Abstract
Background
The current guidelines for treatment of high-risk of lipid disorders do not specify a therapeutic target level of HDL-C for prevention of vascular disease in high-risk populations. However, there is a substantial body of evidence from basic science and epidemiologic studies and from clinical trials, providing the strong, consistent message that raising HDL-C by therapeutic means will effectively and independently reduce cardiovascular risk.
Sources
This review summarizes epidemiologic evidence and the results of a meta-analysis of 23 published, prospective, randomized, placebo-controlled clinical trials. It focuses on the effects of lipid therapies on coronary stenosis progression, as measured by quantitative arteriography and/or, on clinical cardiovascular endpoints.
Findings
Among the 7 drug/treatment classes into which individual study results were categorized and averaged, reduction in stenosis progression and reduction in clinical events are both very highly correlated with the composite lipid variable (%ΔHDL-C - %ΔLDL-C; where %Δ is percent change relative to the placebo group response). This holds true for all lipid drug classes or combinations of lipid drug therapy, with the exception of the unexpectedly anomalous effects of the torcetrapib-atorvastatin combination.
Conclusions
There is a strong and consistent body of evidence that therapeutic HDL-C-raising is at least as effective as comparable percentages of LDL-C-lowering for reduction of atherosclerosis progression or clinical cardiovascular events overa broad range of risk levels. Adoption of this strategy into guidelines probably awaits results of at least one large controlled HDL-C-raising clinical trial, of which two are ongoing and one other is planned.
Keywords: HDL-cholesterol, atherosclerosis, fenofibrate, niacin, torcetrapib, heart disease prevention
The Question: Is Targeted HDL-C-raising Beneficial?
A commonly-voiced, or implied, opinion(1) is that current evidence does not sufficiently justify a recommendation to selectively increase HDL-C above a given level by any of the known pharmacologic approaches. This voice of caution has recently been amplified by the demonstration of a substantial adverse effect of torcetrapib(2), a CETP inhibitor that raises HDL-C by 35–45% in its current dosage, and more substantially raises the proportion of cholesteryl ester in the larger HDL particles. The presently available information is that torcetrapib is linked to a 61% increase (p < 0.001) in total mortality in a large, now-terminated, trial designed to compare atorvastatin with combined torcetrapib and atorvastatin in terms of clinical outcomes. Analyses of possible mechanisms explaining this unexpected finding are under way.
Prior to this finding, the general consensus, based on epidemiologic data and sometimes-small early clinical trials, was that HDL-C-raising is almost certainly beneficial but probably less so than LDL-C-lowering; nevertheless, a large confirming trial is needed. The torcetrapib finding, at the worst, raises the question whether all forms of HDL-C-raising are detrimental and, at the least, changes the above consensus to: HDL-C-raising is most likely to be beneficial, but can be harmful, depending on the means by which it is achieved, or whether there is any lipid-independent toxicity associated with the treatment. What follows is an examination of published evidence supporting the premise of benefit from each of the individual classes of HDL-C-raising lipid therapy.
Goals of this Review
This review summarizes existing epidemiologic and trial evidence addressing the magnitude of clinical and arteriographic benefit from HDL-raising and LDL-C-lowering therapies that, in aggregate, demonstrates the clear benefits of HDL-C-raising as well as LDL-C-lowering therapies. In part, it draws on the findings and conclusions of a recent publication on this subject (3). It then interprets the torcetrapib-atorvastatin findings from the perspective of this large body of data.
