Abstract
The short allele of the serotonin transporter linked polymorphic region (5-HTTLPR) moderates the effects of stress on vulnerability to mood and anxiety disorders. The mechanism by which this occurs may relate to differential sensitivity to stressful life events. Here we explored whether 5-HTTLPR and sex affected behavioral responses to repeated maternal separation in infant rhesus macaques. Behaviors were collected during the acute (Day 1) and the chronic (Days 2–4) phases of the separation, and the effects of duration of separation (acute vs. chronic), genotype (long/long vs. short allele), and sex (male vs. female) on behavioral responses were analyzed across four successive separations. Males increased their levels of locomotion with repeated maternal separation, whereas females exhibited an increase in frequency of self-directed behavior, a measure of “depression-like” behavior. The short-allele predicted increased environmental exploration, particularly during the chronic phase of social separation, indicative of higher arousal. In addition, the short-allele carriers were more likely to increase their levels of self-directed behavior during the chronic phase of separation, as a function of repeated exposures. These findings suggest that the short allele may increase reactivity to repeated, chronic stressors, leaving them more vulnerable to affective psychopathology, with females particularly vulnerable.
Stress is a universal condition of life but, if it is chronic, severe, or occurs during critical developmental windows, it contributes to a variety of disease vulnerabilities, particularly disorders of the brain (McEwen, 2007). In humans, there are known links between stress and a variety of psychiatric disorders, including depression, posttraumatic stress disorder, anxiety disorders, and substance use disorders (Neigh, Gillespie, & Nemeroff, 2009; Sinha, 2008). Genetic variants that influence stress reactivity have been shown to determine whether an individual will be vulnerable to such disorders (Caspi et al., 2003). This may occur by determining environmental sensitivity, magnitude, and/or duration of stress response, or the types of responses exhibited by an individual following stress exposure. Genetic variation that determines whether individuals habituate or sensitize to repeated or chronic stress would be expected to play a major role (Neigh et al., 2009).
A number of studies investigating the influence of genes in susceptibility to depression and other stress-related psychiatric disorders report associations of the short allele of the serotonin transporter linked polymorphic region (5-HTTLPR) with increased risk (Gross & Hen, 2004; Levinson, 2006), particularly in the presence of high cumulative levels of stress exposure (Caspi et al., 2003; Grabe et al., 2009).Moreover, epidemiological data indicate a higher prevalence of mood and anxiety disorders in women compared to men (Garber, 2006; Sloan & Kornstein, 2003) and gender differences in the contributions of the 5-HTTLPR genotype to depression have also been reported (Brummett et al., 2008; Sjöberg et al., 2006). Although a recent meta-analysis has questioned the hypothesis that the 5-HTTLPR genotype (alone or in interaction with stressful life events) is associated with an increased risk for depression (Risch et al., 2009), results from studies examining effects of 5-HTTLPR genotype on highly relevant intermediate phenotypes (Hariri et al., 2002) and those from controlled animal studies (Barr, Newman, Schwandt, et al., 2004) provide evidence that 5-HTTLPR genotype does influence emotional and environmental reactivity (Caspi et al., 2010). Because depression is more common among individuals with high levels of cumulative stress burden, individual differences in stress reactivity that are attributable to the 5-HTTLPR genotype may be more easily appreciated at early developmental time points and with controlled gradations of stress exposure.
In support to this hypothesis, personality traits related to an increased risk for stress-related disorders, such as neuroticism and harm avoidance (Fanous, Neale, Aggen, & Kendler, 2007; Kendler, Neale, Kessler, Heath,&Eaves, 1993), are associated with the short allele (Canli & Lesch, 2007; Lesch et al., 1996). Carriers of the short allele also show greater amygdala activation in response to fearful/negative stimuli compared to individuals homozygous for the long allele (Hariri et al., 2002; Munafò, Brown, & Hariri, 2008) and greater sensitivity to induction of negative mood is also found in children homozygous for the short allele (Hayden et al., 2008). Taken together, these studies suggest that the short allele may be linked to increased stress sensitivity from a time early in development (Lesch et al., 1996; Murphy, Lerner, Rudnick, & Lesch, 2004) and may predict individual differences in environmental reactivity, coping style, and stress adaptation.
Social separation in nonhuman primates is considered a highly stressful event, which induces behavioral and physiological responses of anxiety and stress and has been used as a model of human depression (Mineka & Suomi, 1978; Suomi, 1997). Similarities between the behavioral response of infant macaques and children to separation stress have also been reported (Bowlby, 1969). Upon separation from a caregiver, most children become first upset and agitated; but within a few days the transition from “protest” to “despair,” a response characterized by depression and social withdrawal, is seen in some but not all children (Bowlby, 1969). Similar to children, most infant macaques initially exhibit agitation and arousal, characterized by increased locomotion and distress vocalizations. Although responses to prolonged separation are not universal among subjects, some individuals show “despairlike” behaviors characteristic of human depression (Mineka & Suomi, 1978; Suomi, 1997), during which high levels of behavioral withdrawal and self-directed behavior are reported (Higley, Suomi & Linnoila, 1992). Previous findings in nonhuman primates exposed to repeated exposures to social separation stress demonstrate behavioral habituation in some subjects (Barr et al., 2008; Coe, Glass, Wiener, & Levine, 1983; Hennessy, 1986), but increased sensitivity to separation’s depression-inducing effects in others (Mineka, Suomi, & De-Lizio, 1981; Spinelli et al., 2007, Suomi, Mineka & De Lizio, 1983). These later studies indicate that, although most animals show unaltered or even attenuated responses across repeated separations, some show increased “despair” (shown as increased self-directed behavior and behavioral withdrawal) with repeated separation stressors (Mineka et al., 1981; Suomi et al., 1983). This illustrates an important point: that both the degree to which an infant responds to separation stress and the temporal pattern of these behaviors differ among individuals.
In rhesus macaques (Macaca mulatta), there is a 21-bp insertion/deletion polymorphism (rh-5-HTTLPR) is functionally similar to 5-HTTLPR in humans. The rhesus short allele is linked to increased anxiety-related behaviors beginning early in development, during the neonatal period (Champoux et al., 2002), and continuing into adolescence and early adulthood, providing a valid developmental model for understanding how the rh-5-HTTLPR short allele might modulate stress reactivity. We previously reported interactive effects of rh-5-HTTLPR genotype and early nursery rearing (an established model of early adversity) on reactivity to social separation stress (Barr, Newman, Schwandt, et al., 2004; Barr, Newman, Shannon, et al., 2004; Spinelli et al., 2007). However, whether genotype predicts responses to stress in normally reared primates and across step-wise increases in cumulative stress exposures has not yet been examined. Such an approach could be important because of its potential to be highly sensitive for detecting moderating effects of genotype on depression-related behaviors in a different species. It could also inform us of how genotype predicts tendencies toward habituation and/or sensitization to stress, which could potentially be more easily translated to the human condition.
