Abstract
Childhood trauma is associated with hypothalamic–pituitary–adrenal (HPA) axis dysregulation. Both factors increase risk for suicidal behavior. Corticotropin releasing hormone (CRH) regulates the HPA axis and its actions are moderated by a high-affinity binding protein (CRHBP). We hypothesized that CRHBP variation and interaction with childhood trauma might influence suicidal behavior. Moreover, there might be an additive effect with FKPB5, another HPA axis gene previously associated with suicidality in this dataset. African Americans were recruited: 398 treatment seeking patients with substance dependence (90% men; 120 suicide attempters) and 432 non-substance dependent individuals (40% men; 21 suicide attempters). A total of 474 participants (112 suicide attempters) completed the Childhood Trauma Questionnaire (CTQ). Haplotype-tagging SNPs were genotyped across CRHBP and, for completeness, across CRH, CRHR1 and CRHR2. FKBP5 genotypes were available. Three CRHBP SNPs rs6453267, rs7728378 and rs10474485 showed a nominally significant interaction with the continuous CTQ score to predict suicide attempt; rs7728378 remained significant after FDR correction. There was an additive effect with FKBP5: in the group exposed to high trauma, the prevalence of suicide attempt was 0.35–0.30 in carriers of either the FKBP5 rs3800373 major homozygote or the CRHBP rs7728378 major homozygote and 0.58 in carriers of both major homozygotes. Individuals without either major homozygote were resilient to the effects of childhood trauma (suicide attempt prevalence 0.24). Main effects of CRHBP rs6453267 and CRHR1 rs9900679, both unique to African ancestry, were detected. CRHBP variation may predispose, independently and additively, to suicidal behavior in individuals who have experienced childhood trauma.
Keywords: African Americans, Substance dependence, CTQ, CRH, CRHR1, CRHR2
1. Introduction
Short term activation of the stress-response system, a major element of which is the hypothalamic–pituitary–adrenal (HPA) axis, is essential for survival. In response to acute stress, hypothalamic corticotropin releasing hormone (CRH) stimulates the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary which in turn stimulates the release of the glucocorticoid hormone cortisol from the adrenal cortex. Cortisol binding to the glucorticoid receptor (GR) and the translocation of the GR from the cytoplasm into the nucleus in order to facilitate transcription of stress-response genes is moderated by a large molecular complex that includes FKBP5, a co-chaperone of hsp90 (Binder, 2009). The availability of CRH is regulated in part by a high-affinity binding protein (CRHBP) that is widely distributed throughout the body (Westphal and Seasholtz, 2006). A large proportion of total CRH is complexed with CRHBP and is therefore unavailable for receptor (CRHR1, CRHR2) activation (Behan et al., 1997).
Long term dysregulation of the HPA axis has been noted in several stress-related psychiatric disorders. Moreover, many studies have shown that childhood trauma can result in HPA axis dysregulation in adulthood and long-lasting increased risk for psychopathology (Enoch, 2011; Mann and Currier, 2010). Numerous studies have reported HPA axis dysfunction in individuals attempting or committing suicide (Arató et al., 1989; Coryell and Schlesser, 2001; Coryell et al., 2006; Jokinen et al., 2007; Jokinen and Nordstrom, 2009; Lindqvist et al., 2008a,b; Mann and Currier, 2007; Nemeroff et al., 1988; Pfennig et al., 2005; Roy, 1992; Roy et al., 1986).
Recently, CRHBP has gained prominence as a candidate gene for stress-related disorders (Binder et al., 2010; De Luca et al., 2010; Enoch et al., 2008; Kertes et al., 2010; Ray, 2011). Three of these studies (Binder et al., 2010; De Luca et al., 2010; Kertes et al., 2010) also investigated CRH, CRHR1 and CRHR2 SNPs but found significant results only for CRHBP. Our study participants were African Americans, many of whom had been diagnosed with substance dependence, a known risk factor for suicidal behavior (Roy, 2001, 2002, 2003; Roy and Janal, 2007). We previously showed that childhood trauma predicted suicidality in this sample (Roy et al., 2010). Based upon the results of the studies described above, we hypothesized that CRHBP variation and also the interaction with childhood trauma might influence suicidal behavior. We further hypothesized that we would find phenotype associations with distal CRHBP SNPs since all five of the earlier studies found associations only within this region of the gene. For the sake of completeness we also analyzed the effects of variation in CRH, CRHR1 and CRHR2, but expected negative results based on the earlier findings.