HDL-C-raising: Epidemiologic Predictions and Arteriographic Evidence
Epidemiologic evidence that LDL-C and HDL-C levels are comparably important as predictors of cardiovascular disease events or of other surrogate estimates has emerged from observation of healthy populations. Wilson, et al (4), using Framingham data, assert: “to simplify, a 1% [population] difference in HDL-C, for each sex, or a 1% difference in LDL-C implies a 1% difference in CHD [incidence] over the follow-up interval”. The PROCAM study has nearly-identical findings (5). Indeed, among the statin clinical trials, each 1% reduction in LDL-C has been associated with a nearly 1% reduction in risk, over a wide range of baseline LDL-C levels, clinical risk, and therapeutic LDL-C responses (6,7). In examining HDL-C effects, Gordon, et al (8) found that a 1.0 mg/dl (0.03 mmol/L) greater HDL-C level is associated with an LDL-C-independent 2–4% (median 2.9%) reduced risk of cardiovascular events over the 7-to-10 year follow-up periods in four examined North American populations that totaled nearly 25,000 untreated subjects. Thus, in a subject with baseline HDL-C of 45 mg/dl, a change of one mg/dl (2.2%) would predict a 2.9% risk change (i.e. 1.3% risk reduction for each 1% HDL increase, or vice-versa). That LDL-C and HDL-C levels are statistically independent and comparably important determinants of cardiovascular risk suggests that simultaneous therapeutic changes in each of these variables will result in roughly additive effects on risk reduction. Among patients with established coronary disease, the same principles apply; LDL-C and HDL-C are statistically independent and comparably important risk predictors, although absolute risk is approximately10-fold greater in these patients than in normal subjects, at any given lipoprotein level (9).
Similarly, the progression of coronary stenosis, as seen with angiographic follow-up, has been reduced significantly and independently by comparable percentages of LDL-C-lowering and HDL-C-increase (10, 11), with the influence of HDL-C being somewhat (1.3 times) greater (10).
Meta-analysis: Trial Selection and Analytical Approach
Lipid-altering therapy, in various forms, has been evaluated in many trials whose primary endpoints include either pre-defined clinical event composites or estimates of change in coronary artery stenosis, as measured from serial coronary arteriography. A recently reported meta-analysis (3) has combined evidence from trials that are each randomized, placebo-controlled assessments of lipid therapy benefit for patients with clinically diagnosed native coronary disease or with lipid abnormalities at very high clinical risk. The details of most of these trials have been summarized in a recent publication (12) in terms of their populations, therapies, and primary clinical or arteriographic outcomes; a few more recent trials fitting the above requirements have also been included (13–19). We have excluded two trials (20, 21) in which the control group was an active comparator, not placebo, or for which the presenting diagnosis was hypertension (22), or in which the stenosis change was not directly measured (23). Trials in which the qualifying diagnosis was acute coronary syndrome were excluded, as well as those that were non-pharmacologic studies or in which more than 20% of the population was post-coronary bypass (23). Twenty-three trials met these criteria. In three of these, the principal active therapies were fibric acid derivatives. In one trial each, niacin mono-therapy, or bile acid binding resins, or partial ileal bypass surgery, or statin-resin combination therapy were tested against placebo. Statin mono-therapy was used in 11 trials; combinations of niacin with various LDL-C-lowering agents were used in five. Twelve of these trials were large (838 - 20,536 patients) and long (4.9 – 9.7 years) and designed to show reduction in a pre-specified clinical endpoint. Change with therapy in clinical endpoint frequency was available in all of the 12 trials classified as “clinical” and all but one of the 11 trials classified as “QCA” (quantitative coronary arteriography).
The included trials are specified below by type (QCA or Clinical), drug class, acronym, literature reference, number enrolled, and entry diagnosis.
QCA Trials
Fibric acid derivatives
DAIS (14): 418 patients (pts) with type 2 diabetes and visible coronary stenosis.
Statin monotherapy
PLAC-I (12): 408 pts with clinically-manifest coronary disease (CAD). MARS (12): 280 pts with CAD. MAAS (12): 381 pts with CAD. LCAS (18): 429 pts with CAD. REGRESS (12): 855 men with CAD. CCAIT (12): 331 pts with CAD.
Statin plus resin combination
FATS (L+C) (12): 96 men with CAD and hyper-apoB.
Niacin combined with LDL-C-lowering agents
FATS (N+C) (12): 100 men with CAD and hyper-apoB. HATS (12): 160 pts with coronary disease and low HDL-C (≤35 mg/dl, men; ≤45, women). UC-SCOR (12): 98 asymptomatic subjects with heterozygous Familial Hypercholesterolemia. AFREGS (15): 148 patients with CAD.
Clinical Endpoint Trials
Fibric acid derivatives
VA-HIT (13): 2531 men with CAD. FIELD (16): 9795 patients with type 2 diabetes.
