Abstract
Background
Cardiac cell therapies can yield electrical coupling of unexcitable donor cells to host cardiomyocytes with functional consequences that remain unexplored.
Methods and Results
We micropatterned cell pairs consisting of a neonatal rat ventricular myocyte (NRVM) coupled to an engineered HEK293 cell expressing either connexin-43 (Cx43 HEK) or Kir2.1 and connexin-43 (Kir2.1+Cx43 HEK). The NRVM-HEK contact length was fixed yielding a coupling strength of 68.9±9.7 nS, while HEK size was systematically varied. With increase in Cx43 HEK size, NRVM maximal diastolic potential (MDP) was reduced from −71.7±0.6 mV in single NRVMs to −35.1±1.3 mV in pairs with HEK:NRVM cell surface area ratio of 1.7±0.1, while action potential upstroke ((dVm/dt)max) and duration (APD) decreased to 1.6±0.7 % and increased to 177±32% of the single NRVM values, respectively (N=21 cell pairs). Pacemaking occurred in all NRVM-Cx43 HEK pairs with cell surface area ratios of 1.1-1.9. In contrast, NRVMs coupled with Kir2.1+Cx43 HEKs of increasing size had similar MDPs, exhibited no spontaneous activity, and showed gradual decrease in APD (N=23). Furthermore, coupling single NRVMs to a dynamic clamp model of HEK cell ionic current reproduced the cardiac MDPs and pacemaking rates recorded in cell pairs, while reproducing changes in (dVm/dt)max and APD required coupling to a HEK model that also included cell membrane capacitance.
Conclusions
Size and ionic currents of unexcitable cells electrically coupled to cardiomyocytes distinctly affect cardiac action potential shape and initiation with important implications for the safety of cardiac cell and gene therapies.
Keywords: ion channels, cardiac cell therapy, gap junctions, micropatterning, passive cell
Introduction
Unexcitable stem and somatic cells genetically engineered to express specific ion channels (e.g. HCN11, HCN22, Kv1.33, 4, or Kir2.14) have been proposed as a means to alter the cardiac electrophysiological substrate and prevent reentrant arrhythmias and pacemaking disorders. These cells exhibit a large variety of sizes with membrane capacitances ranging from <20 pF for fibroblasts to 55 pF for mesenchymal stem cells (MSCs).5, 6 Furthermore, distinct sets of endogenous ion channels in these cells yield diverse input resistances at rest ranging from 380 to 25 GΩ and resting potentials ranging from −75 to −20 mV5-9. While it is well-recognized that electrical coupling between cardiomyocytes and endogenous or exogenously added unexcitable cells can profoundly alter cardiac action potential shape and conduction, systematic studies of these interactions in vivo are hampered by the complex geometry of the heart, limited access to interacting cells, and low reproducibility of experimental conditions. Similarly, traditional in vitro systems involve co-culture of cells with random geometry, distribution, and number of homo- and heterotypic contacts rendering the reproducibility and quantification of results difficult. Many of these difficulties can be overcome with the use of cell micropatterning techniques to precisely control the size, geometry, and contact length of interacting cells.10
We previously studied impulse conduction in neonatal rat cardiac monolayers covered with different types of unexcitable cells, including human embryonic kidney (HEK293) cells engineered to express connexin-43, and found that, even at the highest coverage densities, these cells only modestly depolarized cardiomyocytes and did not induce pacemaking activity despite slowing cardiac conduction by as much as 5 times. Similarly, coupling of cardiomyocytes with fibroblasts in computer models did not cause significant cardiac depolarization or pacemaking.11-13 Other studies have, however, reported that covering cardiomyocyte monolayers at a moderate density with myofibroblasts expressing connexin-43 not only slowed cardiac conduction but also induced pacemaking activity and significant cell depolarization from −78 mV to −50 mV.14 Additionally, human MSCs, HeLa cells, and HEK293 cells transfected to express HCN2 current moderately depolarized single adult canine ventricular myocytes from −75 mV to −65 mV and still induced pacemaking activity.9 While together these studies showed that coupling of unexcitable cells to cardiomyocytes can yield diverse functional outcomes, the mechanisms by which specific properties of unexcitable cells determine these outcomes remain largely unknown.
In our previous study, large numbers of micropatterned cell pairs with reproducible shape, size, and region of cell-cell contact were used to quantify the frequency of structural coupling between a neonatal rat ventricular myocyte (NRVM) and different non-myocytes.15 In the current study we modified this in vitro assay to precisely vary the relative size of the non-myocyte vs. cardiomyocyte while keeping contact length (and thus coupling strength) between the two cells constant. We used this system to dissect the roles that unexcitable cell size, resting potential, and ionic vs. capacitive currents play in affecting cardiomyocyte action potential shape and pacemaking behavior. The results of this study shed new light on the roles of heterocellular interactions in cardiac electrophysiology with important implications for current and future cell and gene therapies.
Methods
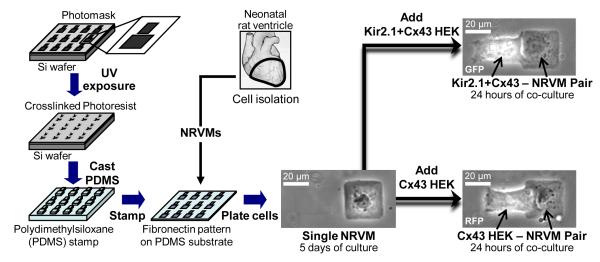
Microcontact printing of fibronectin15 was used to create large numbers of individual heterotypic cell pairs consisting of an NRVM coupled to a monoclonally-derived HEK293 cell engineered to express either connexin-43 (Cx43 HEKs) or Kir2.1 and Cx43 (Kir2.1+Cx43 HEKs) (Figure 1). The ratio of two cell surface areas in the pair was systematically varied over a wide range of values (otherwise unachievable using conventional cell culture techniques), while cell-cell contact length was kept constant. Whole-cell current or voltage clamp recordings were performed in single NRVMs or HEK293s, coupled NRVM-HEK pairs, or NRVMs connected through real-time dynamic clamp software to different HEK cell models (Online Figure I). Electrophysiological recordings were analyzed to determine the dependence of cardiac maximal diastolic potential (MDP), maximum rate of action potential rise ((dVm/dt)max), action potential duration at 80% repolarization (APD80), and pacemaking rate on the ratio of estimated HEK cell membrane surface area to NRVM membrane surface area (HEK:NRVM cell surface area ratio). An expanded Methods section is available in the online data supplement.
