Abstract
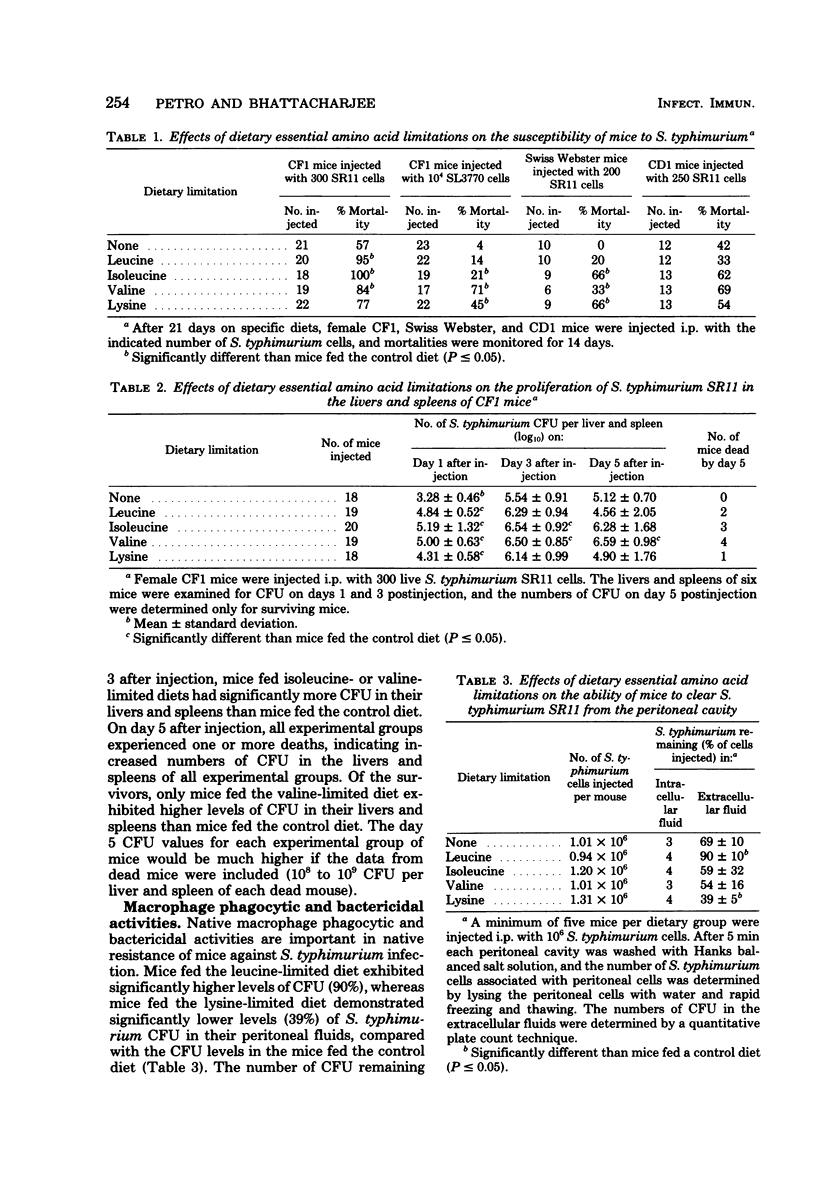
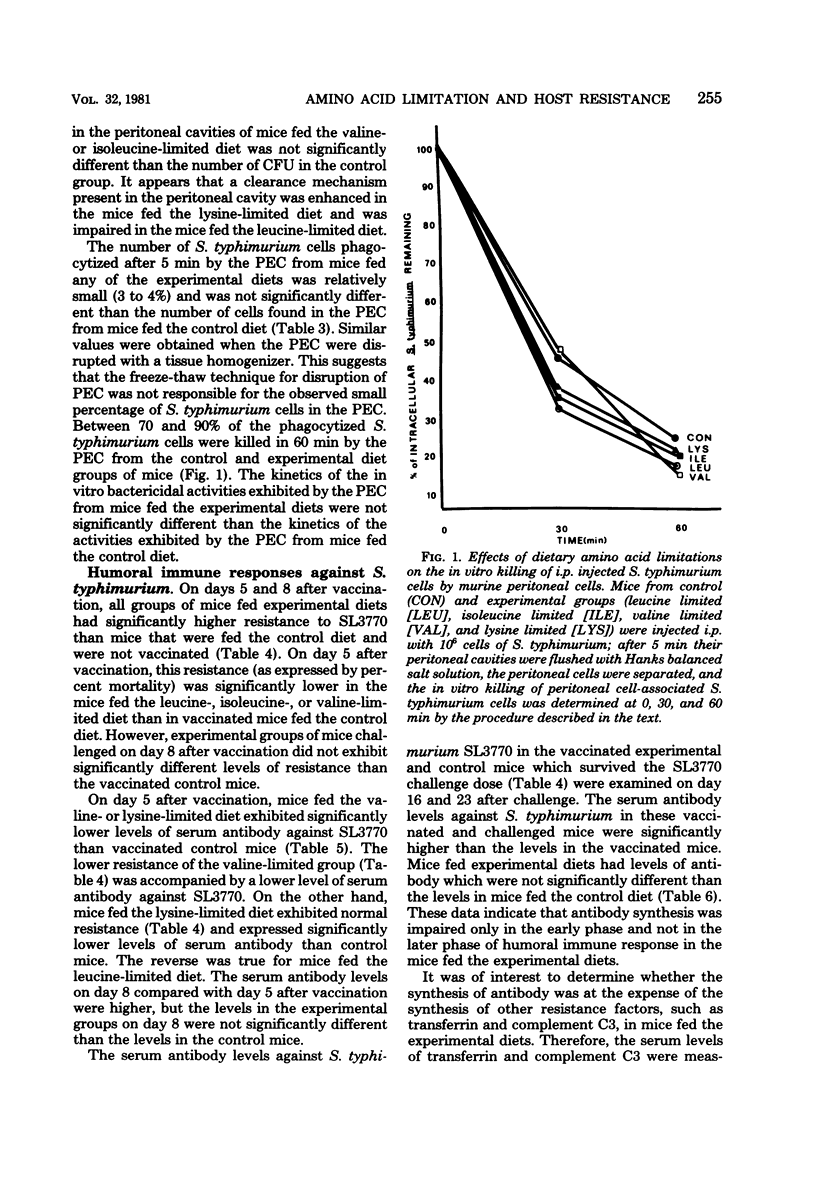
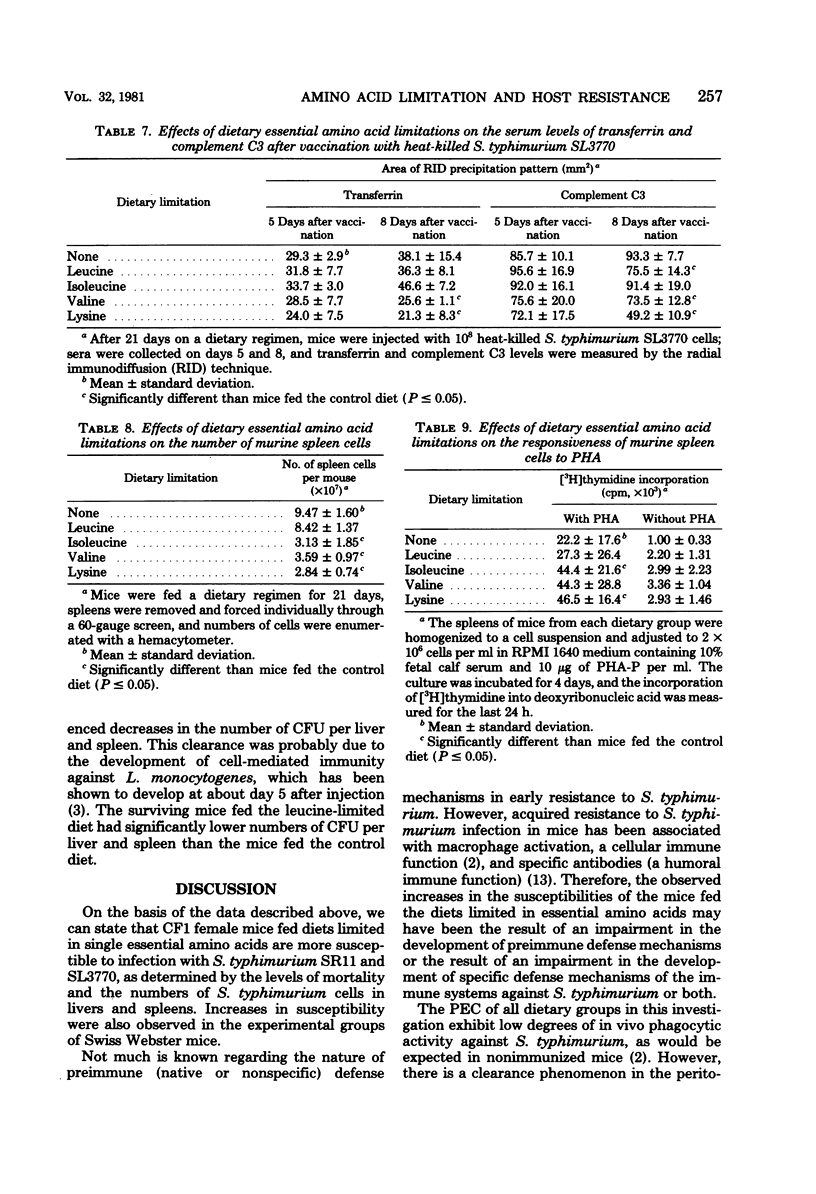
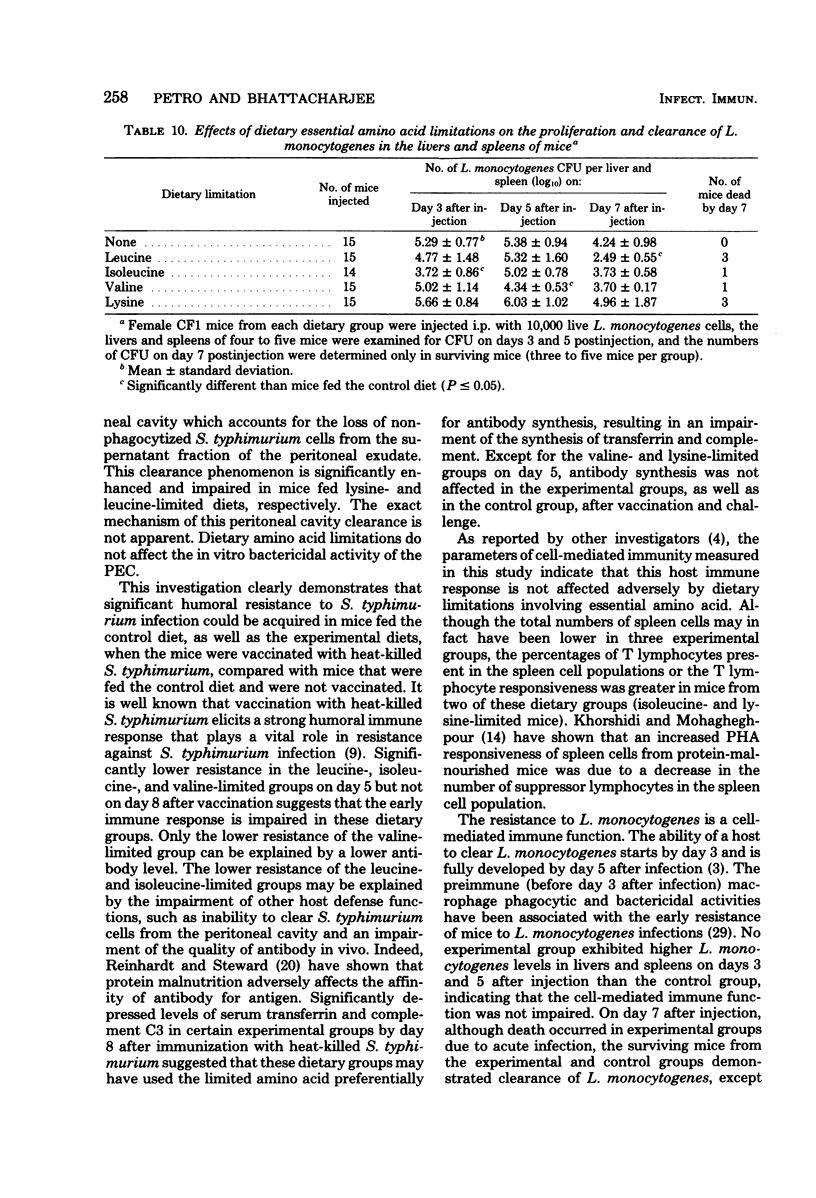
We investigated the effects of dietary essential amino acid limitations on the susceptibility of mice to Salmonella typhimurium infections and on humoral and cellular immune (cell-mediated immune) responses of mice. Mice fed synthetic diets limited (significantly less than optimum concentration) in a single essential amino acid (leucine, isoleucine, valine, or lysine) for 3 weeks after they were weaned exhibited significantly enhanced susceptibility to S. typhimurium infection, as evidenced by the higher levels of mortality and spread of the bacterial cells in their livers and spleens compared with mice fed the control diet. Compared with mice fed the control diet, mice fed the diet limited in leucine had a lower ability to clear S. typhimurium cells from the peritoneal cavity 5 min after intraperitoneal injection, whereas mice fed the diet limited in lysine had a greater ability. The in vivo phagocytosis and in vitro bactericidal kinetics against S. typhimurium cells by peritoneal macrophages were not significantly different in the control group and the groups of mice fed experimental diets. Certain experimental groups exhibited significantly lower resistance and antibody response against