We previously showed that the interaction between childhood trauma and FKBP5 variation influenced suicide attempts in this sample of African Americans (Roy et al., 2010). Since the effectiveness of CRH in stimulating ACTH with resultant cortisol release is governed in part by CRHBP and since the effectiveness of cortisol in influencing gene expression via the GR is moderated by a molecular complex that includes FKBP5, we further hypothesized that there would be an additive effect between CRHBP and FKBP5 variation in the interaction with childhood trauma to predict risk for suicide attempt.
2. Methods
2.1. Participants
The sample consisted of two subsamples of African–American participants recruited from the same geographic area that were pooled for this study. The first subsample consisted of 706 African–American patients (634 men, 72 women) recruited from the Substance Abuse Treatment Program (SATP) at the Department of Veteran Affairs New Jersey Healthcare System (VANJHCS), East Orange Campus. Nearly all were inpatients however a few were recruited from the SATP outpatient clinic or from the methadone clinic. Their mean (SD) age was 45.2 (7.9) years. Criteria for inclusion in the study were that patients were ≥18 years of age, met DSM-IV criteria for alcohol, cocaine, or opiate dependence, self-identified as African American and were abstinent. Exclusion criteria included mental retardation, dementia and acute psychosis. Patients were interviewed by a psychiatrist (AR) using the substance abuse section of the Structured Clinical Interview for DSM-IV (SCID) (Spitzer et al., 1995) to establish lifetime substance dependence diagnoses. The diagnosis of suicide attempt was implemented by A.R. after interviewing the patient with a standard series of clinical questions and reviewing all available collateral information, e.g. from mental health program staff, medical records, treating physicians. A suicide attempt was defined as a self-destructive act with some intent to end one’s life that was not self-mutilatory in nature. Actions without suicide intent such as delicate self cutting and accidental drug overdose were excluded.
The second subsample was recruited as potential controls for genetic studies in substance dependence: 759 African American men (N = 307) and women (N = 452) recruited from a consecutive series of insulin-dependent diabetic outpatients seen at an ophthalmology clinic (54%) at the University of Medicine and Dentistry, New Jersey Medical School (UMDNJ), Newark, NJ and from churches and a blood bank in Newark, NJ, (46%). Their mean (SD) age was 34.3 (10.1) years. The diabetics underwent two physician-conducted structured interviews over a 6 year period that included questions about suicidal behavior and substance abuse (Roy, 2000). Any reports of suicidal behavior were discussed with the medical staff by AR. The participants recruited from churches and a blood bank underwent a semi-structured psychiatric interview conducted by a trained research worker (a former substance abuse counselor) that included a standard series of questions about lifetime suicidal behavior and substance abuse/dependence (reviewed by AR).
After a full description of the study was provided, all participants gave written informed consent to the study that was approved by the Institutional Review Boards of the VANJHCS and UMDNJ.
2.2. Childhood Trauma Questionnaire (CTQ)
The CTQ (28 item version) was completed by 538 patients (92% men) with substance dependence; 166 (90% men) had attempted suicide. The CTQ was completed by 342 non-substance dependent participants (42% men), 32 (28% men) of whom had attempted suicide (see Fig. 1).
Fig. 1.