Niacin monotherapy
CDP(17): 3908 pts with prior myocardial infarction (MI).
Bile acid binding resins
LRC-CPPT (12): 3806 asymptomatic men with high LDL-C, averaging 216 mg/dl.
Partial ileal bypass
POSCH (12): 838 pts with prior MI.
Statin monotherapy
CARE (12): 4159 pts with prior MI. LIPID (12): 9014 patients with CAD. 4S (12): 4444 patients with angina or prior MI and high LDL-C. HPS (7): 20,536 high risk patients (CAD, peripheral vascular disease, type 2 diabetes). WOSCOPS (19): 6595 asymptomatic men with hypercholesterolemia.
Niacin combined with LDL-C-lowering agents
FATS-TT10 (12) 176 men with CAD treated with triple-therapy (niacin, lovastatin, and colestipol for 10 years.
In this analysis, percent changes (%Δ), per-patient, in LDL- and HDL-cholesterol (%ΔLDL-C and %ΔHDL-C) were averaged within each treatment group (active and placebo) for each of the above trials. The difference between active treatment and placebo for each of these two variables were computed for each trial. These two placebo-adjusted lipid change variables, and their differences, (%ΔHDL-C - %ΔLDL-C), were also averaged within each of the seven different drug class/treatment categories, and were examined as determinants of the two outcome variables, % change in frequency of primary clinical event composite (%Δ 1ry Event Rate), or per-patient mean change in percent diameter stenosis (Δ%S) determined over the course of each trial.
Results of the Meta-analyses
Table 1 provides treatment class averages for placebo-adjusted changes in LDL- C, HDL-C, and their difference (HDL-C – LDL-C), as well as averaged changes from baseline in mean proximal stenosis severity (Δ%S) for the QCA studies, and percent reduction in the pre-defined primary clinical endpoint for all trials in which clinical outcomes were provided. In these analyses (3), the lipid, clinical, and arteriographic responses within each drug class were quite consistent, having small standard deviations.
Table 1.
Trial-averaged lipid, stenosis, and clinical outcomes by class of therapy and trial type.
| Drug/treatment Class (total number of patients) | Study Type (Number of studies) | %Δ HDL-C in-Rx | %Δ LDL-C in-Rx | %Δ HDL- %Δ LDL* | Δ Mean %S in- Rx | % Event Red’n# |
|---|---|---|---|---|---|---|
| Placebo** (1350) | QCA (6) | 0 | 0 | 0 | 3.0±0.4 | 0 |
| Fibrates (481) (12,744) |
QCA (1) | 6 | −7 | 13 | 2.1 | --- |
| Clinical (3) | 4±1 | −6±5 | 10±6 | --- | 19±6 | |
| Niacin (3908) | Clinical (1) | 18” | −7” | 25” | --- | 26 |
| Resins (3806) | Clinical (1) | 3 | −16 | 19 | --- | 19 |
| Ileal Bypass (838) | Clinical (1) | 4 | −38 | 42 | --- | 36 |
| Statins (2703) (47,462) |
QCA (6) | 7±2 | −29±5 | 36±7 | 1.4±0.5 | --- |
| Clinical (11) | 6±2 | −29±4 | 35±6 | --- | 30±10 | |
| Statin+Resin (96) | QCA (1) | 10 | −44 | 54 | −0.7 %S (reg’n) | 66 |
| Niacin Combinations (518) (693) |
QCA (4) | 28±7 | −31±4 | 59±3 | − 0.9±0.4 %S regn | --- |
| Clinical (5) | 28±7 | −31±4 | 59±3 | --- | 66±8 |
Sum of % reduction in LDL-C and % increase in HDL-C from baseline (eg. 30% ↓LDL & 30%↑HDL = 60%). Placebo lipid effects are subtracted in all studies.
Results from 6 placebo groups in statin monotherapy angio trials.
“ Estimated from dose.
Event rate reduction in each trial relative to corresponding placebo group.