Figure 1.
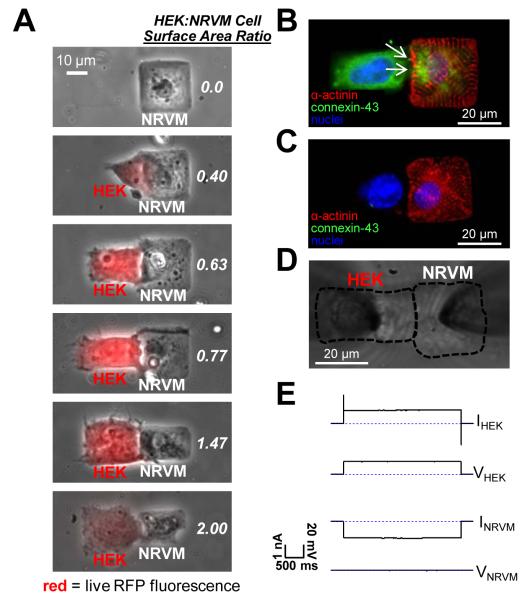
Microfabrication of heterotypic cell pairs. Microcontact printing techniques were used to stamp PDMS-coated coverslips with a large number of two-rectangle fibronectin patterns. Seeding of neonatal rat ventricular myocytes (NRVMs) at low density yielded a significant number of patterns where a single NRVM was spread on only one of the rectangles. HEK-293 cells transfected to express connexin-43 and red fluorescence protein (RFP) mCherry (Cx43 HEKs) or Kir2.1, connexin-43, and GFP (Kir2.1+Cx43 HEKs) were added after 5 days to NRVM cultures and allowed to attach and form heterotypic pairs. While micropatterned cell surface areas were varied in different pairs, cell contact length was kept constant. Electrophysiological experiments were performed after 24 hours of co-culture.
Results
Single cell electrophysiological properties
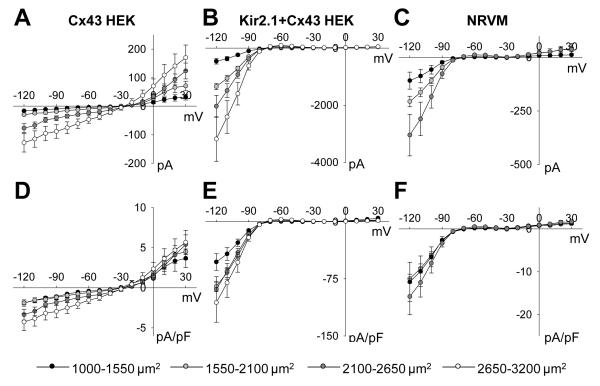
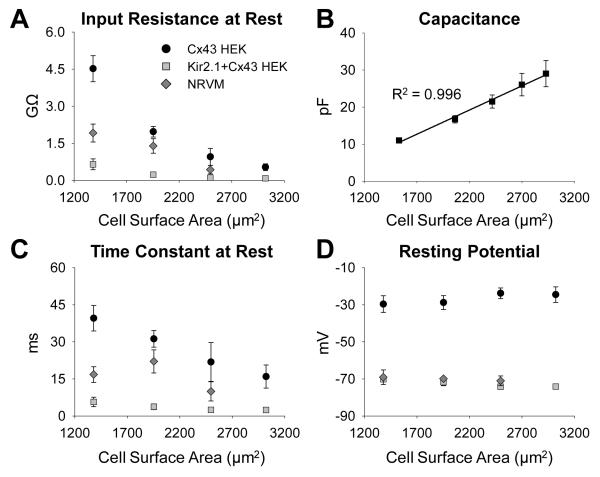
Whole-cell membrane currents were measured in single micropatterned cells with systematically varied surface area (Online Figures II and III). As cell surface area increased from 1000-1550 μm2 to 2100-2650 μm2, input resistance in NRVMs, Cx43 HEK cells, and Kir2.1+Cx43 HEK cells respectively decreased from 1.92±0.36, 4.52±0.53, and 0.649±0.22 GΩ to 0.433±0.17, 0.951±0.34, and 0.107±0.017 GΩ (Figure 2B). Simultaneously, with the increase in cell surface area, membrane capacitance of all cells increased linearly (slope 1.3 μF/cm2, Figure 2B) while resting membrane potential remained unaffected (Figure 2D) and on average was −71.7±0.6, −26.5±1.9, and −72.7±0.8 mV in NRVMs, Cx43 HEK cells, and Kir2.1+Cx43 HEK cells, respectively. As expected, inward and outward steady-state current magnitudes in different cells increased with cell surface area (Figure 3A-C). Interestingly, larger cells also exhibited larger membrane current density (Figure 3D-F). No significant difference in any electrophysiological parameter was found in single NRVMs vs. those cultured in the vicinity of HEK cells, suggesting an absence of paracrine effects of HEK cells on NRVM electrical properties, similar to our previous findings16, 17.
Figure 2.
Dependence of resting membrane properties on cell size. (A) Input resistance at rest in Cx43 HEKs (N=23 cells), Kir2.1+Cx43 HEKs (N=19 cells), and NRVMs (N=10 cells) significantly decreased with increase in cell membrane surface area. (B) Membrane capacitance of all three cell types increased linearly with cell surface area allowing data for all cells to be combined in a single plot. Different x-axis values resulted from data re-binning. (C) Membrane time constant at rest also decreased with increased cell size. (D) In all studied cell types, resting potential remained unaffected by changes in cell size.
Figure 3.
Dependence of current-voltage relationships on cell size. Steady-state current-voltage relationships were constructed from whole-cell patch clamp recordings for cells with total cell surface area between 1000-1550 μm2 (black), 1550-2100 μm2 (light grey), 2100-2650 μm2 (dark grey), and 2650-3200 μm2 (white) in (A) Cx43 HEKs (N=23 cells), (B) Kir2.1+Cx43 HEKs (N=19 cells), and (C) NRVMs (N=10 cells, no 2650-3200 μm2 bin). Normalized current per membrane capacitance in (D) Cx43 HEKs, (E) Kir2.1+Cx43 HEKs, and (F) NRVMs increased with increase in cell size. Data points are connected by straight lines for improved clarity.