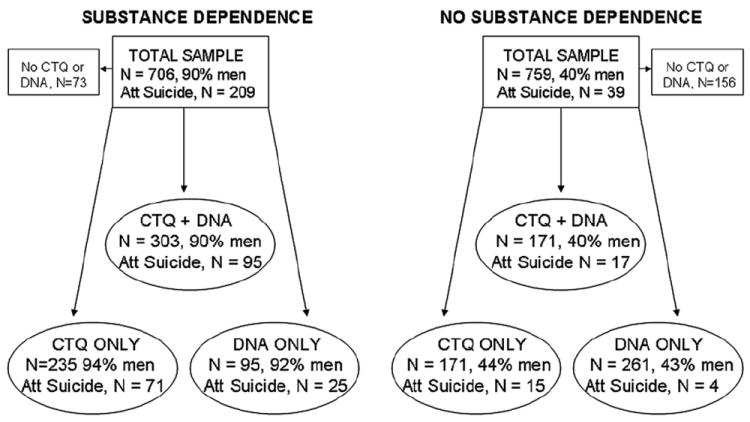
Flow chart detailing dataset.
The CTQ yields scores (5–25 each) for physical abuse, physical neglect, emotional abuse, emotional neglect, and sexual abuse, as well as a total score (25–125). Reliability and validity of the CTQ has been demonstrated, including in drug abusers and African American populations (Bernstein et al., 1994, 1997, 2003; Bernstein and Fink, 1998; Scher et al., 2001; Thombs et al., 2007). The CTQ was used as a continuous measure in all logistic regression analyses.
A dichotomous total CTQ score was derived solely to illustrate the direction of interaction effects on suicide attempt. A total CTQ score ≥ 1 SD above the mean CTQ score of non-substance dependent/no suicide attempt participants (36.49 (11.67), i.e. ≥48) was designated ‘high trauma’ (N = 290); lower total CTQ scores (<48) were designated ‘low trauma’ (N = 590). This approach has been used in our earlier studies: Enoch et al. (2010) and Roy et al. (2010).
2.3. Genotyping
Genomic DNA was isolated from blood using standard protocols. A genomic region containing sequence 5 kb upstream and 1 kb downstream of CRHBP was retrieved from NCBI Human Build 35.1. Haplotype-tagging SNPs, derived from the African HapMap sample, were identified using a previously described design pipeline (Hodgkinson et al., 2008). Eight CRHBP SNPs, listed in Table 1, were genotyped using the Illumina GoldenGate platform (Hodgkinson et al., 2008). Using the identical methodology, four CRH, nine CRHR1 and eleven CRHR2 haplotype-tagging SNPs were genotyped (Table 1). All SNPs were in Hardy–Weinberg equilibrium other than CRHBP rs32897 (p = 0.0001) that was excluded from analyses.
Table 1.
The CRH gene complex: description of SNPs.
| SNPs
|
Location
|
Base variation | MAF in AAs | Allele Freqa in HapMap, CEU–Asian | |
|---|---|---|---|---|---|
| CRHBP | Chr 5 | Gene | |||
| rs3792738 | 76283540 | 5′ UTR | C > A | 0.08 | 0.06–0.18 |
| rs328967b | 76286728 | Intron | A > G | 0.31 | 0.10–0.18 |
| rs6453267 | 76291512 | Intron | G > A | 0.22 | 0.00–0.01 |
| rs7728378 | 76295106 | Intron | C > T | 0.23 | 0.58–0.48 |
| rs1875999 | 76300738 | 3′UTR | A > G | 0.43 | 0.36–0.53 |
| rs10474485 | 76306609 | Alt isoform | C > A | 0.39 | 0.18–0.37 |
| rs7704995c | 76310293 | Alt isoform | C > T | 0.30 | 0.65–0.43 |
| rs1500 | 76312594 | Alt isoform | C > G | 0.30 | 0.65–0.44 |
| CRH | Chr 8 | ||||
| rs6996265 | 67248904 | Intron | A > G | 0.42 | 0.93–1.00 |
| rs3176921 | 67253933 | 5′ region | C > T | 0.41 | 0.87–1.00 |
| rs6472257 | 67254734 | 5′ region | C > T | 0.34 | 0.07–0.00 |
| rs5030875 | 67256620 | Intergenic | T > G | 0.18 | 0.04–0.00 |
| CRHR1 | Chr 17 | ||||
| rs9900679 | 41223923 | Intron | G > C | 0.06 | 0.00–0.00 |
| rs4792887 | 41232791 | Intron | C > T | 0.33 | 0.07–0.00 |
| rs110402 | 41235818 | Intron | C > T | 0.33 | 0.45–0.87 |