We wanted to explore whether behavioral responses to repeated maternal separation stress would be influenced by rh-5-HTTLPR genotype and sex in infant rhesus macaques and whether these responses differed as a function of duration and repetition (number of exposures) of the stressor.
Methods
Subjects and care
One hundred thirty-two (64 males, 68 females) rhesus macaques (representing nine birth cohorts) were born and housed at the National Institutes of Health Animal Center in Poolesville, Maryland. The monkeys were reared for the first 6 months of life with their mothers and fathers in social groups comprising 8 to 12 adult females (about half of whom had same-aged infants) and 2 adult males, a condition that approximates natural rhesus experiences (described in detail by Shannon et al., 2005). Protocols for the care and use of experimental animals were approved by the Institutional Animal Care and Use Committee of the National Institute on Alcohol Abuse and Alcoholism and the National Institute of Child Health and Human Development, National Institutes of Health.
Maternal separation
When the animals reached 6–7 months of age, they were subjected to four sequential, 4-day maternal separations, each followed by with 3 days of reunion with their mothers in their social group (Higley et al., 1991). Infants were separated from their mothers by removing the mother from the social group leaving the infant in the home cage with other adult females, males, and infants (no maternal siblings or older juveniles were present). Based on previous studies, Day 1 (Monday) of each separation week was designated as the “acute” phase of separation, whereas Days 2 through 4 (Tuesday through Thursday) were designated as the “chronic” phase of separation (Higley et al., 1991). Following each separation week, subjects were reunited with their mothers early on Friday morning and separated again at noon on Monday.
Behavioral observations
During each separation week, a total of nine observations were made. Three observations were made on Day 1: two immediately following separation and one an hour later. Two observations were made each day for Days 2, 3, and 4. Each observation period was 300 s in duration. All observations were recorded as durations in seconds, except vocalization that was recorded as frequencies. Behavioral data were collected by multiple observers trained by a senior behavior coder to a standard criteria with at least 85% interobserver reliability between each of the raters. They were checked at least yearly for continued reliability. Each objectively defined collected behavior is defined in Table 1.
Table 1.
Definition of behaviors scored during maternal separation
| Behavior | Definition |
|---|---|
| Self-directed behavior | Includes self-groom, grooming of one’s own body, self-scratching, biting or cleaning nails; self-clasp, firm manual or pedal gripping of self, which is not a component of an ongoing behavior, i.e., self-groom; self-mouth, sucking (not biting) at any bodily appendage or own fur; should be scored with motionless, locomotion, or social. Self-directed behavior can be scored with any other behavior as long as it is not part of another ongoing behavior such as stypic or stereotypy. |
| Behavioral withdrawal | Absence of directed movement, social behaviors, and environmental manipulation (i.e., no simultaneously occurring social or nonsocial behaviors, except self-directed behaviors, or vocalizations); includes bouncing in place |
| Locomotion | Any movement across the substrate; includes changes in location by means of walking, running, dropping from ceiling to floor, swinging, and bouncing, rolling, hopping on all fours, bouncing around the cage, and “displays.” Note: if a motor pattern is repeated more than three times, it is scored as stereotypy. |
| Environmental exploration | Any active manual, oral, or pedal examination, exploration, or manipulation of the physical environment, or the attempt to do the same; includes manipulating or playing with chow while eating or drinking; does not include active play on the substrate, chewing chow, or passively holding object (food or other) |
| Vocalization | Any vocal sound emitted by the subject; includes coo, bark, screech, squeal, etc.; can be scored with any other behavior. Sounds made by coughing or sneezing are not vocalizations. |
Genotyping
Using standard extraction methods, DNA was isolated from whole blood collected from the femoral vein under ketamine anesthesia (15 mg/kg, intramuscular). The rh-5-HTTLPR was amplified from 25 ng of genomic DNA with flanking oligonucleotide primers (stpr5, 5′-GGCGTTGCCGCTCTGAATGC; intl, 5′-CAGGGGAGATCCTGGGAGGG; as described in Barr, Newman, Shannon, et al., 2004). Amplicons were separated by electrophoresis on a 10% polyacrylamide gel, and the short (398-bp) and long (419-bp) alleles were identified by direct visualization following ethidium–bromide staining. The frequency of the short allele was 16%, and genotype frequencies did not deviate from Hardy–Weinberg equilibrium. Genotyping was performed in duplicate in 10% of subjects, and genotype completion and accuracy were 100% and 99%, respectively. Of the 132 infants included in the study, 89 were homozygous for the long allele (45 females) and 43 were long/short (l/s) (23 females).
Statistical analyses
For each separation week, three scores (Day 1) were averaged to provide the acute mean score for each behavior, and six scores (Days 2–4) were averaged to provide the mean score for the chronic phase. Repeated-measures analyses of variance (ANOVAs) were used to perform analyses, with phase (acute and chronic) and separation number (S1–S4) as the within-subjects variable and sex (males vs. females) and genotype (long/long [l/l] vs. l/s, as short/short [s/s] subjects were too few in number) as the between-subjects variable. StatView 5.0.1 (SAS Institute, Inc., Cary, NC) was used for all statistical analyses. Based on previous studies in our laboratory, five behaviors were analyzed representing measures of despair and anxiety. To control for multiple comparisons, post hoc tests were performed using Tukey–Kramer tests. All data are reported as mean ± standard error of the mean, and criterion for significance was set at p ≤ .05.
Results
Effects of duration of stress exposure
There were main effects of phase on environmental exploration, F (1, 351) = 587, p < .0001 (Figure 1), locomotion, F (1, 351) = 20.0, p ≤ .0001 (Figure 3), behavioral withdrawal, F (1, 351) = 17.1, p < .0001 (Figure 4), and vocalization, F (1, 351) = 116.8, p < .0001 (Figure 5), but not for self-directed behavior, F (1, 351) = 0.142, p = .71. From the acute to the chronic phases of separation, levels of locomotion, vocalization, and behavioral withdrawal decreased, whereas those for environmental exploration increased (Tukey–Kramer, p < 0.05).