Formation of heterotypic cell pairs
After optimization of NRVM and HEK seeding densities, heterotypic NRVM-HEK pairs formed on approximately 1% of available fibronectin islands. The HEK:NRVM cell surface area ratio in micropatterned cell pairs ranged from 0.4 to 2.0 (Figure 4A), with individual cell surface areas that were in the range of those studied in single micropatterned cells. A 20 μm long, 1 μm wide micropatterned gap between two rectangular fibronectin islands was used to confine the site of contact between NRVMs and HEK cells to the region of the gap, resulting in a relatively reproducible cell contact length of 19.6±1.3 μm.
Figure 4.
Control of HEK:NRVM cell surface area ratio and assessment of gap junctional coupling in cell pairs. (A) HEK:NRVM cell surface area ratio was varied from 0.0 (single NRVM) to 2.0 using cell micropatterning. HEK cells were identified by live mCherry fluorescence (red overlay). (B) Connexin-43 (green) immunostaining resulted in a punctate fluorescence pattern (white arrows) at the contact between a sarcomeric alpha-actinin positive NRVM (red) and a Cx43 HEK cell (identified by blue DAPI stained nucleus), indicating the presence of gap junctions. (C) No connexin-43 staining was observed between wild type HEK cells and NRVMs. Dual patch clamp recordings (D) revealed strong gap junction coupling (Gj=68.9±9.7 nS) between engineered HEKs and NRVMs as evidenced from a flow of large transjunctional current upon application of a voltage between the two cells (E).
Gap junction coupling in cell pairs
The ability of NRVMs and HEKs to structurally and functionally couple through gap junctions was investigated by immunostaining (Figure 4B&C) and dual whole-cell patch clamp recordings (Figure 4D&E). Connexin-43 gap junctions formed at the cell border between NRVMs and HEKs (Figure 4B) and were also internalized within both cell types, consistent with our previous studies.15 In contrast, pairs formed between NRVMs and wild type HEK cells showed no detectable connexin-43 staining (Figure 4C).16 Applying a 20 mV voltage gradient across the cell-cell contact through the HEK patch electrode created a junctional current measured through the NRVM electrode (Figure 4E). Due to a reproducible cell contact length, coupling conductance Gj of 68.9±9.7 nS was reproducibly measured in different pairs, and did not differ between NRVM-Cx43 HEK (67.1±13.0 nS) and NRVM-Kir2.1+Cx43 HEK pairs (71.3±17.6 nS). This was also expected because Kir2.1+Cx43 HEK line was derived from a monoclonal Cx43 HEK line. In the presence of gap junctional uncoupler (200 μM carbenoxolone), no fluorescence recovery after photobleaching (FRAP) occurred in the cell pairs demonstrating gap junctional origin of the measured Gj (Online Figure IV).
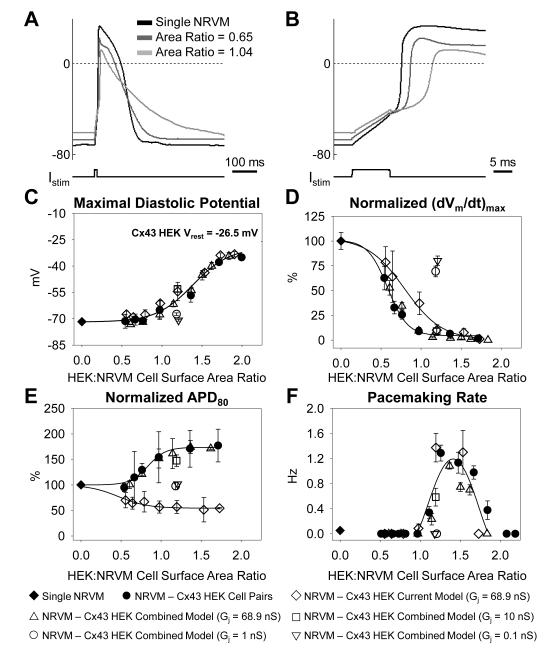
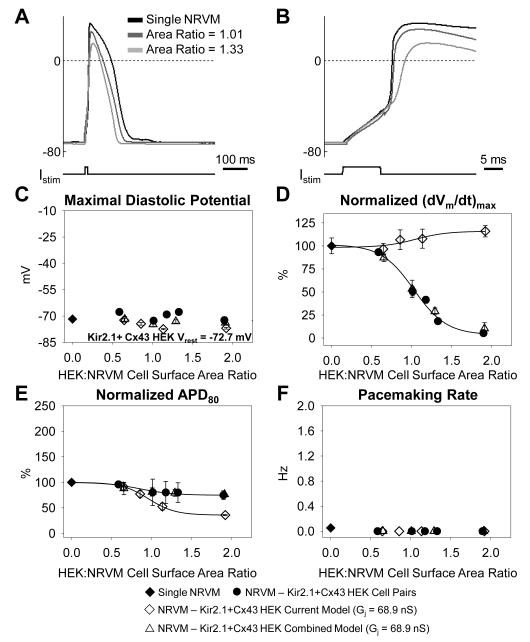
Changes in NRVM action potential shape due to coupling with a Cx43 HEK cell and dynamic clamp models
Electrical coupling of NRVMs to Cx43 HEK cells of different size yielded apparent qualitative changes in cardiac action potential shape including depolarization of resting membrane potential, decrease of action potential upstroke, and increase in action potential duration (Figure 5A&B). Quantitatively, we found that maximum diastolic potential (MDP) of NRVMs exhibited a sigmoidal dependence on HEK:NRVM cell surface area ratio, depolarizing from −71.7±0.6 mV in single NRVMs to −37.8±0.7 mV in pairs with cell surface area ratio 1.71±0.09 (Figure 5C). This dependence was fitted with a Boltzmann function with half-maximum cell surface area ratio x1/2=1.40±0.06 and slope b=0.18±0.05. When single NRVMs were connected to dynamic clamp models containing either ionic current alone or combined ionic current and capacitance of different size Cx43 HEK cells (Online Figure V), the resulting dependence of cardiac MDP on HEK:NRVM ratio (Figure 5C) and fit parameters (not shown) did not statistically differ from those measured in cell pairs.