| rs242924 | 41241147 | Intron | C > A | 0.32 | 0.45–0.87 |
| rs8072451 | 41249496 | Intron | C > T | 0.07 | 0.19–0.00 |
| rs81189 | 41250579 | Intron | G > C | 0.37 | 0.46–0.84 |
| rs242939 | 41251360 | Intron | A > G | 0.31 | 0.07–0.00 |
| rs173365 | 41256855 | Intron | T > C | 0.45 | 0.60–0.88 |
| rs17689918 | 41265869 | Intron | G > A | 0.05 | 0.15–0.00 |
| CRHR2 | Chr 7 | ||||
| rs3779250 | 30467500 | Intron | G > A | 0.15 | 0.70–0.49 |
| rs973002 | 30472144 | Intron | A > G | 0.12 | 0.16–0.39 |
| rs8192498 | 30475052 | Val240Leu | G > A | 0.02 | 0.00–0.00 |
| rs2190242 | 30482715 | Intron | A > C | 0.19 | 0.20–0.58 |
| rs2284217 | 30486848 | Intron | G > A | 0.37 | 0.19–0.47 |
| rs2014663 | 30488813 | Intron | A > G | 0.18 | 0.14–0.44 |
| rs6967702 | 30495736 | 5′ region | G > C | 0.10 | 0.00–0.00 |
| rs4723002 | 30498941 | Intergenic | A > G | 0.16 | 0.08–0.22 |
| rs255102 | 30504404 | Intergenic | T > A | 0.47 | 0.71–0.85 |
| rs255105 | 30505347 | Intergenic | T > C | 0.18 | 0.71–0.61 |
| rs255125 | 30516251 | Intergenic | G > A | 0.47 | 0.24–0.54 |
AAs = African Americans in this study (N = 830). The 3 distal CRHBP SNPs are located in an alternative isoform, expressed in brain.
Frequency in HapMap European (CEU) and Asian populations of the minor allele in African Americans.
This SNP was not in Hardy–Weinberg equilibrium and was therefore excluded from analyses.
Now rs1715747.
2.4. Summary of dataset
An illustration of the dataset used in this study is provided in Fig. 1. DNA was available for a total of 830 participants: 141 suicide attempters and 689 non-suicide attempters. For G × E analyses, both DNA and CTQ scores were available for a total of 474 individuals, including 112 suicide attempters and 362 non-suicide attempters. Missing DNA and CTQ data was random and showed no selection bias.
2.5. Assessment of population stratification using ancestry informative markers
The samples were genotyped for 186 HapMap derived ancestry informative markers (AIMS) (Hodgkinson et al., 2008). The same AIMS were also genotyped in 1051 individuals from the 51 worldwide populations represented in the HGDP-CEPH Human Genome Diversity Cell Line Panel (http://www.cephb.fr/HGDP-CEPH-Panel). Structure 2.2 (http://pritch.bsd.uchicago.edu/software.html) was run simultaneously using the AIMS genotypes from our sample and the 51 CEPH populations to identify population substructure and compute individual ethnic factor scores. This ancestry assessment identifies seven ethnic factors that have been previously described (Hodgkinson et al., 2008). In this sample of African Americans, the mean African factor score was 0.77 (median = 0.81) and the mean European factor score was 0.09 (median value = 0.04). Both a Mid East factor and an Asian factor had a mean score of 0.06 (median 0.04).