Arteriographic Findings
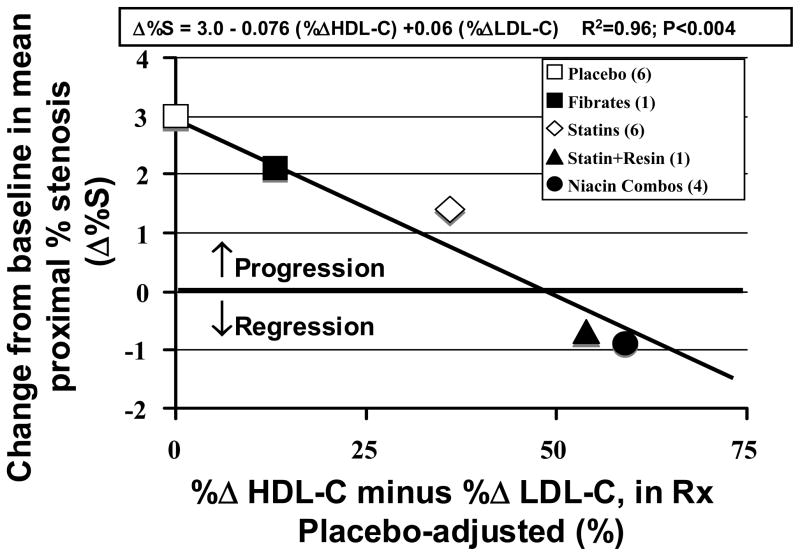
As seen in Figure 1, the mean proximal coronary stenosis severity progressed about +3%S in the six statin placebo groups over the typical 2.5–3 year QCA trials. Progression rate was slowed slightly by fenofibrate, was slowed by about half with statins, and there was a small amount of actual stenosis regression, of the order of -0.4 to -0.9 %S, with the one statin-resin and the four niacin combination trials. Stenosis change, averaged within each drug class, was surprisingly well correlated with the placebo-adjusted variable (%ΔHDL-C - %ΔLDL-C). For the multivariate linear regression model entering both LDL-C and HDL-C, R2 was 0.96.
Figure 1.
Effect of various drug classes on coronary stenosis progression, or regression, as related to combined in-treatment changes in HDL-C and LDL-C.
Clinical event reduction
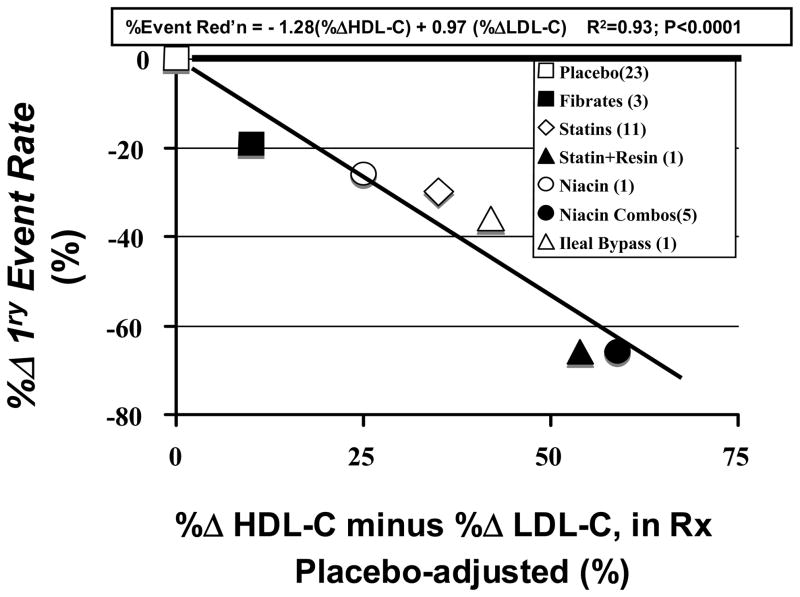
In a plot (not shown), of the %ΔLDL-C variable against the percent reduction in the pre-defined primary clinical event composite, averaged over the 7 different drug/treatment classes, there was a good correlation between %ΔLDL-C and event reduction for drug classes in which the principal lipid effect was LDL-C-lowering (statins, resins, partial ileal bypass). But drug classes with mixed LDL-C and HDL-C effects (fibrates, niacin, statin-resin, and niacin combinations) fell well below the line of identity (1% LDL-C-lowering equals 1% risk reduction); those trials actually had greater event reduction than predicted by %ΔLDL-C. Overall, the R2 for the relationship between event reduction vs LDL-C reduction, by drug/treatment class was 0.70 (p<0.01) (3). Figure 2 shows that the variable (%ΔHDL-C - %ΔLDL-C) is much more predictive of primary event reduction, with an R2 = 0.93 (p<0.0001). Not surprisingly, the linear regression model (3) describing this relationship is virtually that predicted by the epidemiological risk gradients for LDL-C and HDL-C:
Figure 2.