S. typhimurium SL3770 on day 5 after immunization with heat-killed S. typhimurium SL3770. On day 8 after immunization, the levels of serum antibody against S. typhimurium in the mice fed the experimental diets were comparable to the levels in mice fed the control diet. However, the levels of serum transferrin and complement C3 were significantly lower in mice fed certain experimental diets. The cellular immune capacities of mice fed any of the experimental diets were not impaired compared with the capacities of mice fed the control diet, as measured by spleen cell responsiveness to phytohemagglutinin and the ability to clear infecting Listeria monocytogenes cells from livers and spleens.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binder H. J., Whiting D. S. Inhibition of small-intestinal sugar and amino acid transport by the enterotoxin of Shigella dysenteriae I. Infect Immun. 1977 May;16(2):510–512. doi: 10.1128/iai.16.2.510-512.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Mackaness G. B., Collins F. M. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., McKenzie I. F., Pavlov H., Waid C., York J. Resistance and susceptibility of mice to bacterial infection: course of listeriosis in resistant or susceptible mice. Infect Immun. 1978 Mar;19(3):763–770. doi: 10.1128/iai.19.3.763-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper W. C., Good R. A., Mariani T. Effects of protein insufficiency on immune responsiveness. Am J Clin Nutr. 1974 Jun;27(6):647–664. doi: 10.1093/ajcn/27.6.647. [DOI] [PubMed] [Google Scholar]