2.6. Statistical analyses
Logistic regression analyses with attempted suicide as the dependent variable were undertaken using JMP 7 software and yielded likelihood ratio (L-R) χ2 results. Backward stepwise regression was performed with predictor variables being eliminated from the model in an iterative process if the level of significance was p > 0.1. The continuous CTQ score and European ethnic factor score and the dichotomous variables: age, sex and substance dependence diagnosis were initially included as predictor variables. Age and sex had no significant effects in any model. Substance dependence and the continuous CTQ score were included in all models since they had a significant effect.
There was a significant substance dependence × CTQ interaction for all SNPs, therefore this interaction term was included in all analyses. The genotype × CTQ interaction term was included where p ≤ 0.1. There was no substance dependence × genotype interaction (p = 0.5–0.9) nor was there any association between CRHBP SNP genotypes and substance dependence.
Backward stepwise regression was performed for each of the seven CRHBP SNPs, as shown in Table 2; likewise for the CRH, CRHR1 and CRHR2 SNPs.
Table 2.
The influence of CRHBP SNPs and childhood trauma on attempted suicide.
| SNPs | Gene effect
|
European ethnic factor
|
Childhood trauma effect
|
G × E interaction
|
Whole model
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| L-R χ2 | p value | L-R χ2 | p value | L-R χ2 | p value | L-R χ2 | p value | df | p value | |
| rs3792738 | 2.8 | 0.094 | 29.3 | <0.0001 | 5 | <0.0001 | ||||
| rs6453267 | 7.2 | 0.0072 [0.0504] | 3.4 | 0.065 | 31.3 | <0.0001 | 4.4 | 0.0365 [0.0852] | 6 | <0.0001 |
| rs7728378 | 4.4 | 0.035 | 23.8 | <0.0001 | 6.7 | 0.0098 [0.0490] | 6 | <0.0001 | ||
| rs1875999a | 2.8 | 0.095 | 32.1 | <0.0001 | 4.6 | 0.0986 [0.1232] | 8 | <0.0001 | ||
| rs10474485a | 2.7 | 0.100 | 29.8 | <0.0001 | 6.9 | 0.0320 [0.0800] | 8 | <0.0001 | ||
| rs7704995 | 4.1 | 0.042 | 24.1 | <0.0001 | 2.2 | 0.1396 [0.1396] | 6 | <0.0001 | ||
| rs1500 | 4.0 | 0.047 | 26.6 | <0.0001 | 3.1 | 0.0776 [0.1232] | 6 | <0.0001 | ||
Results are derived from logistic regression analyses for each CRHBP SNP and are for effect likelihood ratio (L-R) tests. Results for p ≤ 0.1 are presented. FDR adjusted p values are provided in parentheses. df = degrees of freedom. N = 474. Childhood trauma was measured as a continuous variable using the childhood trauma questionnaire (CTQ). Substance dependence (yes/no) was included as an independent variable in all SNP analyses (L-R χ2 = 17.0–22.1, p < 0.0001). The substance dependence × CTQ interaction was included in all analyses (L-R χ2 = 5.7–8.4, p = 0.017–0.004). The whole model analysis for each SNP accounted for 0.13–0.14 of the variance.
Bold values indicates significant findings.
All 3 genotypes were included in analyses for these two SNPs since the MAF were >0.30. For the remaining SNPs the minor homozygotes and heterozygotes were combined to increase the power of the analyses.
In order to prevent spurious G × E results due to small sample sizes, for each of the SNPs that had minor allele frequencies (MAF) of ≤0.30 the minor homozygotes and heterozygotes were combined as one group.
Haplotype frequencies were estimated using a Bayesian approach implemented with PHASE (Stephens and Donnelly, 2003). Haploview version 2.04 Software (Whitehead Institute for Biomedical Research, USA) was used to produce linkage disequilibrium (LD) matrices. Since rare and uncommon haplotypes are subject to estimation errors because of increased sampling variance, all analyses were conducted with haplotypes ≥5% frequency. These haplotypes were included in models for CRHR1 and for CRH together with the predictor variables listed above and backward stepwise regression was performed.