Effect of various drug/treatment classes on trial primary clinical event rate, relative to placebo rate, plotted against combined in-treatment changes in HDL-C and LDL-C.
The torcetrapib finding as seen from the perspective of other HDL-C-raising trials
While the exact details of LDL-C and HDL-C change with the atorvastatin-torcetrapib combination in ILLUMINATE are not yet available, we can roughly estimate from published data (24) that, as compared to atorvastatin alone, there would be at least -10% reduction from baseline in LDL-C and at least +35% increase in HDL-C. The difference variable defined above would come to +45%. Imagine plotting the the +61% excess mortality risk attributable to this drug combination on the vertical axis of Figure 2, against +45% on the horizontal axis. This point would lie many standard deviations off the best-fit line, which is predicted by epidemiologic studies and followed closely by each of the drug/treatment classes or their combinations. Thus the clinical effect of the torcetrapib-atorvastatin combination is strikingly anomalous when compared with these 23 widely recognized lipid therapy trials. As we await analyses evaluating the mechanism(s) of harm, a number of key questions remain unanswered: 1) Are all types of HDL-C-raising dangerous? This is unlikely; the data from trials shown in Table 1 and Figures 1 and 2 strongly suggest that HDL-C-raising is at least as beneficial as LDL-C-lowering for cardiovascular event reduction and for slowing or reversing progressive coronary obstruction. 2) Is the specific HDL-C-raising mechanism of torcetrapib (CETP inhibition) dangerous? This possibility can only be resolved by further careful examination of other compounds that inhibit CETP. Indeed, the mechanism of torcetrapib may be viewed as interfering with the process of “reverse cholesterol transport”, which might be an adverse effect. 3) Does torcetrapib have toxicity(ies) unrelated to its mechanism of HDL-C-raising? This is also a possibility that requires further study. Clearly the rise in systolic blood pressure is undesirable, although it seems hard to link a 3–4 mmHg rise in SBP to a 61% increase in total mortality over 14 months. 4) Are the HDL particles created by torcetrapib dysfunctional? This is a possibility for which some evidence is available. We have described studies on the altered size distribution of HDL particles in patients with coronary disease (25, 26) or at risk for the disease (27), as compared to healthy or disease-free subjects. We have also described the response of HDL particles to simvastatin plus niacin (26). In these analyses, the HDL particles were first separated by immuno-affinity column into Lp(A-I) and Lp(A-I,A-II), defined by the absence or presence of apoA-II, respectively. Particles in each of these two subgroups were further classified by their percent distribution in one of four size categories based on laser densitometry of the protein staining of these particles separated in a sizing gel: small (7.0–8.2 nm), medium (8.2–9.2 nm), large (9.2–11.2 nm) and very large (11.2–17.0 nm). Coronary disease subjects and those at risk for the disease have a smaller percent distribution of large particles when compared to the HDL of healthy individuals, and some have a relative enrichment of very large particles (25–27). In the HATS study (26,28), simvastatin and niacin significantly increase (double) the apoA-I, cholesterol, and phospholipid content of the Lp(A-I) particles, with virtually no effect on these variables in Lp(A-I,A-II). Of interest, the principal effect of simvastatin plus niacin in HATS (26) in both apo-specific subclasses was to significantly increase the percentage distribution of the large particles, and reduce that of the small particles without significant effect on the distribution of the medium or the very large particles. Slowing of coronary stenosis progression, or regression was strongly correlated with the sum of medium sized, plus the large Lp(A-I) particle concentration (28), and with the similar sized α-1, α-2 and pre-α-1,pre-α-2 particles by the 2-D gel method of Asztalos et al (29).