Figure 5.
Dependence of NRVM action potential parameters on coupling to Cx43 HEK cells of different size. (A) NRVM resting potential and action potential shape in NRVM-Cx43 HEK pairs are altered as a function of relative HEK size (HEK:NRVM cell surface area ratio). (B) Close-up of the upstroke of the action potential shown in A. (C) Maximal cardiac diastolic potential, (D) normalized (dVm/dt)max, (E) normalized APD80, and (F) average pacemaking rate in NRVM–Cx43 HEK cell pairs (N=21 pairs, solid circles), single NRVMs (N=38 cells, solid diamonds), NRVMs connected to the Cx43 HEK current model (N=12 cells, diamonds), or NRVMs connected to the Cx43 HEK combined model with Gj=68.9 nS (N=7 cells, upward triangles) Gj=10 nS (N=6 cells, squares), Gj=1 nS (N=10 cells, circles), or Gj=0.1 nS (N=10 cells, downward triangles), plotted against HEK:NRVM cell surface area ratio.
Concurrent with MDP depolarization, the maximum action potential upstroke (dVm/dt)max of NRVMs decreased in a sigmoidal fashion from 138.3±10.2 V/s in single NRVMs to 2.2±1.0 V/s in NRVM-Cx43 HEK cell pairs with cell surface area ratio 1.71±0.09. The dependence of normalized (dVm/dt)max on HEK:NRVM cell surface area ratio was fitted with a Boltzmann function with x1/2=0.57±0.05 and b=-0.15±0.05 (Figure 5D). When single NRVMs were connected to dynamic clamp models containing only ionic current of Cx43 HEK cells, the sigmoidal dependence of (dVm/dt)max on HEK:NRVM cell surface area ratio was significantly right-shifted (x1/2=0.73±0.05). This shift was absent when the combined dynamic clamp models containing both ionic currents and corresponding membrane cell capacitances were connected to NRVMs (Figure 5D).
As a consequence of coupling with Cx43 HEK cells, the NRVM APD80 was significantly prolonged, increasing from 130.1±7.9 ms in single NRVMs to 230.5±24.1 ms in pairs with a HEK:NRVM cell surface area ratio of 1.71±0.09 (Figure 5E). The Boltzmann function slope parameter fit to the normalized APD80 in cell pairs (b=0.21±0.11) was significantly different from the ionic current model (b=-0.12±0.07) but not different from that found in NRVMs connected to the combined model. Other fit parameters were not significantly different between cell pairs and dynamic clamp models.
In addition to changes in action potential shape, we investigated the dependence of NRVM pacemaking rate on HEK:NRVM cell surface area ratio. From combined assessment by patch clamp measurements and video recordings, we observed pacemaking activity in only 2 of 38 (5.3%) single NRVMs compared to 25 of 50 (50%) in NRVM-Cx43 HEK cell pairs with all 25 spontaneously active pairs having HEK:NRVM cell surface area ratio between 1.08 and 1.94 (Online Figure VI). In these spontaneously active cell pairs the average pacemaking rate depended on HEK:NRVM cell surface area ratio in a biphasic manner with a peak beating rate of 1.29±0.13 Hz measured in NRVM-Cx43 HEK cell pairs with HEK:NRVM cell surface area ratio 1.26±0.04 (Figure 5F). The use of either the Cx43 HEK ionic current model or the combined model reproduced the dependence of pacemaking rate on HEK:NRVM cell surface area ratio with no significant difference in the fourth order polynomial fit parameters between the two models and cell pairs. The gap junction blocker carbenoxolone (100 μM) stopped pacemaking in 10/10 spontaneously active cell pairs with different HEK:NRVM cell surface area ratios (not shown).
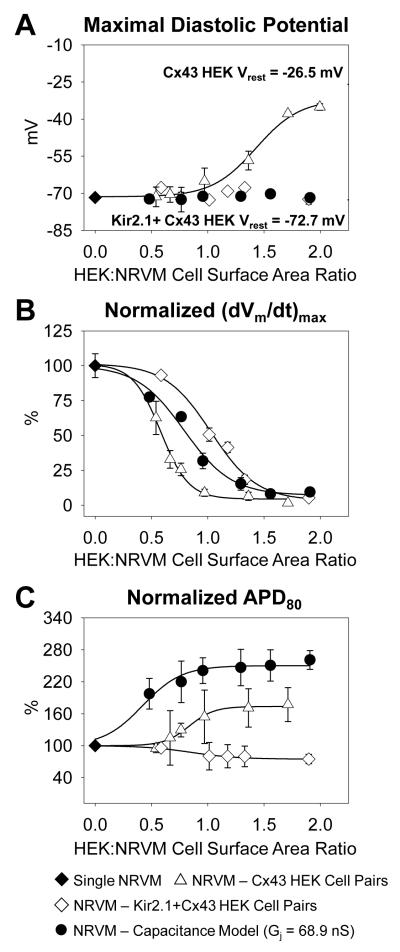
Changes in NRVM action potential shape due to coupling with Kir2.1+Cx43 HEK cells and dynamic clamp models
Next, we investigated how the NRVM action potential shape was altered due to coupling with Kir2.1+Cx43 HEK cells of different size (Figure 6A&B). MDP in NRVMs coupled to Kir2.1+Cx43 HEK cells or to corresponding dynamic clamp models (Online Figure VII) remained unchanged compared to that of single NRVMs, and equaled −70.1±0.8 mV when averaged over all studied NRVM-Kir2.1+Cx43 HEK pairs (Figure 6C). Despite no change in MDP, (dVm/dt)max of NRVMs was significantly decreased down to 7.2±1.6 V/s in pairs with HEK:NRVM cell surface area ratio 1.90±0.05. This sigmodial dependence of (dVm/dt)max on HEK:NRVM cell surface area ratio was fit with a Boltzmann function with x1/2=1.04±0.01 and b=-0.18±0.01. Interestingly, when NRVMs were coupled to Kir2.1+Cx43 HEK ionic current models, the resulting (dVm/dt)max increased with increase in HEK:NRVM cell surface area ratio. The use of the combined model was required to obtain the same dependence as measured in cell pairs (Figure 6D). Simultaneously, APD80 of NRVMs was significantly shortened with increase in HEK:NRVM cell surface area ratio, equaling to 97.3±7.6 ms in pairs with cell surface area ratio 1.90±0.05 (Figure 6E). Coupling the NRVMs with Kir2.1+Cx43 HEK ionic current models yielded a sigmodial dependence of APD80 on HEK:NRVM cell surface area ratio with Boltzmann fit parameters that significantly differed from those obtained for NRVM-Kir2.1+Cx43 HEK cell pairs (x1/2=0.92±0.03 vs. 1.31±0.13 and b=-0.16±0.03 vs. −0.39±0.12). In contrast, the use of the combined dynamic clamp models replicated the results obtained in the cell pairs. Finally, no pacemaking activity was observed in any NRVM coupled to a Kir2.1+Cx43 HEK cell or connected to either the ionic current or combined Kir2.1+Cx43 HEK cell model (Figure 6F and Online Figure VI).