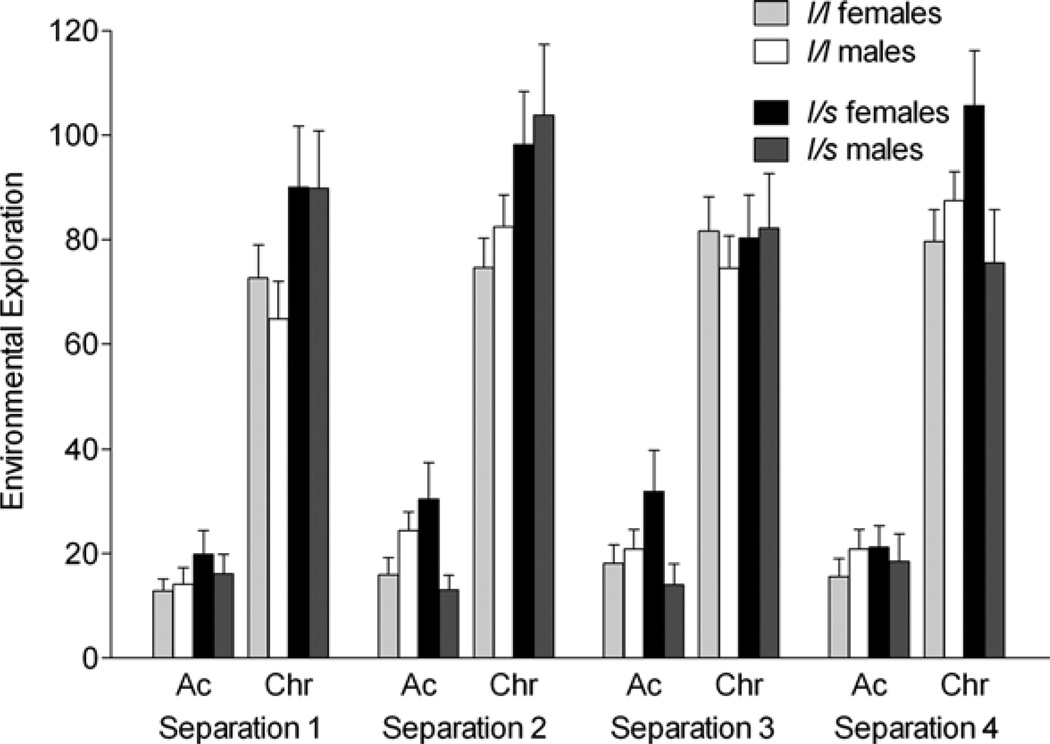
Figure 1.
The effects of the rhesus serotonin transporter linked polymorphic region (rh-5-HTTLPR) polymorphism and sex on environmental exploration (duration of seconds in 300-s session) during the acute (Ac) and chronic phase (Chr) of four repeated separation stress. Environmental exploration showed no effect of repeated separation. Environmental exploration was higher in short carriers compared to long/long (l/l) animals (p < .05). This effect was driven by the chronic phase of separation, during which short carriers showed higher levels compared to l/l animals (p < .05). In addition, females showed higher levels of environmental exploration during S4 compared to males and compared to S1 (p < .05). Bars depict mean ± SEM, and all post hoc tests were performed using Tukey–Kramer.
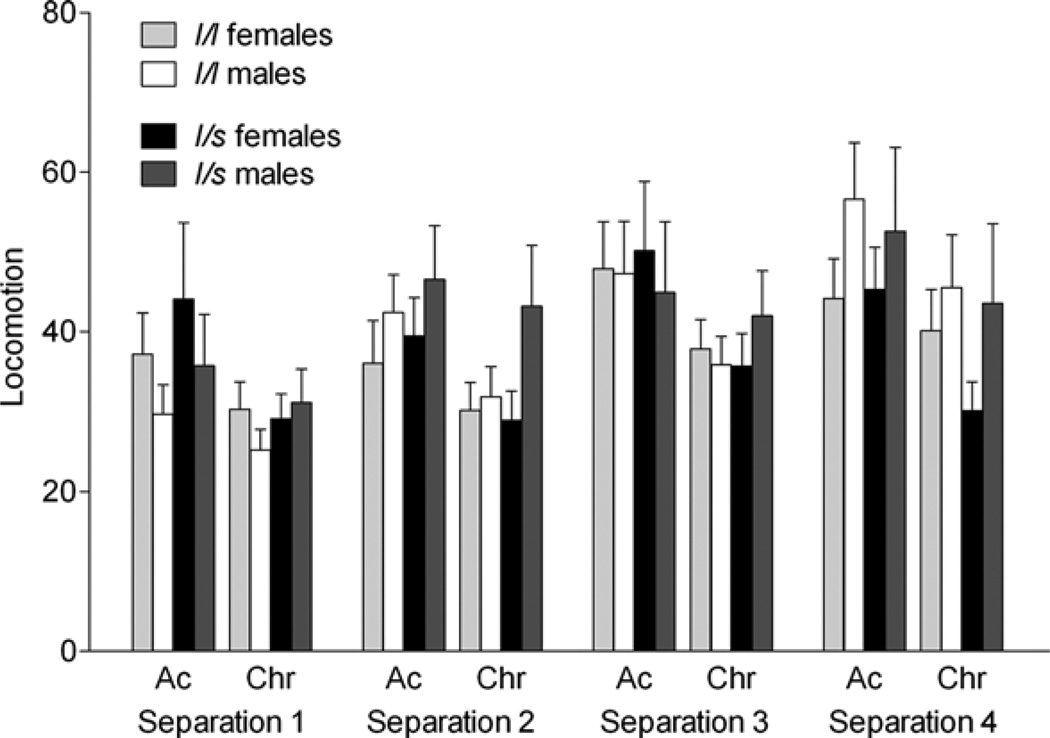
Figure 3.
The effects of the rhesus serotonin transporter linked polymorphic region (rh-5-HTTLPR) polymorphism and sex on locomotion (duration of seconds in a 300-s session) during the acute (Ac) and chronic phase (Chr) of four repeated separation stress. During the acute phase levels of locomotion were lower for S1 and S2 than S3 and S4; similarly during the chronic phase levels for S1 were lower than S3 and S4 and, for S2 were lower than S4 (p < .05). Compared to males, females showed higher levels of locomotion during S1 (p < .05). However, both sexes showed increased locomotion over repeated separations (in females values for S1 were lower than S2 and S4, values for S2 were higher than S3; in males values for S1 were lower than S2, S3, and S4, values for S2 were lower than S4; p < .05). Bars depict mean ± standard error of the mean, and all post hoc tests were performed using Tukey–Kramer.