- DUBOS R. J., SCHAEDLER R. W. Effect of dietary proteins and amino acids on the susceptibility of mice to bacterial infections. J Exp Med. 1958 Jul 1;108(1):69–81. doi: 10.1084/jem.108.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Moon R. J. Hepatic clearance of Salmonella typhimurium in silica-treated mice. Infect Immun. 1977 Jun;16(3):1005–1012. doi: 10.1128/iai.16.3.1005-1012.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadimi H., Kumar S., Abaci F. Endogenous amino acid loss and its significance in infantile diarrhea. Pediatr Res. 1973 Mar;7(3):161–168. doi: 10.1203/00006450-197303000-00008. [DOI] [PubMed] [Google Scholar]
- Herzberg M., Nash P., Hino S. Degree of immunity induced by killed vaccines to experimental salmonellosis in mice. Infect Immun. 1972 Jan;5(1):83–90. doi: 10.1128/iai.5.1.83-90.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochadel J. F., Keller K. F. Protective effects of passively transferred immune T- or B-lymphocytes in mice infected with Salmonella typhimurium. J Infect Dis. 1977 May;135(5):813–823. doi: 10.1093/infdis/135.5.813. [DOI] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- John A. M., Bell J. M. Amino acid requirements of the growing mouse. J Nutr. 1976 Sep;106(9):1361–1367. doi: 10.1093/jn/106.9.1361. [DOI] [PubMed] [Google Scholar]