Figure 6.
Dependence of NRVM action potential parameters on coupling to Kir2.1+Cx43 HEK cells of different size. (A) NRVM resting potential and action potential shape in NRVM-Kir2.1+Cx43 HEK pairs are altered as a function of relative HEK size (HEK:NRVM cell surface area ratio). (B) Close-up of the upstroke of the action potential shown in A. (C) Maximal cardiac diastolic potential, (D) normalized (dVm/dt)max, (E) normalized APD80, and (F) average pacemaking rate in NRVM–Kir2.1+Cx43 HEK cell pairs (N=23 pairs, solid circles), single NRVMs (N=38 cells, solid diamonds), NRVMs connected to the Kir2.1+Cx43 HEK current model (N=7 cells, diamonds), or NRVMs connected to the Kir2.1+Cx43 HEK combined model with Gj = 68.9 nS (N=11 cells, triangles) plotted against HEK:NRVM cell surface area ratio.
Changes in NRVM action potential shape due to coupling with dynamic clamp capacitance model
To dissect the specific roles that unexcitable cell capacitance plays in the alteration of cardiac action potential shape, NRVMs were coupled to the pure capacitance model (Online Figure I). The resulting capacitive loading significantly altered NRVM action potential shape (Online Figure VIII) without changing its MDP (Figure 7A). Despite no change in MDP, (dVm/dt)max of NRVMs decreased in a sigmoidal fashion with increase in loading capacitance corresponding to an increase in HEK:NRVM cell surface area ratio. The resulting x1/2=0.75±0.06 in this case was intermediate to and significantly different from those obtained for NRVM-Cx43 HEK and NRVM-Kir2.1+Cx43 HEK cell pairs (Figure 7B). In addition, applying capacitance models corresponding to increased HEK:NRVM cell surface area ratio significantly prolonged cardiac APD80 (Figure 7C) and yielded a sigmoidal dependence of APD80 on HEK:NRVM cell surface area ratio with significantly different fit parameters of x1/2=0.45±0.08 and b=0.12±0.05. Finally, this pure capacitive loading of NRVMs using dynamic clamp software did not induce any pacemaking activity.
Figure 7.
Dependence of NRVM action potential parameters on coupling to the capacitance model. Action potentials were recorded from NRVMs connected to the capacitance model (N=14 cells, solid circles) and were compared to action potentials recorded from NRVM–Cx43 HEK cell pairs (N=21 pairs, triangles), NRVM–Kir2.1+Cx43 HEK cell pairs (N=23 pairs, diamonds), and single NRVMs (N=38 cells, solid diamonds). (A) Maximal diastolic potential, (B) normalized (dVm/dt)max, and (C) normalized APD80 plotted against HEK:NRVM cell surface area ratio. Cell pair data (triangles and diamonds) are repeated from Figures 5 and 6 for comparison.
Discussion
In this study we utilized novel in vitro assays consisting of NRVMs coupled to real or modeled unexcitable cells to dissect the effects of unexcitable cell size and ionic currents on cardiac action potential shape and the occurrence of pacemaking activity. Specifically, we created a reproducible and well-controlled experimental setting by employing: (1) cell micropatterning techniques to systematically vary the relative sizes of HEK cells and NRVMs while keeping their coupling strength constant, (2) genetic engineering of HEK cells to enable expression of gap junctions and specific ion channels in a monoclonal fashion, and (3) dynamic clamp models of engineered HEK cells to dissect the relative roles of unexcitable cell capacitance and ionic currents in shaping the action potential of NRVMs. Using this system we showed for the first time that coupling of cardiomyocytes to unexcitable cells of increasing size (i.e. increased capacitive loading) strongly depresses cardiac action potential upstroke and prolongs action potential duration. Specific ionic currents of unexcitable cells further modulate the action potential upstroke and duration, and determine cardiac maximal diastolic potential and pacemaking activity.
Choice of unexcitable cell type
We chose HEK cells as the unexcitable cell type for the described studies because they are permissive to the stable monoclonal expression of exogenous ion channel and gap junction proteins and were able to occupy a large range of micropatterned cell surface areas. Two monoclonal cell lines were selected: 1) Cx43 HEKs because their depolarized resting potential of −26.5 mV falls in the range of those reported for bone marrow-derived MSCs6 and skeletal myoblasts18 used in cell therapies for infarct repair as well as cardiac fibroblasts8 and myofibroblasts5 that recently have been proposed to electrically couple with cardiomyocytes19, 20, and 2) Kir2.1+Cx43 HEKs because their resting membrane potential of −72.7 mV is similar to that of genetically modified cells proposed for cell therapies for tachyarrhythmias.4, 7
Dependence of HEK cell electrophysiological properties on cell size
Varying the size of individual HEK cells or NRVMs caused no significant change in their resting membrane potentials, while the cell capacitance increased linearly with patterned cell surface area with slope of 1.3 μF/cm2 that was comparable to reported specific membrane capacitance values of ~1.1 μF/cm2 for HEK cells.21 The measured values for Cx43 HEK input resistance at rest were consistent with those previously reported for unpatterned HEK cells (1–5 GΩ22). Membrane ionic currents in HEK cells increased with cell capacitance, but curiously, despite the use of monoclonal cells, this increase was non-linear, as the larger cells showed increased membrane current densities (Fig. 3D-E), similar to what has been previously reported in rabbit sinoatrial node cells23. This non-linear change in membrane currents with cell size required that the dynamic clamp models of engineered HEK cells incorporate exactly measured current-voltage relationships instead of simply injecting currents with amplitudes proportional to the modeled cell capacitance.