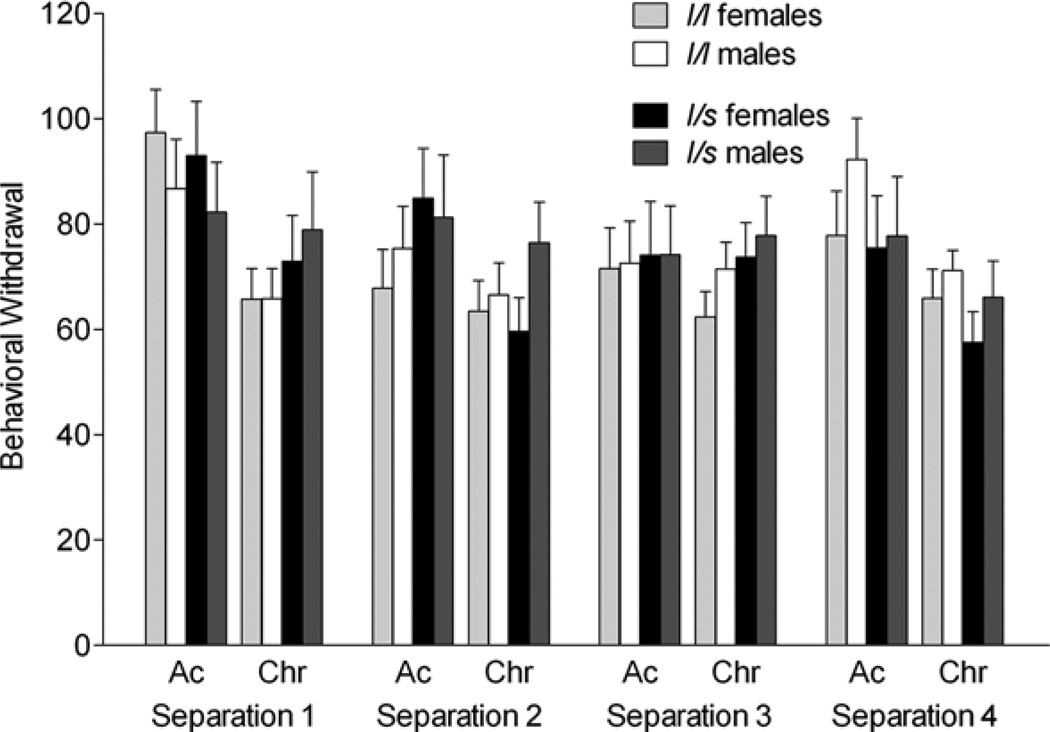
Figure 4.
The effects of the rhesus serotonin transporter linked polymorphic region (rh-5-HTTLPR) polymorphism and sex on behavioral withdrawal (duration of seconds in a 300-s session) during the acute (Ac) and chronic phase (Chr) of four repeated separation stress. During the acute phase, levels for S1 were higher than S2 and S3 (p < .05). Bars depict mean ± SEM, and all post hoc tests were performed using Tukey–Kramer.
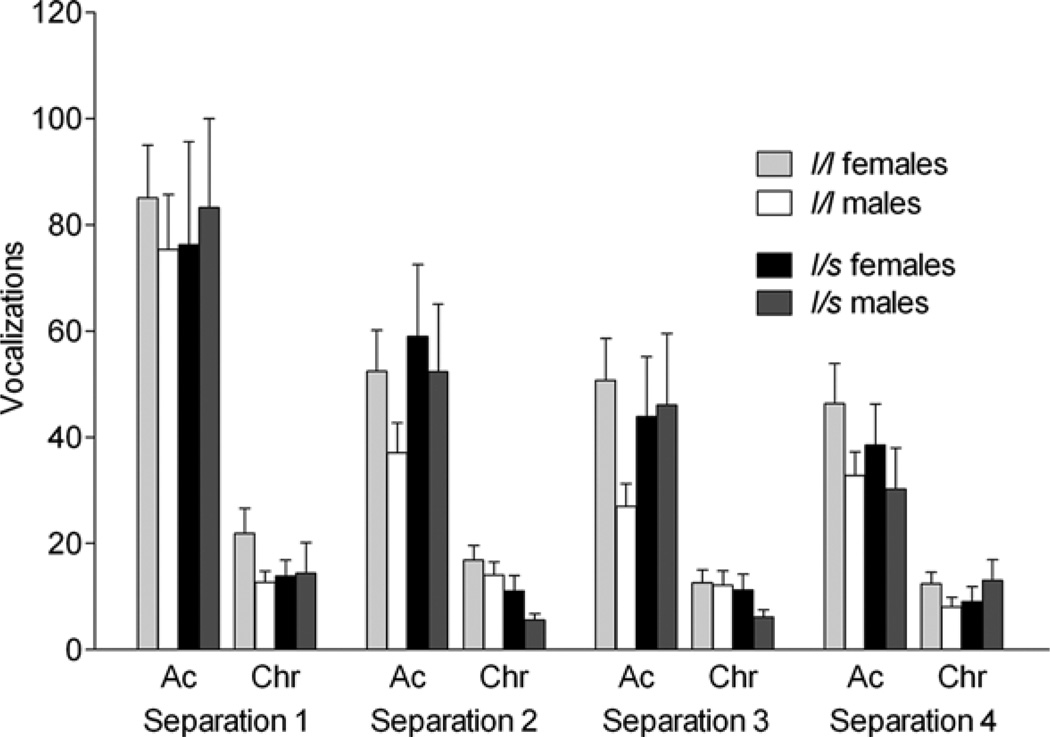
Figure 5.
The effects of the rhesus serotonin transporter linked polymorphic region (rh-5-HTTLPR) polymorphism and sex on vocalization (frequency in a 300-s session) on during the acute (Ac) and chronic phase (Chr) of four repeated separation stress. During the acute phase, levels for S1 were higher than S2 and S3; during the chronic phase, levels for S1 were higher than S3 and S4 (p < .05). Bars depict mean ± standard error of the mean, and all post hoc tests were performed using Tukey–Kramer.
Effects of repeated exposure to separation stress
We observed changes in frequencies of all behaviors assessed as a function of repeated exposures to separation stress: environmental exploration, F (3, 351) = 2.9, p < 0.04; locomotion, F (3, 351) = 11.1, p < 0.0001; vocalization, F (3, 351) = 32.7, p < .0001; behavioral withdrawal, F (3, 351) = 3.5, p < .02; self-directed behavior, F (3, 351) = 3.6, p < 0.02. There were patterns of increased environmental exploration, locomotion, and self-directed behavior with repeated stress, whereas levels of vocalization and behavioral withdrawal showed patterns of decline. For vocalization and behavioral withdrawal, there were also two-way Phase × Week of Separation interactions, F (3, 351) = 20.0, p < .0001 and F (3, 351) = 3.2, p < 0.03, respectively. For both behaviors, more marked declines in frequency/duration were observed from the acute to the chronic phases of the first separation week (Figures 4 and 5).