Based on earlier studies our a priori hypothesis was that we would find phenotypic associations within the distal region of CRHBP. Nevertheless, in the African Americans the five distal SNPs were not in LD and therefore we could not predict which of these SNPs might have an effect. Therefore we applied a False Discovery Rate (FDR) correction (Benjamini et al., 2001) for comparisons across the five SNPs. In contrast, based on earlier publications our hypothesis was that there would be no main or G × E effects on suicidality of the two proximal SNPs, rs3792738 and rs6453267 and therefore we applied FDR corrections for all 7 CRHBP SNPs. The analysis of additive effects of FKBP5 and CRHBP variation was strongly hypothesis-driven and no corrections were warranted. For CRHR1, one haplotype block extended distally across the gene and included seven SNPs. There were two proximal SNPs that were not in LD and were therefore independent of each other and of the haplotype block. Therefore an adequate Bonferroni correction for these 2 SNPs would be 0.05/3 = 0.017.
3. Results
Table S1 provides the mean (SD) total CTQ and subscale scores for the groups of participants who had and had not, attempted suicide. Fig. 2 shows that, irrespective of substance dependence and gender, suicidal behavior was associated with higher childhood trauma scores and that the proportion of women attempting suicide was numerically but not significantly greater compared with men in both the substance dependent group (0.40 vs 0.30, p = 0.265) and the non-substance dependent group (0.12 vs 0.06, p = 0.524).
Fig. 2.
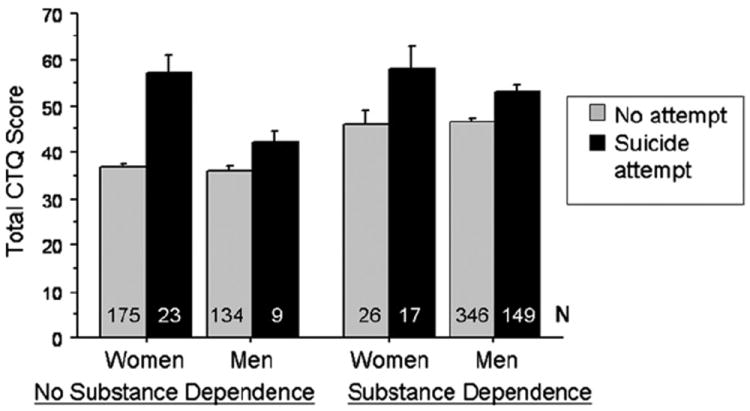
Suicidal behavior is associated with increased childhood trauma in both substance dependent and non dependent men and women. Error bars are standard errors. N = number of individuals in each group.
3.1. CRHBP
There was no haplotype block structure in the African Americans (Fig. 3). Therefore analyses were conducted with the individual SNPs.
Fig. 3.
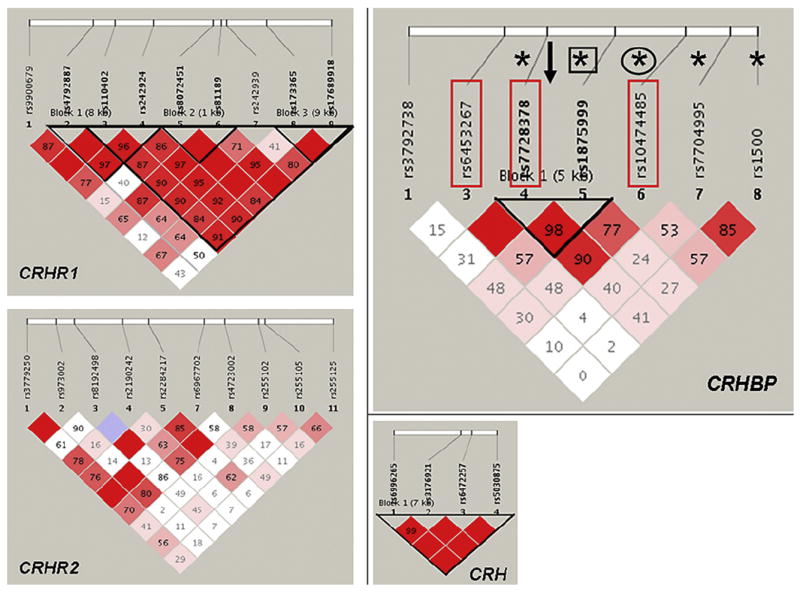
CRHBP, CRH, CRHR1 and CRHR2 Haplotype block structure. The numbers in the squares refer to pairwise linkage disequilibrium measured as D′. Haplotype blocks were defined using a setting of average pairwise D′ within-block of ≥0.80. CRHBP: The direction of gene transcription is from left to right. Rectangles denote SNPs that had a main or G × E effect on suicidal behavior in the current study. *denotes SNPs that had significant effects on alcohol use disorders, anxiety disorders and EEG alpha power (Enoch et al., 2008). Circle denotes a SNP implicated in depressive symptoms (Kertes et al., 2010, Binder et al., 2010) and square denotes a SNP implicated in suicide in schizophrenics (De Luca et al., 2010). An arrow indicates the location of a SNP associated with stress-induced craving in heavy drinkers (Ray, 2011).