Relevance of strong heterocellular coupling to cardiac cell therapies
Forced expression of Cx43 in HEK cells in conjunction with controlled length of their contact with NRVMs yielded strong heterocellular gap junctional coupling with a reproducible conductance of ~69 nS that was significantly larger than the resting input conductances of micropatterned HEK cells and cardiomyocytes (0.22–19.6 nS, Figure 2A). The observed coupling strength was in the range of strong coupling values previously reported between pairs of neonatal or adult cardiac myocytes or unexcitable cells engineered to overexpress Cx43.24-27 Dynamic clamp experiments further showed that the measured electrophysiological effects for Gj=69 nS remained unchanged when Gj was reduced to 10 nS, but were significantly attenuated when Gj was further reduced to 1 nS and 0.1 nS (Figure 5C-F). This finding is in agreement with modeling studies of cardiomyocyte loading by fibroblasts which have suggested that for Gj>8 nS, the loading effects of fibroblasts on cardiac conduction velocity, APD, and (dVm/dt)max remain unchanged (saturated).11 While the exact Gj when this saturation effect occurs will depend on the unexcitable cell and cardiomyocyte input resistances, we speculate that our findings could be directly relevant for therapies that utilize unexcitable cells able to strongly couple with host cardiomyocytes, such as MSCs (Gj=9 nS9), or cells genetically engineered to overexpress connexin proteins.24
The effect of unexcitable cells on cardiac MDP
Increasing HEK cell size reduced the HEK input resistance relative to that of NRVMs yielding an MDP depolarization in NRVMs coupled to Cx43 HEK cells with a relatively positive resting potential, or no change of MDP in NRVMs coupled to Kir2.1+Cx43 HEK cells with resting potential similar to that of NRVMs. Specifically, depolarization of cardiac MDP resulted from the presence of increased inward currents (at Vm<-26.5mV) in larger Cx3 HEK cells (Figure 3A). These results were fully reproduced in dynamic clamp studies using the HEK models with ion currents alone, signifying the dominant role that steady state current-voltage (I-V) relationships in unexcitable cells strongly coupled to cardiomyocytes play in setting the cardiac MDP, not only in quiescent but also in pacemaking cells (at least for the resulting pacemaking rates with cycle lengths significantly longer than the cell membrane time constants (Figures 2C and 5F)).
The effect of unexcitable cells on cardiac action potential upstroke
On the other hand, cardiac action potential parameters that depended on relatively fast changes in Vm (i.e., (dVm/dt)max and APD80) showed significant dependence on the HEK capacitive current. Specifically, increasing the size of Cx43 HEK cells slowed the (dVm/dt)max by the additive contribution of both HEK ionic current and capacitance (Figures 5D and 7B). While HEK ionic currents contributed to upstroke slowing by increasing cardiac MDP and, as a result, inactivating sodium channels, the HEK capacitance further reduced (dVm/dt)max by diverting a fraction of the depolarizing charge away from the NRVM. In NRVMs connected to Kir2.1+Cx43 HEK cells, in which no change in cardiac MDP was observed, increased capacitive load was the only source of upstroke slowing (Figures 6D and 7B). This significant contribution of capacitive loading to decrease in (dVm/dt)max is consistent with our previous studies where a large decrease in conduction velocity in NRVM monolayers loaded with Cx43 HEK cells was accompanied by only a moderate depolarization of cardiac resting potential.16
Taken together, these results indicate that implantation of any unexcitable cells of sufficient size able to strongly couple to host cardiomyocytes, including those that match cardiac resting potential (e.g. by forced expression of potassium currents), would be expected to significantly reduce cardiac (dVm/dt)max, locally slow conduction, and potentially generate a pro-arrhythmic substrate. Genetic engineering of small unexcitable cells (that do not impose significant capacitive load) along with the use of strong promoters to express large ionic currents would give the least non-specific effects caused by endogenous membrane properties of the implanted unexcitable cells. In addition, the forced stable expression of both inward and outward currents in unexcitable cells (as done in our recent study24) will be needed to prevent negative effects on action potential upstroke and permit safe and efficient cell therapies.
The effect of unexcitable cells on cardiac action potential duration
Increase in unexcitable cell capacitance not only yielded slowing of action potential upstroke, but also significantly prolonged cardiac action potential repolarization (Figure 7C) because the discharge of HEK and NRVM capacitances took longer than the discharge of NRVM capacitance alone. In the cell pairs, the APD increase due to pure capacitive loading of NRVMs was opposed by the flow of outward currents at Vm>-26.5 mV in Cx43 HEKs (Figure 3A and 5E), aiding early cardiac repolarization (Figure 5A), or at Vm>-72.7 mV in Kir2.1+Cx43 HEK cells (Figure 3B), aiding both early and late repolarization (Figure 6A). Collectively, these results show that the net APD prolongation or shortening in cardiomyocytes coupled to unexcitable cells will be determined by the specific unexcitable cell current-voltage relationship while unexcitable cell capacitance (size) will only act to prolong the APD. Consistent with previous modeling studies of fibroblasts coupled to cardiomyocytes11, 12 and dynamic clamp studies of a cardiomyocyte loaded by an RC circuit with battery28, the unexcitable cells with hyperpolarized resting potential (relative to that of NRVMs) shortened cardiac APD while those with relatively depolarized resting potential prolonged cardiac APD. Beside the resting potential, our studies establish unexcitable cell size (capacitance) and expression of specific ion currents as additional independent and potent modulators of cardiac APD.