Moderating effects of sex and rh-5-HTTLPR genotype
There were no main effects of sex on any of the behaviors scored during the study. However, there were Separation Number × Sex interactions for levels of locomotion, F (3, 351) = 4.2, p < 0.006 and self-directed behavior, F (3, 351) = 4.5, p < 0.04. Males were more likely to increase their levels of locomotion as a function of repeated stress exposure, whereas females were more likely to exhibit increased self-directed behavior (Tukey–Kramer, p < 0.05).
There was a main effect of rh-5-HTTLPR genotype on levels of environmental exploration, F (1, 351) = 4.8, p = .03, with carriers of the short allele exhibiting higher levels than those homozygous for the long allele (Tukey–Kramer, p < .05). There was also an interaction between phase and rh-5-HTTLPR genotype, F (1, 351) = 4.0, p < 0.05, and a Phase×Separation Number×Sex×rh-5-HTTLPR interaction, F (3, 351) = 3.1, p < .03. Carriers of the short allele exhibited higher levels of exploration, particularly during the chronic phase of separation stress (Figure 1). Higher levels of exploration during the chronic phase were observed in female short-allele carriers (vs. males), but only for the final separation.
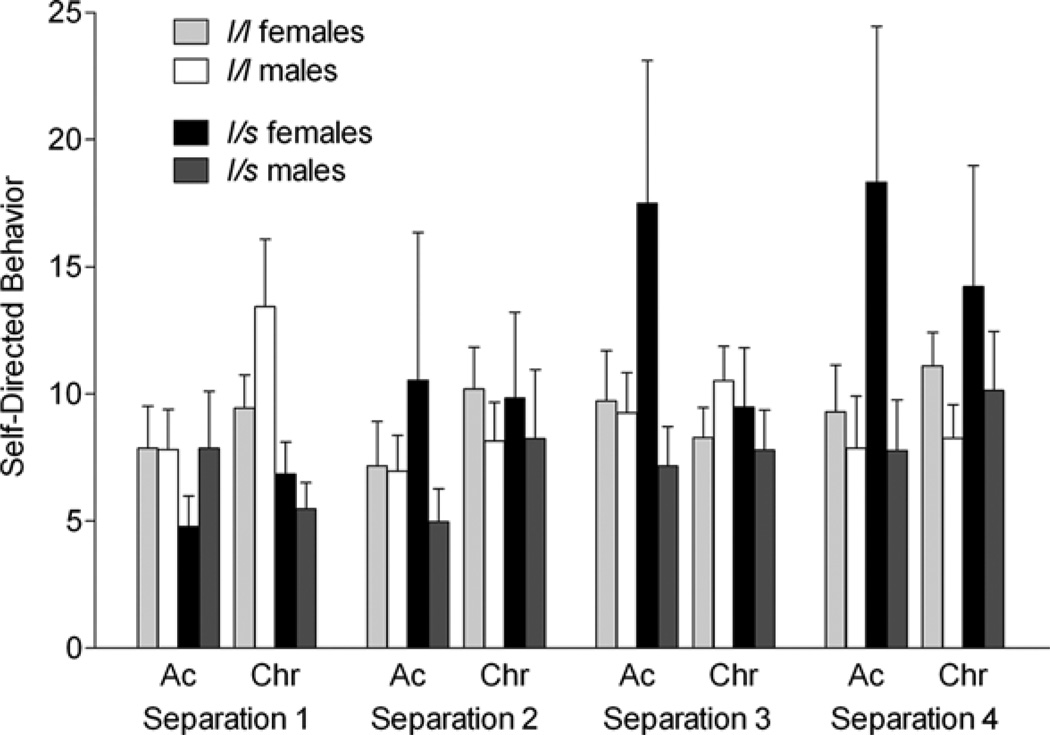
There was no main effects of genotype on self-directed behavior, but there was a two-way week of Separation × rh-5-HTTLPR Genotype interaction, F (1, 351) = 3.7, p = .01, for levels of self-directed behavior. In contrast to subjects with the l/l genotype, carriers of the short allele showed increased levels of self-directed behavior as a function of repeated separation (Figure 2). There were neither main nor interactive effects of rh-5-HTTLPR genotype on levels of locomotion (Figure 3), behavioral withdrawal (Figure 4), or vocalization (Figure 5).
Figure 2.
The effects of the rhesus serotonin transporter linked polymorphic region (rh-5-HTTLPR) polymorphism and sex on self-directed behavior (duration of seconds in a 300-s session) during the acute (Ac) and chronic phase (Chr) of four repeated separation stress. Across separations, levels of self-directed behavior were similar in the short-allele carriers and long/long (l/l) allele animals. However, long/short (l/s) allele animals showed lower levels during the first separation (S1; p < .05) but increased as a function of repeated maternal separation (levels during S4 were higher than S1; p < .05), whereas it remained stable in l/l animals during both phases. Bars depict mean ± standard error of the mean, and all post hoc tests were performed using Tukey–Kramer.
Discussion
The existence of two basic behavioral phenotypes, low (dove) and high (hawk) aggressive individuals, has been observed in a wide variety of animal species, including humans (Korte, Koolhaas, Wingfield, & McEwen, 2005). Although the former is aggressive and bold, adopts a more proactive coping style, and fight or flight responses to stress, the latter is more harm avoidant and exhibits reactive, anxietylike behaviors following stress (i.e., are more likely to freeze or hide). A conceptual framework has been put forth that suggests that differences in behavioral strategies may have implications not only in terms of selection, but in terms of vulnerability to stress-related pathology (Korte et al., 2005). In humans, functional genetic variants predict individual differences in susceptibilities to various forms of psychopathology (Caspi et al., 2002, 2003). Here, we report that rh-5-HTTLPR genotype and sex interact with duration and number of stress exposures to predict the patterns and types of behavioral responses that macaque infants exhibit during early development. Such findings may inform us of potential pathways through which 5-HTTLPR genotype could impact risk for stress-related disorders in humans.
In the present study, we report that macaque infants exhibit patterns of behavior that are potentially representative of both habituation and sensitization to prolonged, repeated maternal separation. Across subjects, levels of distress vocalizations and behavioral withdrawal decreased as a function of repeated separation stress (particularly from the first to second separations), whereas levels of environmental exploration increased. These changes could be interpreted as being representative of coping with the separation stressor (i.e., displacement behavior) or habituation. Conversely, behavior associated with arousal (locomotion), increased as a function of repeated exposure, suggesting there to be some sensitization as well.
Our analyses indicate that both sex and genotype play moderating roles on behavioral responses to stress in infant macaques. Of interest, these were prominent among those behaviors that exhibited sensitization (locomotion and self-directed behavior). Because selective pressures differ between the two sexes, males and females also exhibit differences in their responses to environmental challenge (Eme, 2007; Wood, Cowan & Baker, 2002). In some ways, the strategies adopted by males and females parallel those described in the hawk–dove model: a bold strategy of coping and adjustment; opposed by amore anxious, cautious strategy (Korte et al., 2005).
