DOI] [PubMed] [Google Scholar]
- Ashwell JD. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nat. Rev. Immunol. 2006;6:532–540. doi: 10.1038/nri1865. [DOI] [PubMed] [Google Scholar]
- Avgustinovich DF, Gorbach OV, Kudryavtseva NN. Comparative analysis of anxiety-like behavior in partition and plus-maze tests after agonistic interactions in mice. Physiol. Behav. 1997;61:37–43. doi: 10.1016/s0031-9384(96)00303-4. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Padgett DA, Dhabhar FS, Stark JL, Kramer KA, Engler H, Sheridan JF. Expression of glucocorticoid resistance following social stress requires a second signal. J. Leukoc. Biol. 2003;74:507–513. doi: 10.1189/jlb.0303090. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Dhabhar FS, Padgett DA, Sheridan JF. Social disruption-induced glucocorticoid resistance. kinetics and site specificity. J. Neuroimmunol. 2002;124:54–61. doi: 10.1016/s0165-5728(02)00010-3. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Sheridan JF. Social stress induces glucocorticoid resistance in subordinate animals. Horm. Behav. 2001;39:247–257. doi: 10.1006/hbeh.2001.1653. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Engler H, Powell ND, Padgett DA, Sheridan JF. Repeated social defeat increases the bactericidal activity of splenic macrophages through a Toll-like receptor-dependent pathway. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R1180–R1190. doi: 10.1152/ajpregu.00307.2007. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Engler H, Sheridan JF. Stress induces the translocation of cutaneous and gastrointestinal microflora to secondary lymphoid organs of C57BL/6 mice. J. Neuroimmunol. 2006;171:29–37. doi: 10.1016/j.jneuroim.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Kierstein S, Sharma S, Spaits M, Kinsey SG, Tliba O, Amrani Y, Sheridan JF, Panettieri RA, Haczku A. Social stress enhances allergen-induced airway inflammation in mice and inhibits corticosteroid responsiveness of cytokine production. J. Immunol. 2009a;182 doi: 10.4049/jimmunol.0800891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, Kinsey SG, Padgett DA, Sheridan JF, Leblebicioglu B. Social stress enhances IL-1beta and TNF-alpha production by Porphyromonas gingivalis lipopolysaccharide-stimulated CD11b+ cells. Physiol. Behav. 2009b;98:351–358. doi: 10.1016/j.physbeh.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkqvist K. Social defeat as a stressor in humans. Physiol. Behav. 2001;73:435–442. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- Bosisio D, Polentarutti N, Sironi M, Bernasconi S, Miyake K, Webb GR, Martin MU, Mantovani A, Muzio M. Stimulation of Toll-like receptor 4 expression in human mononuclear phagocytes by interferon-gamma: a molecular basis for priming and synergism with bacterial lipopolysaccharide. Blood. 2002;99:3427–3431. doi: 10.1182/blood.v99.9.3427. [DOI] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Regulation and dysregulation of the hypothalamic-pituitary-adrenal axis. The corticotropin-releasing hormone perspective. Endocrinol. Metab. Clin. North Am. 1992;21:833–858. [PubMed] [Google Scholar]
- Curry JM, Hanke ML, Piper MG, Bailey MT, Bringardner BD, Sheridan JF, Marsh CB. Social disruption induces lung inflammation. Brain Behav. Immun. 2010;24:394–402. doi: 10.1016/j.bbi.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve–an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Engler H, Bailey MT, Engler A, Sheridan JF. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J. Neuroimmunol. 2004;148:106–115. doi: 10.1016/j.jneuroim.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Engler H, Engler A, Bailey MT, Sheridan JF. Tissue-specific alterations in the glucocorticoid sensitivity of immune cells following repeated social defeat in mice. J. Neuroimmunol. 2005;163:110–119. doi: 10.1016/j.jneuroim.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Gold SM, Irwin MR. Depression and immunity: inflammation and depressive symptoms in multiple sclerosis. Neurol. Clin. 2006;24:507–519. doi: 10.1016/j.ncl.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Hebert MA, Evenson AR, Lumley LA, MeyerhoV JL. Effects of acute social defeat on activity in the forced swim test: parametric studies in DBA/2 mice using a novel measurement device. Aggress. Behav. 1998;24:257–269. [Google Scholar]
- Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin S, Rabah D, Kinet JP, H.S. S. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat. Neurosci. 2010;13 doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- Johnson RR, Storts R, Welsh TH, Welsh CJ, Meagher MW. Social stress alters the severity of acute Theiler's virus infection. J. Neuroimmunol. 2004;148:74–85. doi: 10.1016/j.jneuroim.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Bluthé RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain Behav. Immun. 2003;17:S112–118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Bailey MT, Sheridan JF, Padgett DA. The inflammatory response to social defeat is increased in older mice. Physiol. Behav. 2008;93:628–636. doi: 10.1016/j.physbeh.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Bailey MT, Sheridan JF, Padgett DA, Avitsur R. Repeated social defeat causes increased anxiety-like behavior and alters splenocyte function in C57BL/6 and CD-1 mice. Brain Behav. Immun. 2007;21:458–466. doi: 10.1016/j.bbi.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Wilson SP, Dzielak DJ, Yang WH, Viveros OH. Opioid peptides and noradrenaline co-exist in large dense-cored vesicles from sympathetic nerve. Neuroscience. 1982;7:2255–2261. doi: 10.1016/0306-4522(82)90135-x. [DOI] [PubMed] [Google Scholar]