3.1.1. Main effects of CRHBP SNPs
Using the whole dataset of genotyped individuals (N = 830) and including the dichotomous ‘substance dependence diagnosis’ as a covariate, there was no main effect of any of the CRHBP SNPs on suicidal behavior.
3.1.2. Gene–environment (G × E) interactions
These analyses were conducted on the 474 participants who had both CTQ data and DNA (Fig. 1). From Table 2 it can be seen that within the whole logistic regression model there was a main effect of rs6453267. This variant allele, unique to individuals of African ancestry (Table 1), was more common in non-suicide (0.23 vs 0.19, p = 0.007). The result remained of trend significance after FDR correction (p = 0.0504).
All the interactive analyses shown in Table 2 were performed with the total CTQ score, a continuous variable. There were nominally significant G × E interactive effects for rs7728378, rs10474485 and rs6453267 but after FDR correction, only the results for rs7728378 remained significant.
For rs7728378 and rs10474485 the major homozygote interacted with total CTQ score to predict suicide attempt. In order to illustrate the direction of this interactive effect on suicide attempt the dichotomous total CTQ score was used, the derivation of which was described earlier. Since the MAF was high (0.39) for rs10474485 we were able to identify the direction of effect of all three genotypes. The prevalence of suicide attempt in carriers of the major homozygote, heterozygote and minor homozygote was as follows: high trauma: 0.54, 0.27, 0.32; low trauma: 0.17, 0.20, 0.17.
3.1.3. G × E interactions: participants with substance dependence
The logistic regression analyses were repeated in the substance dependent individuals (N = 303). Even though the sample size was smaller than the total sample (N = 474) similar G × E results were obtained for the CRHBP SNPs. Substance dependent vs total sample: rs1500: p = 0.026 vs 0.078; rs10474485: p = 0.038 vs 0.032; rs7728378: p = 0.054 vs 0.0098. The main effect of rs6453267 remained significant: p = 0.037 (uncorrected).
3.2. Independent and additive effects of CRHBP and FKBP5
3.2.1. Independent effects
In order to test for an additive effect with CRHBP we selected the FKBP5 SNP rs3800373 that had the greatest G × E interactive effect within the logistic regression model (L-R χ2 = 6.6, 1df, p = 0.0103) in our earlier study (Roy et al., 2010). For this analysis we selected CRHBP rs7728378 that showed the strongest G × E result in the current study and in our earlier study (Enoch et al., 2008).
Fig. 4B illustrates the results of independent analyses in FKBP5 rs3800373 and CRHBP rs7728378 using the derived dichotomous CTQ score. For the sake of simplicity we designated the FKBP5 rs3800373 genotype groups as: major homozygote = FF; minor homozygote + heterozygote = ff. Likewise for the CRHBP rs7728378 genotype groups: major homozygote = CC; minor homozygote + heterozygote = cc. In the case of high childhood trauma, the prevalence of suicide attempt was 0.49 in carriers of the FKBP5 rs3800373 major homozygote (FF) and 0.40 in carriers of the CRHBP rs7728378 major homozygote (CC) whereas the prevalence in carriers of the minor homozygote/heterozygote was 0.28 (ff) and 0.31 (cc) respectively. Without exposure to childhood trauma, genotype had no effect on suicide risk (prevalence = 0.17–0.20).