Of interest, the hawk–dove model maps well onto the proposed genetic structure of externalizing and internalizing human psychiatric disorders (Kendler, Prescott, Myers, & Neale, 2003; Schwandt et al., 2006). We found that, whereas males were more likely to exhibit increased levels of locomotion with repeated separation stress, females were more likely to increase their levels of self-directed behavior. Self-directed behavior is widely held as a measure of affective psychopathology or despair in the social separation model. This finding of more self-directed behaviors in females is consistent with humans studies showing increased risk for depression in females. This may also be of relevance to Taylor’s model, in which females are more likely to exhibit “tend and befriend” responses, whereas males adopt a “fight or flight” strategy (Taylor et al., 2000). Other studies performed in rhesus infants have reported higher levels of self-directed behavior and locomotion during separation stress among infants that also showed higher withdrawal behaviors in the stressfree home cage environment (Erickson et al., 2005). Because behaviorally inhibited infants are considered more reactive to stress, high levels of self-directed behavior and locomotion during separation are suggestive of higher stress reactivity. Although sex differences in self-directed behavior and locomotion were not reported in study cited above (Erickson et al., 2005, we have previously reported sexually dichotomous effects of genotype in behavioral and endocrine responses to stress (Barr, Newman, Shannon, et al., 2004; Schwandt et al., 2010). Such differences may not only represent sex-biased adaptations to stress, but are quite relevant to the sex differences in the incidence of externalizing and internalizing disorders that are observed in humans (Kendler et al., 2003).
Studies in both macaques and humans have shown that 5-HTTLPR genotype is associated with harm avoidance and anxiety-like behavior (Becker,El-Faddagh,Schmidt,&Laucht, 2007; Koller et al., 2008; Monteleone et al., 2006; Spinelli et al., 2007).We found that rh-5-HTTLPR short-allele carriers showed higher levels of environmental exploration compared to l/l infants, particularly during the chronic phases of separation stress. This finding may also map well onto the hawk–dove model and the genetic structure of externalizing and internalizing human psychiatric disorders (Kendler et al., 2003; Schwandt et al., 2006) described above. Although stress generally decreases levels of environmental exploration in nonhuman primates (Suomi et al., 1983), an observed increase in the occurrence of this behavior may be reflective of the type of behavioral response/adaptation exhibited by short-allele carriers suggestive of an externalizing coping strategy.
Consistent with our original hypothesis, we found that sensitization to repeated maternal separation stress was moderated by the rh-5-HTTLPR genotype. As was observed with females, short-allele carriers exhibited increased self-directed behavior following repeated maternal separation, similar to the internalizing response reported in women. Self-directed behavior is a form of behavioral pathology that is most often observed in highly reactive animals and in animals that are prone to despair, at times necessitating treatment with anxiolytic or antidepressant compounds (Hugo et al., 2003; Kraemer & Clarke, 1990; Suomi, 1991). In rhesus infants, if separations are prolonged in duration, the frequency of this behavior increases, and, for this reason, prolonged separation is often used to model how various factors contribute to this “depression-like” behavior in primates. Our study demonstrates that this behavior increases with repeated separations, particularly among females and short carriers. This finding is consistent with the fact that the incidence of depression is increased among women and that there is greater vulnerability for depression in short carriers. Although sensitization to repeated stress exposures would be expected to be an adaptive behavioral response, it may also increase risk for development of psychopathology in the face of high levels of cumulative stress exposure. Our findings in nonhuman primates represent evidence of an increased sensitivity to repeated and/or chronic stress in short carriers from a very early developmental time point, providing support for the 5-HTTLPR × Stress interaction reported in humans (Caspi et al., 2003; Grabe et al., 2009; Stein, Schork, & Gelernter, 2008).
The 5-HTTLPR genotype may be a predictor of environmental sensitivity, a trait that would be predicted to be under selection. With regard to the relevance to genotype moderated effects of the environment on vulnerability to psychopathology in humans, it has been hypothesized that the short allele increases sensitivity not only to stressful/negative life events but in some cases and situations to positive environmental stimuli as well (Belsky et al., 2009; Taylor et al., 2006). In this context, carriers of the short allele may also be more protected when surrounded by a supporting/positive environment. In this study, mothers were removed from the social group, whereas infants stayed behind in the home cage, along with other members of the social group. In line with the increased environmental susceptibility hypothesis (Belsky et al., 2009), our data in nonhuman primates show that during the first separation levels of self-directed behavior were higher in l/l animals compared to short-allele carriers, suggesting that during the first stress exposure long homozygotes may have been less able to cope with the stress of maternal absence, even in an enriched/positive social environment. However, whereas in l/l animals’ levels of self-directed behavior remained stable, l/s subjects increased the level of this behavior over repeated separations, paralleling Caspi et al.’s (2003) findings that effects of the short allele is exaggerated when individuals are exposed to increased or prolonged stressors.
A major advantage to studying nonhuman primates is the ability to follow animals prospectively from birth and to then control stress exposure. By examining how behavioral responses to stress occur over time in controlled animal studies, we can examine the trajectory through which stress-induced behavioral changes occur, even from very early developmental time points. Although we have previously investigated whether the rh-5-HTTLPR short allele predicts phenotypic outcomes in the face of severe early stress exposure and have repeatedly demonstrated Gene × Environment interactions that translate to the human condition (Barr & Goldman, 2006), the present data are unique and particularly important for several reasons. First, the study was a within-subjects study, conducted in infant macaques raised with their mothers in social groups, rather than comparing mother-reared to nursery-reared animals. Second, the behavioral results reflect the response to a stressor that commonly occurs in nature, both in macaques and in humans (maternal separation). Third, we were able to examine how behavioral responses of varying chronicity and frequency were moderated by genotype. Therefore, the present findings may be easily translatable to and highly informative of the human condition.
Previous studies from our laboratory have repeatedly shown influences of the rh-5-HTTLPR short allele in combination with an adverse rearing environment (Barr, Newman, Schwandt, et al, 2004; Barr, Newman, Shannon, et al., 2004; Spinelli et al., 2007). However, in the context of a “normal” early-life environment, the effect of the 5-HTTLPR genotype has been reported in some studies (Bethea et al., 2004; Trefilov, Berard, Krawczak, & Schmidtke, 2000), but not in others (Rogers, Shelton, Shelledy, Garcia, & Kalin, 2008). Bethea et al. (2004) reported that the short allele predicted increased anxiety-related behaviors using various behavioral paradigms known to elicit fearful/anxious responses. However, although Rogers et al. (2008) demonstrated heritability of fear responses, they did not observe an effect of rh-5-HTTLPR genotype. Although somewhat speculative, one difference between these studies and ours is that our subjects were studied under the conditions of a repeated chronic stressor. It may be that the phenotypic effect of the short allele is only present under conditions of chronic or repeated stress.