- Kohut ML, Martin AE, Senchina DS, Lee W. Glucocorticoids produced during exercise may be necessary for optimal virus-induced IL-2 and cell proliferation whereas both catecholamines and glucocorticoids may be required for adequate immune defense to viral infection. Brain Behav. Immun. 2005;19:423–435. doi: 10.1016/j.bbi.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva NN, Bondar NP, Avgustinovich DF. Effects of repeated experience of aggression on the aggressive motivation and development of anxiety in male mice. Neurosci. Behav. Physiol. 2004;34:721–730. doi: 10.1023/b:neab.0000036013.11705.25. [DOI] [PubMed] [Google Scholar]
- Madden KS. Catecholamines, sympathetic innervation, and immunity. Brain Behav. Immun. 2003;17:S5–S10. doi: 10.1016/s0889-1591(02)00059-4. [DOI] [PubMed] [Google Scholar]
- Madden KS, Sanders VM, Felten DL. Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annu. Rev. Pharmacol. Toxicol. 1995;35:417–448. doi: 10.1146/annurev.pa.35.040195.002221. [DOI] [PubMed] [Google Scholar]
- Maes M, Scharpé S, Meltzer HY, Bosmans E, Suy E, Calabrese J, Cosyns P. Relationships between interleukin-6 activity, acute phase proteins, and function of the hypothalamic-pituitary-adrenal axis in severe depression. Psychiatry Res. 1993;49:11–27. doi: 10.1016/0165-1781(93)90027-e. [DOI] [PubMed] [Google Scholar]
- Mays JW, Bailey MT, Hunzeker JT, Powell ND, Papenfuss T, Karlsson EA, Padgett DA, Sheridan JF. Influenza virus-specific immunological memory is enhanced by repeated social defeat. J. Immunol. 2010;184:2014–2025. doi: 10.4049/jimmunol.0900183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher MW, Johnson RR, Young EE, Vichaya EG, Lunt S, Hardin EA, Connor MA, Welsh CJ. Interleukin-6 as a mechanism for the adverse effects of social stress on acute Theiler's virus infection. Brain Behav. Immun. 2007;8 doi: 10.1016/j.bbi.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot E, Moze E, Dantzer R, Neveu PJ. Cytokine production by spleen cells after social defeat in mice. Activation of T cells and reduced inhibition by glucocorticoids. Stress. 2004;7:55–61. doi: 10.1080/1025389042000208150. [DOI] [PubMed] [Google Scholar]
- Miura S, Kawanaka K, Kai Y, Tamura M, Goto M, Shiuchi T, Minokoshi Y, Ezaki O. An increase in murine skeletal muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) mRNA in response to exercise is mediated by beta-adrenergic receptor activation. Endocrinology. 2007;148:3441–3448. doi: 10.1210/en.2006-1646. [DOI] [PubMed] [Google Scholar]
- Powell ND, Bailey MT, Mays JW, Stiner-Jones LM, Hanke ML, Padgett DA, Sheridan J. Repeated social defeat activates dendritic cells and enhances Toll-like receptor dependent cytokine secretion. Brain Behav. Immun. 2009;23:225–231. doi: 10.1016/j.bbi.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, Avitsur R, Stark JL, He L, Lai W, Dhabhar F, Sheridan JF. Molecular mechanisms of glucocorticoid resistance in splenocytes of socially stressed male mice. J. Neuroimmunol. 2003;137:51–58. doi: 10.1016/s0165-5728(03)00042-0. [DOI] [PubMed] [Google Scholar]
- Quan N, Avitsur R, Stark JL, He L, Shah M, Caligiuri M, Padgett DA, Marucha PT, Sheridan JF. Social stress increases the susceptibility to endotoxic shock. J. Neuroimmunol. 2001;115:36–45. doi: 10.1016/s0165-5728(01)00273-9. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmächer T. Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychol. Rev. 1998;105:325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- Samama P, Cotecchia S, Costa T, Lefkowitz RJ. A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J. Biol. Chem. 1993;268:4325–4636. [PubMed] [Google Scholar]
- Schroeder S, Wichers M, Klingmuller D, Hofer M, Lehmann LE, von ST, Hering R, Putensen C, Hoeft A, Stuber F. The hypothalamicpituitary-adrenal axis of patients with severe sepsis: altered response to corticotropin-releasing hormone. Crit. Care Med. 2001;29:310–316. doi: 10.1097/00003246-200102000-00017. [DOI] [PubMed] [Google Scholar]
- Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu. Rev. Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan JF, Padgett DA, Avitsur R, Marucha PT. Experimental models of stress and wound healing. World J. Surg. 2004;28:327–330. doi: 10.1007/s00268-003-7404-y. [DOI] [PubMed] [Google Scholar]
- Stark JL, Avitsur R, Hunzeker J, Padgett DA, Sheridan JF. Interleukin-6 and the development of social disruption-induced glucocorticoid resistance. J. Neuroimmunol. 2002;124:9–15. doi: 10.1016/s0165-5728(02)00004-8. [DOI] [PubMed] [Google Scholar]
- Stark JL, Avitsur R, Padgett DA, Campbell KA, Beck FM, Sheridan JF. Social stress induces glucocorticoid resistance in macrophages. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R1799–R1805. doi: 10.1152/ajpregu.2001.280.6.R1799. [DOI] [PubMed] [Google Scholar]
- Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, Flood PM. β2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-kappaB-independent mechanisms. Cell. Signal. 2007;19:251–260. doi: 10.1016/j.cellsig.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol. Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J. Neurosci. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla E, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligma n.D.A., Schmidt K. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav. Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]