- Kenny K., Herzberg M. Antibody response and protection induced by immunization with smooth and rough strains in experimental salmonellosis. J Bacteriol. 1968 Feb;95(2):406–417. doi: 10.1128/jb.95.2.406-417.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorshidi M., Mohagheghpour N. Effect of protein deficiency on suppressor cells. Infect Immun. 1979 Jun;24(3):770–773. doi: 10.1128/iai.24.3.770-773.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvach J. T., Wiles T. I., Mellencamp M. W., Kochan I. Use of transferrin-iron enterobactin complexes as the source of iron by serum-exposed bacteria. Infect Immun. 1977 Nov;18(2):439–445. doi: 10.1128/iai.18.2.439-445.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Moore R. N., Johnson B. A., Berry L. J. Nutritional effects of salmonellosis in mice. Am J Clin Nutr. 1977 Aug;30(8):1289–1293. doi: 10.1093/ajcn/30.8.1289. [DOI] [PubMed] [Google Scholar]
- Newberne P. M., Hunt C. E., Young V. R. The role of diet and the reticuloendothelial system in the response of rats to Salmonella typhilmurium infection. Br J Exp Pathol. 1968 Oct;49(5):448–457. [PMC free article] [PubMed] [Google Scholar]
- Petro T. M., Bhattacharjee J. K. Effect of dietary essential amino acid limitations upon native levels of murine serum immunoglobulins, transferrin, and complement. Infect Immun. 1980 Feb;27(2):513–518. doi: 10.1128/iai.27.2.513-518.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt M. C., Steward M. W. Antibody affinity and clearance function studies in high and low antibody affinity mice. The effect of protein deficiency. Immunology. 1979 Dec;38(4):735–739. [PMC free article] [PubMed] [Google Scholar]
- SRIRAMACHARI S., GOPALAN C. Nutrition and tuberculosis. III. Effect of some nutritional factors on resistance to tuberculosis. Indian J Med Res. 1958 Jan;46(1):105–112. [PubMed] [Google Scholar]
- Scrimshaw N. S., Taylor C. E., Gordon J. E. Interactions of nutrition and infection. Monogr Ser World Health Organ. 1968;57:3–329. [PubMed] [Google Scholar]
- Venneman M. R., Berry L. J. Cell-mediated resistance induced with immunogenic preparations of Salmonella typhimurium. Infect Immun. 1971 Oct;4(4):381–387. doi: 10.1128/iai.4.4.381-387.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannemacher R. W., Jr Key role of various individual amino acids in host response to infection. Am J Clin Nutr. 1977 Aug;30(8):1269–1280. doi: 10.1093/ajcn/30.8.1269. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Blanden R. V. Macrophage activation in mice lacking thymus-derived (T) cells. Experientia. 1975 May 15;31(5):591–593. doi: 10.1007/BF01932477. [DOI] [PubMed] [Google Scholar]