The effect of unexcitable cells on cardiac pacemaking
Spontaneous activity in NRVMs coupled to Cx43 HEK cells was induced for a range of HEK cell sizes (HEK:NRVM cell surface area ratio of 1.08-1.94) with pacemaking rates that depended in a biphasic fashion on the amplitude of HEK ion currents and did not depend on HEK cell capacitance (Figure 5F). Specifically, increased inward currents in larger HEK cells (for Vm<−26.5 mV, Figure 3A) yielded NRVM depolarization which decreased the threshold for activation of inward currents (first sodium and then L-type calcium) to promote pacemaking, but also inactivated these currents to ultimately prevent any excitation. Significantly, pacemaking in NRVM-Cx43 HEK pairs was induced and modulated solely by the relative difference in the sizes of the two cells (and endogenous HEK currents) without a need for the additional expression of any exogenous pacemaker currents in HEK cells. Conceivably, this pacemaking activity was driven by the inflow of current from Cx43 HEK cells into NRVMs through gap junction channels29 and may have been additionally contributed by the activity of the cardiac sodium-calcium exchanger, similar to modeling studies in cardiomyocytes with reduced IK1.30
Limitations
Cells used for cardiac therapies, such as MSCs, often have larger sizes compared to HEK cells (55.2 pF6 capacitance in MSCs compared to the maximum HEK capacitance studied here of 31.0 pF). However, size and IK1 expression in adult cardiomyocytes are also larger than in the neonatal myocytes studied here31, so the net loading effects in adult myocardium may be attenuated compared to those found in this study. In the adult setting, the most readily observed effect of coupling to unexcitable cells is expected to be the decrease in the cardiac (dVm/dt)max since this change occurred at the smallest HEK:NRVM cell surface area ratios and independently of the unexcitable cell MDP. Decreased (dVm/dt)max would be further expected to yield conduction slowing with potential for arrhythmogenic outcomes.
In addition to findings from our simplified in vitro assay, the electrophysiological effects of host-donor coupling in situ will also depend on other factors including the number of unexcitable cells coupled per myocyte, spatial distribution of this coupling, and position of engrafted unexcitable cells relative to the direction of propagation. Different tissue engineering strategies10 may be used in the future to systematically study functional roles of these more complex heterocellular interactions and design safer and more efficient cell and gene therapies.
Supplementary Material
Recent studies have suggested the application of genetically engineered unexcitable stem and somatic cells for the treatment of cardiac arrhythmias. The potential clinical safety and effectiveness of this approach are yet to be explored, but initial results in animal models have been encouraging. The cell types proposed for these therapies exhibit a wide range of passive electrical properties in terms of membrane capacitance, input resistance, resting potential, and cell-cell coupling strength. While it is well recognized that electrical coupling between cardiomyocytes and unexcitable cells can alter cardiomyocyte electrophysiological properties, traditional in vitro and in vivo systems lack sufficient flexibility and reproducibility to allow systematic and quantitative studies of these phenomena. The current study applies cell micropatterning and genetic engineering techniques to independently control geometry, electrical properties, and coupling strength of cardiomyocytes and unexcitable cells in individual cell pairs. Studies in these cell pairs reveal that electrical coupling between cardiomyocytes and unexcitable cells of increasing size strongly depresses cardiac action potential upstroke and prolongs action potential duration, while specific ionic currents of unexcitable cells further modulate the action potential upstroke and duration and determine cardiac maximal diastolic potential and pacemaking activity. Furthermore, cardiac action potential upstroke is found to be the most sensitive to coupling of cardiomyocytes with large unexcitable cells. The implantation of small, well-coupled unexcitable cells engineered to express large ionic currents is expected to yield safest and most effective cardiac therapies.
Acknowledgments
R. Kirkton for assistance with plasmid construction, cell transfection, and FRAP analysis; D. Christini and RTXI (www.rtxi.org) for dynamic clamp software; and A. Krol for NRVM isolation.
Funding Sources: American Heart Association Predoctoral Fellowship to L.C.M. and NIH-NHLBI grants HL104326 and HL106203 to N.B.
Footnotes
Conflict of Interest Disclosures: None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cho HC, Kashiwakura Y, Marban E. Creation of a biological pacemaker by cell fusion. Circ Res. 2007;100:1112–1115. doi: 10.1161/01.RES.0000265845.04439.78. [DOI] [PubMed] [Google Scholar]
- 2.Plotnikov AN, Shlapakova I, Szabolcs MJ, Danilo P, Jr., Lorell BH, Potapova IA, Lu Z, Rosen AB, Mathias RT, Brink PR, Robinson RB, Cohen IS, Rosen MR. Xenografted adult human mesenchymal stem cells provide a platform for sustained biological pacemaker function in canine heart. Circulation. 2007;116:706–713. doi: 10.1161/CIRCULATIONAHA.107.703231. [DOI] [PubMed] [Google Scholar]
- 3.Feld Y, Melamed-Frank M, Kehat I, Tal D, Marom S, Gepstein L. Electrophysiological modulation of cardiomyocytic tissue by transfected fibroblasts expressing potassium channels: A novel strategy to manipulate excitability. Circulation. 2002;105:522–529. doi: 10.1161/hc0402.102661. [DOI] [PubMed] [Google Scholar]
- 4.Yankelson L, Feld Y, Bressler-Stramer T, Itzhaki I, Huber I, Gepstein A, Aronson D, Marom S, Gepstein L. Cell therapy for modification of the myocardial electrophysiological substrate. Circulation. 2008;117:720–731. doi: 10.1161/CIRCULATIONAHA.106.671776. [DOI] [PubMed] [Google Scholar]
- 5.Chilton L, Ohya S, Freed D, George E, Drobic V, Shibukawa Y, Maccannell KA, Imaizumi Y, Clark RB, Dixon IM, Giles WR. K+ currents regulate the resting membrane potential, proliferation, and contractile responses in ventricular fibroblasts and myofibroblasts. Am J Physiol Heart Circ Physiol. 2005;288:H2931–2939. doi: 10.1152/ajpheart.01220.2004. [DOI] [PubMed] [Google Scholar]