Conversely, and of interest to the torcetrapib question, the relative concentration of the very large Lp(AI) particles, although normally averaging about 10% of the particles in Lp(A-I) and 6% in Lp(A-I,A-II), is significantly predictive of stenosis progression (unpublished analyses from HATS). Asztalos et al (30) have examined HDL particle size and electrophoretic mobility in normal control subjects and patients with genetic CETP deficiencies. They find that compound heterozygotes and homozygotes, with CETP mass concentrations that were 37%, and less than10%, of control, have a striking increase in large and very large α-migrating particles not seen in controls or in heterozygotes with 70% of control CETP mass. These unusual particles with modal diameters of 12.9–17.6 nm contain apoA-II, apoC’s, and apoE as well as apoA-I. At least some of these particles would thus be classified as Lp(A-I,A-II) (25). Brousseau, et al (31) report that torcetrapib at 120 mg qd (twice the dose used in ILLUMINATE), with and without atorvastatin 20 mg qd, increase the apoA-I content of the “α1-migrating” particles by 136–153%, mainly due to reduced apoA-I catabolism. However, the size range of these “α1-migrating” particles was not provided in their report. Plasma CETP level has been found to be inversely correlated with the very large Lp(AI,AII) particles (27). If the torcetrapid-induced α1-migrating particles have composition and size similar to those in the more severe genetic CETP deficiency syndromes, they may be dysfunctional, or atherogenic, by mechanisms yet to be defined. The hypothesis that CETP inhibition creates a very large, atherogenic HDL particle, enriched in apolipoproteins E, C, and A-II as well as A-I, is consistent with the higher rate of stenosis progression associated with increased particle concentration in this very large size range.
Conclusions
This review of epidemiologic, arteriographic and clinical trial evidence supports the idea that raising HDL-C is at least as important as a comparable percentage reduction of LDL-C. Indeed, the lipid response variable (%ΔHDL-C - %ΔLDL-C) is very strongly associated with slowing of coronary stenosis progression and reduction of clinical coronary events. This observation appears to apply to all FDA-approved pharmaceutical agents analyzed. Given these observations, a strong LDL-C-lowering agent, such as a statin, in combination with niacin - presently the most effective approved HDL-C-raising drug - would appear to provide the greatest potential for cardiovascular disease prevention. Current expert opinion holds that HDL-C-raising agents have not been proven beyond doubt to reduce cardiovascular risk either as monotherapy or when added to LDL-C-lowering agents. However, the evidence, as presented here, strongly supports this hypothesis in its straightforward logic and its fit with epidemiologic and with clinical trial data. If the relationships emerging from this analysis are proven true, risk reductions in the range of 60–75% would be achievable using currently-available drug combinations, a major advance over the 25–35% risk reduction expected with statin monotherapy. Two trials are presently ongoing to examine this possibility. ACCORD will compare fenofibrate plus any statin with statin monotherapy in 9,750 patients with type 2 diabetes. AIM-HIGH will compare simvastatin plus Niaspan® with simvastatin monotherapy in 3300 patients with cardiovascular disease, low HDL-C and high triglycerides. Both trials are due to complete in the 2009–2011 time-frame.
Acknowledgments
Financial Support and Conflicts of Interest:
B. Greg Brown, MD, PhD: Supported in part by grants from the National Institutes of Health (R01-HL49546, U01-HL081616), in part by the Clinical Nutrition Research Unit (NIH DK 35816), in part by the Diabetes Endocrinology Research Center (NIH DK 17047). A portion of this research was performed in the Clinical Research Center (NIH MO1 00037) at the University of Washington. Dr Brown has been largely supported by NHLBI over many years. Within the past 5 years, he has also received research grants from Merck, Inc and Kos Pharmaceuticals, and has served on advisory boards for Merck, Pfizer, and Merck-Schering/Plough.
Xue-Qiao Zhao, MD: Supported in part by grants from the National Institutes of Health (R01-HL49546, R01-HL063895, U01-HL081616), in part by the Clinical Nutrition
Research Unit (NIH DK 35816), in part by the Diabetes Endocrinology Research Center (NIH DK 17047). A portion of this research was performed in the Clinical Research Center (NIH MO1 00037) at the University of Washington. Within the past 5 years, Dr. Zhao has also received research grants from Pfizer, AstraZeneca, and Kos Pharmaceuticals, and has served on advisory boards for Pfizer.