One of the limitations of our study is that, due to the limited number of s/s animals in our colony, we could not analyze the effect of repeated maternal separations in what may be the most sensitive group of subjects. Finally, we did not have physiological measures of stress reactivity or brain–behavioral correlates to address possible mechanisms underlying the reported behavioral differences. Neuroimaging studies have shown that the short allele predicts increased amygdala activation in response to negative stimuli (Hariri et al., 2002; Munafò et al., 2008) and impaired top-down modulation of amygdala response (i.e., decreased amygdala–prefrontal cortex (PFC) coupling). The 5-HTTLPR has been shown to mediate cortisol reactivity to stress in humans (Way & Taylor, 2010) and nonhuman primates (Barr, Newman, Schwandt, et al., 2004). Cortisol is known to increase amygdala activation, a mechanism that could drive anticipatory angst and sensitization to stress (Schulkin, 2006), especially in individuals in which PFC-mediated modulation of amygdala reactivity is impaired. Further studies are warranted to investigate the neurobiological mechanism by which the 5-HTTLPR may affect behavior during chronic/repeated stress exposure.
Although evidence in humans for a direct link between mood disorders and the 5-HTTLPR short allele remains controversial (Caspi et al., 2003; Caspi, Hariri, Holmes, Uher,&Moffitt, 2010; Hamet & Tremblay, 2005; Levinson, 2006; Risch et al., 2009), the increased risk for psychopathology that has been observed among short carriers may be rooted in 5-HTTLPR-mediated differences in individual responses to stressful life events (Murphy et al., 2004). Our data in nonhuman primates support this hypothesis and suggest that in children, the short allele may increase reactivity to repeated, chronic stressors, leaving them more vulnerable to affective psychopathology. This may make such individuals more reactive during early development, may influence the trajectory of responses over periods of adjustment or stress and may predict vulnerability to psychopathology later in life.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism (NIAAA), and National Institute of Child Health and Development (NICHD). Subjects were treated in accordance with the Guide for the Care and Use of Laboratory Animals, as adopted and promulgated by the National Institutes of Health (NIH). The procedures performed in this study were approved by the Institutional Animal Care and Use Committee. We thank the NIAAA and NICHD research fellows and the animal care staff at the NIH Animal Center for their assistance in data collection.
References
- Barr CS, Goldman D. Non-human primate models of inheritance vulnerability to alcohol use disorders. Addiction Biology. 2006;11:374–385. doi: 10.1111/j.1369-1600.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Schwandt M, Shannon C, Dvoskin RL, Lindell SG, et al. Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. Proceedings of the National Academy of Science of the United States of America. 2004;101:12358–12363. doi: 10.1073/pnas.0403763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, et al. Rearing condition and rh5-HTTLPR interact to influence limbic–hypothalamic–pituitary–adrenal axis response to stress in infant macaques. Biological Psychiatry. 2004;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Barr CS, Schwandt ML, Lindell SG, Higley JD, Maestripieri D, Goldman D, et al. Variation at the mu-opioid receptor gene (OPRM1) influences attachment behavior in infant primates. Proceeding of the National Academy of Science of the United States of America. 2008;105:5277–5281. doi: 10.1073/pnas.0710225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K, El-Faddagh M, Schmidt MH, Laucht M. Is the serotonin transporter polymorphism (5-HTTLPR) associated with harm avoidance and internalising problems in childhood and adolescence? Journal of Neural Transmission. 2007;114:395–402. doi: 10.1007/s00702-006-0577-4. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Molecular Psychiatry. 2009;14:746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Streicher JM, Coleman K, Pau FKY, Moessner R, Cameron JL. Anxious behavior and fenfluramine-induced prolactin secretion in young rhesus macaques with different alleles of the serotonin reuptake transporter polymorphism. Behavor Genetics. 2004;34:295–307. doi: 10.1023/B:BEGE.0000017873.61607.be. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment and loss. New York: Basic Books; 1969. [Google Scholar]
- Brummett BH, Boyle SH, Siegler IC, Kuhn CM, Ashley-Koch A, Jonassaint CR, et al. Effects of environmental stress and gender on associations among symptoms of depression and the serotonin transporter gene linked polymorphic region (5-HTTLPR) Behavior Genetics. 2008;38:34–43. doi: 10.1007/s10519-007-9172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: The serotonin transporter in emotion regulation and social cognition. Nature Neuroscience. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Molecular Psychiatry. 2002;7:1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- Coe CL, Glass JC, Wiener SG, Levine S. Behavioral, but not physiological, adaptation to repeated separation in mother and infant primates. Psychoneuroendocrinology. 1983;8:401–409. doi: 10.1016/0306-4530(83)90019-7. [DOI] [PubMed] [Google Scholar]
- Eme RF. Sex differences in child-onset, life-course-persistent conduct disorder. A review of biological influences. Clinical Psychology Review. 2007;27:607–627. doi: 10.1016/j.cpr.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Erickson K, Gabry KE, Lindell S, Champoux M, Schulkin J, Gold P, et al. Social withdrawal behaviors in nonhuman primates and changes in neuroendocrine and monoamine concentrations during a separation paradigm. Development Psychobiology. 2005;46:331–339. doi: 10.1002/dev.20061. [DOI] [PubMed] [Google Scholar]
- Fanous AH, Neale MC, Aggen SH, Kendler KS. A longitudinal study of personality and major depression in a population-based sample of male twins. Psychological Medicine. 2007;37:1163–1172. doi: 10.1017/S0033291707000244. [DOI] [PubMed] [Google Scholar]
- Garber J. Depression in children and adolescents: Linking risk research and prevention. American Journal of Preventive Medicine. 2006;6(Suppl. 1):S104–S125. doi: 10.1016/j.amepre.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Grabe HJ, Spitzer C, Schwahn C, Marcinek A, Frahnow A, Barnow S, et al. Serotonin transporter gene (SLC6A4) promoter polymorphisms and the susceptibility to posttraumatic stress disorder in the general population. American Journal of Psychiatry. 2009;166:926–933. doi: 10.1176/appi.ajp.2009.08101542. [DOI] [PubMed] [Google Scholar]
- Gross C, Hen R. The developmental origins of anxiety. Nature Reviews Neuroscience. 2004;5:545–552. doi: 10.1038/nrn1429. [DOI] [PubMed] [Google Scholar]