- 6.Heubach JF, Graf EM, Leutheuser J, Bock M, Balana B, Zahanich I, Christ T, Boxberger S, Wettwer E, Ravens U. Electrophysiological properties of human mesenchymal stem cells. J Physiol. 2004;554:659–672. doi: 10.1113/jphysiol.2003.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Boer TP, van Veen TA, Houtman MJ, Jansen JA, van Amersfoorth SC, Doevendans PA, Vos MA, van der Heyden MA. Inhibition of cardiomyocyte automaticity by electrotonic application of inward rectifier current from kir2.1 expressing cells. Med Biol Eng Comput. 2006;44:537–542. doi: 10.1007/s11517-006-0059-8. [DOI] [PubMed] [Google Scholar]
- 8.Rook MB, van Ginneken AC, de Jonge B, el Aoumari A, Gros D, Jongsma HJ. Differences in gap junction channels between cardiac myocytes, fibroblasts, and heterologous pairs. Am J Physiol. 1992;263:C959–977. doi: 10.1152/ajpcell.1992.263.5.C959. [DOI] [PubMed] [Google Scholar]
- 9.Valiunas V, Kanaporis G, Valiuniene L, Gordon C, Wang HZ, Li L, Robinson RB, Rosen MR, Cohen IS, Brink PR. Coupling an hcn2-expressing cell to a myocyte creates a two-cell pacing unit. J Physiol. 2009;587:5211–5226. doi: 10.1113/jphysiol.2009.180505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bursac N, Kirkton RD, McSpadden LC, Liau B. Characterizing functional stem cell-cardiomyocyte interactions. Regen Med. 2010;5:87–105. doi: 10.2217/rme.09.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacquemet V, Henriquez CS. Loading effect of fibroblast-myocyte coupling on resting potential, impulse propagation, and repolarization: Insights from a microstructure model. Am J Physiol Heart Circ Physiol. 2008;294:H2040–2052. doi: 10.1152/ajpheart.01298.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maccannell KA, Bazzazi H, Chilton L, Shibukawa Y, Clark RB, Giles WR. A mathematical model of electrotonic interactions between ventricular myocytes and fibroblasts. Biophys J. 2007;92:4121–4132. doi: 10.1529/biophysj.106.101410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sachse FB, Moreno AP, Abildskov JA. Electrophysiological modeling of fibroblasts and their interaction with myocytes. Ann Biomed Eng. 2008;36:41–56. doi: 10.1007/s10439-007-9405-8. [DOI] [PubMed] [Google Scholar]
- 14.Miragoli M, Gaudesius G, Rohr S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ Res. 2006;98:801–810. doi: 10.1161/01.RES.0000214537.44195.a3. [DOI] [PubMed] [Google Scholar]
- 15.Pedrotty DM, Klinger RY, Badie N, Hinds S, Kardashian A, Bursac N. Structural coupling of cardiomyocytes and noncardiomyocytes: Quantitative comparisons using a novel micropatterned cell pair assay. Am J Physiol Heart Circ Physiol. 2008;295:H390–400. doi: 10.1152/ajpheart.91531.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McSpadden LC, Kirkton RD, Bursac N. Electrotonic loading of anisotropic cardiac monolayers by unexcitable cells depends on connexin type and expression level. Am J Physiol Cell Physiol. 2009;297:C339–351. doi: 10.1152/ajpcell.00024.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedrotty DM, Klinger RY, Kirkton RD, Bursac N. Cardiac fibroblast paracrine factors alter impulse conduction and ion channel expression of neonatal rat cardiomyocytes. Cardiovasc Res. 2009;83:688–697. doi: 10.1093/cvr/cvp164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernheim L, Liu JH, Hamann M, Haenggeli CA, Fischer-Lougheed J, Bader CR. Contribution of a non-inactivating potassium current to the resting membrane potential of fusion-competent human myoblasts. J Physiol. 1996;493:129–141. doi: 10.1113/jphysiol.1996.sp021369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rohr S. Myofibroblasts in diseased hearts: New players in cardiac arrhythmias? Heart Rhythm. 2009;6:848–856. doi: 10.1016/j.hrthm.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 20.Kohl P, Camelliti P, Burton FL, Smith GL. Electrical coupling of fibroblasts and myocytes: Relevance for cardiac propagation. J Electrocardiol. 2005;38(Suppl):45–50. doi: 10.1016/j.jelectrocard.2005.06.096. [DOI] [PubMed] [Google Scholar]
- 21.Gentet LJ, Stuart GJ, Clements JD. Direct measurement of specific membrane capacitance in neurons. Biophys J. 2000;79:314–320. doi: 10.1016/S0006-3495(00)76293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmermann D, Terpitz U, Zhou A, Reuss R, Muller K, Sukhorukov VL, Gessner P, Nagel G, Zimmermann U, Bamberg E. Biophysical characterisation of electrofused giant hek293-cells as a novel electrophysiological expression system. Biochem Biophys Res Commun. 2006;348:673–681. doi: 10.1016/j.bbrc.2006.07.112. [DOI] [PubMed] [Google Scholar]
- 23.Boyett MR, Honjo H, Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc Res. 2000;47:658–687. doi: 10.1016/s0008-6363(00)00135-8. [DOI] [PubMed] [Google Scholar]
- 24.Kirkton RD, Bursac N. Engineering biosynthetic excitable tissues from unexcitable cells for electrophysiological and cell therapy studies. Nat Commun. 2011;2:300. doi: 10.1038/ncomms1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. Direct association of the gap junction protein connexin-43 with zo-1 in cardiac myocytes. J Biol Chem. 1998;273:12725–12731. doi: 10.1074/jbc.273.21.12725. [DOI] [PubMed] [Google Scholar]
- 26.Yao JA, Hussain W, Patel P, Peters NS, Boyden PA, Wit AL. Remodeling of gap junctional channel function in epicardial border zone of healing canine infarcts. Circ Res. 2003;92:437–443. doi: 10.1161/01.RES.0000059301.81035.06. [DOI] [PubMed] [Google Scholar]
- 27.Desplantez T, McCain ML, Beauchamp P, Rigoli G, Rothen-Rutishauser B, Parker KK, Kleber AG. Connexin43 ablation in foetal atrial myocytes decreases electrical coupling, partner connexins, and sodium current. Cardiovasc Res. 2012;94:58–65. doi: 10.1093/cvr/cvs025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan RC, Joyner RW. Electrotonic influences on action potentials from isolated ventricular cells. Circ Res. 1990;67:1071–1081. doi: 10.1161/01.res.67.5.1071. [DOI] [PubMed] [Google Scholar]
- 29.Miragoli M, Salvarani N, Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res. 2007;101:755–758. doi: 10.1161/CIRCRESAHA.107.160549. [DOI] [PubMed] [Google Scholar]
- 30.Silva J, Rudy Y. Mechanism of pacemaking in i(k1)-downregulated myocytes. Circ Res. 2003;92:261–263. doi: 10.1161/01.RES.0000057996.20414.C6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wahler GM. Developmental increases in the inwardly rectifying potassium current of rat ventricular myocytes. Am J Physiol. 1992;262:C1266–1272. doi: 10.1152/ajpcell.1992.262.5.C1266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.