Marian C. Cheing, PhD: Supported in part by grants from the National Institutes of Health HL-49546, HL-30086, and DK-35816 (Clinical Nutrition Research Unit)
Glossary
- LDL-C
Low density lipoprotein cholesterol
- HDL-C
High density lipoprotein cholesterol
- TG
Triglycerides
- apo
apolipoprotein
- QCA
Quantitative coronary arteriography, CAD, Coronary artery disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ Coordinating Committee of the National Cholesterol Education Program. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44(3):720–32. doi: 10.1016/j.jacc.2004.07.001. Review. [DOI] [PubMed] [Google Scholar]
- 2.New York Times. Dec 4, 2006. ILLUMINATE Data-Safety Monitoring Board news release, Dec 2; p. A1. [Google Scholar]
- 3.Brown BG, Hinckley-Stukovsky K, Zhao X-Q. Simultaneous LDL-C-lowering and HDL-C-raising for optimum cardiovascular disease prevention with various drug classes, and their combinations. Curr Opin Lipidol. 2006 doi: 10.1097/MOL.0b013e32800ff750. [DOI] [PubMed] [Google Scholar]
- 4.Wilson PW, Anderson KM, Castelli WP. Twelve-year incidence of coronary heart disease in middle-aged adults during the era of hypertensive therapy. The Framingham Offspring Study. Am J Med. 1991;90:11–16. doi: 10.1016/0002-9343(91)90500-w. [DOI] [PubMed] [Google Scholar]
- 5.Assman G, Cullen P, Schulte H. The Munster Heart Study (PROCAM): Results of Follow-up at 8 years. Eur HeartJ. 1998;19(Suppl A):A2–A11. [PubMed] [Google Scholar]
- 6.LaRosa JC, He J, Vupputuri S, et al. Effect of statins on risk of coronary disease: A meta-analysis of randomized controlled trials. JAMA. 1999;282:2340–06. doi: 10.1001/jama.282.24.2340. [DOI] [PubMed] [Google Scholar]
- 7.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 20,536 high-risk individuals:a randomized placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 8.Gordon DJ, Probstfield JL, Garrison RJ, et al. High density lipoprotein cholesterol and cardiovascular disease Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 9.Pekkanen J, Linn S, Heiss G, et al. Ten-year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N Engl J Med. 1990;322:1700–07. doi: 10.1056/NEJM199006143222403. [DOI] [PubMed] [Google Scholar]
- 10.Brown G, Albers JJ, Fisher LD, et al. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990;323:1289–98. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]
- 11.Phillips NR, Waters D, Havel RJ. Plasma lipoproteins and progression of coronary artery disease evaluated by angiography and clinical events. Circulation. 1993;88:2762–70. doi: 10.1161/01.cir.88.6.2762. [DOI] [PubMed] [Google Scholar]
- 12.Brown BG, Gotto AM. Impact of Management on Stabilization of Plaque and Reduction of Cardiovascular Events. In: Fuster V, Topol EJ, Nabel EG, editors. Chapter 12 in Atherothrombosis and Coronary Artery Disease. Lipincott Williams and Wilkins; Phila: 2005. p. 153. [Google Scholar]
- 13.Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high density lipoprotein cholesterol. N Engl J Med. 1999;341:410–18. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 14.Diabetes Atherosclerosis Intervention Study Investigators. Effect of fenofibrate on progression of coronary artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomized study. Lancet. 2001;357:905–10. [PubMed] [Google Scholar]
- 15.Whitney EJ, Krasuski RA, Personius BE, Michalek JE, Maranian AM, Kolusa MW, Monick E, Brown BG, Gotto AM. A randomized trial of a strategy for increasing high-density lipoprotein cholesterol levels: Effects on progression of coronary heart disease and clinical events. Ann Intern Med. 2005;142:95–104. doi: 10.7326/0003-4819-142-2-200501180-00008. [DOI] [PubMed] [Google Scholar]
- 16.The FIELD study investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomized controlled trial. Lancet. 2005;366:1849–61. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 17.Coronary Drug Project Research Group. Clofibrate and niacin in coronary heart disease. JAMA. 1975;231:360–81. [PubMed] [Google Scholar]