- Hamet P, Tremblay J. Genetics and genomics of depression. Metabolism. 2005;54(Suppl. 1):10–15. doi: 10.1016/j.metabol.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Dougherty LR, Maloney B, Olino TM, Sheikh H, Durbin CE, et al. Early-emerging cognitive vulnerability to depression and the serotonin transporter promoter region polymorphism. Journal of Affective Disorder. 2008;107:227–230. doi: 10.1016/j.jad.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB. Multiple, brief maternal separations in the squirrel monkey: Changes in hormonal and behavioral responsiveness. Physiology & Behavior. 1986;36:245–250. doi: 10.1016/0031-9384(86)90011-9. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. CSF monoamine metabolite concentrations vary according to age, rearing, and sex, are influenced by the stressor of social separation in rhesus monkeys. Psychopharmacology (Berlin) 1991;103:551–556. doi: 10.1007/BF02244258. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biological Psychiatry. 1992;32:127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Hugo C, Seier J, Mdhluli C, Daniels W, Harvey BH, Du Toit D, et al. Fluoxetine decreases stereotypic behavior in primates. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2003;27:639–643. doi: 10.1016/S0278-5846(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A longitudinal twin study of personality and major depression in women. Archives of General Psychiatry. 1993;50:853–862. doi: 10.1001/archpsyc.1993.01820230023002. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Koller G, Zill P, Skoruppa T, Bondy B, Preuss UW, Soyka M. Low level of harm avoidance is associated with serotonin transporter functional haplotype in alcohol-dependent individuals. Psychiatric Genetics. 2008;18:59–63. doi: 10.1097/YPG.0b013e3282f60333. [DOI] [PubMed] [Google Scholar]
- Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: Benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neuroscience and Biobehavioral reviews. 2005;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Kraemer GW, Clarke AS. The behavioral neurobiology of selfinjurious behavior in rhesus monkeys. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 1990;14:S141–S168. doi: 10.1016/0278-5846(90)90092-u. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Levinson DF. The genetics of depression: A review. Biological Psychiatry. 2006;60:84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Mineka S, Suomi SJ. Social separation in monkeys. Psychological Bulletin. 1978;85:1376–1400. [PubMed] [Google Scholar]
- Mineka S, Suomi SJ, DeLizio R. Multiple separations in adolescent monkeys: An opponent-process interpretation. Journal of Experimental Psychology: General. 1981;110:56–85. doi: 10.1037//0096-3445.110.1.56. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Santonastaso P, Mauri M, Bellodi L, Erzegovesi S, Fuschino A, et al. Investigation of the serotonin transporter regulatory region polymorphism in bulimia nervosa: Relationships to harm avoidance, nutritional parameters, and psychiatric comorbidity. Psychosomatic Medicine. 2006;68:99–103. doi: 10.1097/01.psy.0000195746.52074.63. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: A meta-analysis. Biological Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DL, Lerner A, Rudnick G, Lesch KP. Serotonin transporter: Gene, genetic disorders, and pharmacogenetics. Molecular Interventions. 2004;4:109–123. doi: 10.1124/mi.4.2.8. [DOI] [PubMed] [Google Scholar]
- Neigh GN, Gillespie CF, Nemeroff CB. The neurobiological toll of child abuse and neglect. Trauma Violence Abuse. 2009;10:389–410. doi: 10.1177/1524838009339758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. Journal of the American Medical Association. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Shelton SE, Shelledy W, Garcia R, Kalin NH. Genetic influences on behavioral inhibition and anxiety in juvenile rhesus macaques. Genes, Brain and Behavior. 2008;7:463–469. doi: 10.1111/j.1601-183X.2007.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwandt ML, Erickson K, Barr CS, Lindell SG, Suomi SJ, Higley JD. Effects of early experience and lack of social support on the endocrine and behavioral responses of rhesus macaques (Macaca mulatta) to separation stress. Neuropsychopharmacology. 2006;31(Suppl. 1):147. [Google Scholar]
- Shannon C, Schwandt ML, Champoux M, Shoaf SE, Suomi SJ, Linnoila M, et al. Maternal absence and stability of individual differences in CSF 5-HIAA concentrations in rhesus monkey infants. American Journal of Psychiatry. 2005;162:1658–1664. doi: 10.1176/appi.ajp.162.9.1658. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use vulnerability to addiction. Annals of the New York Academy of Science. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöberg RL, Nilsson KW, Nordquist N, Ohrivik J, Leppert J, Lindström L, et al. Development of depression: Sex and the interaction between environment and a promotor polymorphism of the serotonin trarnsporter gene. International Journal of Neuropsychopharmacology. 2006;9:443–449. doi: 10.1017/S1461145705005936. [DOI] [PubMed] [Google Scholar]
- Sloan DM, Kornstein SG. Gender differences in depression and response to antidepressant treatment. Psychiatry Clinic of North America. 2003;26:581–594. doi: 10.1016/s0193-953x(03)00044-3. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Schwandt ML, Lindell SG, Newman TK, Heilig M, Suomi SJ, et al. Association between the serotonin transporter linked polymorphic region and behavior in rhesus macaques during a separation paradigm. Development and Psychopathology. 2007;19:977–987. doi: 10.1017/S095457940700048X. [DOI] [PubMed] [Google Scholar]
- Stein MB, Schork NJ, Gelernterc J. Gene-by-environment (serotonin transporter and childhood maltreatment) interaction for anxiety sensitivity, an intermediate phenotype for anxiety disorders. Neuropsychopharmacology. 2008;33:312–319. doi: 10.1038/sj.npp.1301422. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Primate separation models of affective disorders. In: Madden JI, editor. Neurobiology of learning, emotion and affect. New York: Raven Press; 1991. pp. 195–214. [Google Scholar]
- Suomi SJ. Early determinants of behaviour: Evidence from primate studies. British Medical Bulletin. 1997;53:170–184. doi: 10.1093/oxfordjournals.bmb.a011598. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Mineka S, De Lizio RD. Short- and long-term effects of repetitive mother–infant separations on social development in rhesus monkeys. Developmental Psychology. 1983;19:770–786. [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychological Review. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biological Psychiatry. 2006;60:671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Trefilov A, Berard J, Krawczak M, Schmidtke J. Natal dispersal in rhesus macaques is related to serotonin transporter gene promoter variation. Behavior Genetics. 2000;30:295–301. doi: 10.1023/a:1026597300525. [DOI] [PubMed] [Google Scholar]
- Way B, Taylor SE. The serotonin transporter promoter polymorphism is associated with cortisol response to psychosocial stress. Biological Psychiatry. 2010;67:487–492. doi: 10.1016/j.biopsych.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JJ, Cowan PA, Baker BL. Behavior problems and peer rejection in preschool boys and girls. Journal of Genetic Psychology. 2002;163:72–88. doi: 10.1080/00221320209597969. [DOI] [PubMed] [Google Scholar]