- 18.Herd JA, Ballantyne CM, Farmer JA, et al. Effects of Fluvastatin on coronary atherosclerosis in patients with mild to moderate cholesterol elevations. (Lipoprotein and coronary atherosclerosis study [LCAS]) Am J Caridology. 1997;80:278–286. doi: 10.1016/s0002-9149(97)00346-9. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333:1301–07. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen TR, Faergeman O, Kastelein JJP, et al. High dose atorvastatin vsusual-dose simvastatin forsecondary prevention after myocardial infarction. The IDEAL study: A randomized controlled trial. JAMA. 2005;294:2437–45. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
- 21.LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. The TNT study. N Engl J Med. 2005;352:1425–35. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 22.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in moderately hypercholesterolemic hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT) JAMA. 2002;288:2998–3007. doi: 10.1001/jama.288.23.2998. [DOI] [PubMed] [Google Scholar]
- 23.Blankenhorn DH, Nessim SA, Johnson RL, et al. Beneficial effects of combined colestipol-niacin therapy on on coronary atherosclerosis and coronary venous bypass grafts. JAMA. 1987;257:3233–40. [PubMed] [Google Scholar]
- 24.McKenney JM, Davidson MH, Shear CL, Revkin JL. Efficacy and Safety of Torcetrapib, a Novel Cholesteryl Ester Transfer Protein Inhibitor in Individuals With Below-Average High-Density Lipoprotein Cholesterol Levels on a Background of Atorvastatin. J Am Coll Cardiol. 2006;48:1782–1790. doi: 10.1016/j.jacc.2006.06.066. [DOI] [PubMed] [Google Scholar]
- 25.Cheung MC, Brown BG, Wolf AC, Albers JJ. Altered particle size distribution of A-1-containing lipoproteins in subjects with coronary artery disease. J Lipid Res. 1991;32:383–394. [PubMed] [Google Scholar]
- 26.Cheung MC, Zhao X-Q, Chait A, Albers JA, Brown BG. Anti-oxidant supplements block the response of HDL to simvastatin-niacin therapy in patients with coronary artery disease and low HDL. Arterioscler Thromb Vasc Biol. 2001;21:1320–1326. doi: 10.1161/hq0801.095151. [DOI] [PubMed] [Google Scholar]
- 27.Cheung MC, Austin MA, Moulin P, Wolf AC, Cryer D, Knopp RH. Effects of pravastatin on apolipoprotein-specific high density lipoprotein subpopulations and low density lipoprotein subclass phenotypes in patients with primary hypercholesterolemia. Atherosclerosis. 1993:102, 107–119. doi: 10.1016/0021-9150(93)90089-d. [DOI] [PubMed] [Google Scholar]
- 28.Brown BG, Zhao X-Q, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, Frohlich J, Albers JJ. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–92. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 29.Asztalos BF, Batista M, Horvath KV, Cox CE, Dallal GE, Morse JS, Brown BG, Schaefer E. Change in α1 HDL concentration predicts regression in coronary artery stenosis. Arterioscler Thromb Vasc Biol. 2003;23:847–52. doi: 10.1161/01.ATV.0000066133.32063.BB. [DOI] [PubMed] [Google Scholar]
- 30.Asztalos BF, Horvath KV, Kajinami K, Nartsupha C, Cox CE, Batista M, Schaefer EJ, Inazu A, Mabuchi H. Apolipoprotein composition of HDL in cholesteryl ester transfer protein deficiency. J Lipid Res. 2004;45:448–455. doi: 10.1194/jlr.M300198-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Brousseau ME, Diffenderfer MR, Millar JS, Nartsupha C, Asztalos BF, Welty FK, Wolfe ML, Rudling M, Bjorkhem I, Angelin B, Mancuso JP, Digenio AG, Rader DJ, Schaefer EJ. Effects of cholesteryl ester transfer protein inhibition on high density lipoprotein subspecies, apolipoprotein A-I metabolism, and fecal sterol excretion. Arterioscler Thromb Vasc Biol. 2005;25:1057–66. doi: 10.1161/01.ATV.0000161928.16334.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]