Fig. 4.
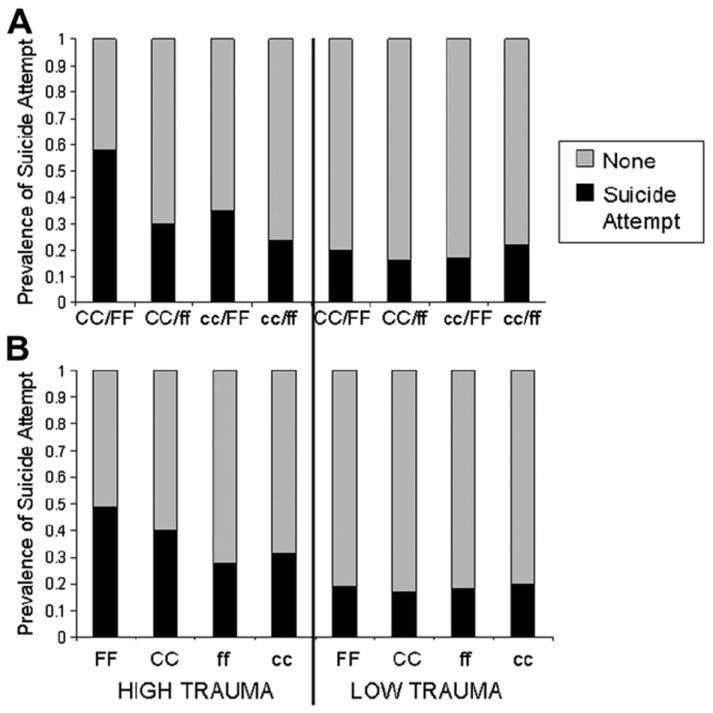
(A) Additive and (B) independent effects of CRHBP rs7728378 and FKBP5 rs3800373 variation on suicide risk after exposure to high childhood trauma. CRHBP rs7728378: CC/cc genotypes; FKBP5 rs3800373: FF/ff genotypes. CC, FF: major homozygote; cc, ff: minor homozygote + heterozygote. High level of childhood trauma defined as total CTQ score ≥ 1SD (≥48) above mean of non-substance dependent/no suicide attempt participants. Low level of childhood trauma defined as total CTQ score < 48. Panel A High Trauma: N’s: CC/FF (33), CC/ff (63), cc/FF (17), cc/ff (37); Panel A Low Trauma: N’s: CC/FF (60), CC/ff (109), cc/FF (47), cc/ff (68). Panel B High Trauma: N’s: FF (51), CC (103), ff (104), cc (61); Panel B Low Trauma: N’s: FF (108), CC (186), ff (181), cc (133). Note: the N’s do not correspond exactly since some individuals had genotypes for only one of the SNPs.
3.2.2. Additive effects
An analysis of the interaction of total CTQ score (continuous variable) with combined FKBP5 rs3800373 and CRHBP rs7728378 genotypes for each individual was significant within the logistic regression model: L-R χ2 =11.6, 3 df, p = 0.0087. The interaction term contributed 2.4% of the variance within the total model. This represents the sum of the interaction term variance in the independent FKBP5 rs3800373 (1.4%) and CRHBP rs7728378 (1.2%) models.
Fig. 4A illustrates the results using the derived dichotomous total CTQ score and shows that the prevalence of suicide attempt in individuals exposed to high childhood trauma was 0.35 in carriers of only the FKBP5 rs3800373 major homozygote (cc/FF) and 0.30 in carriers of only the CRHBP rs7728378 major homozygote (CC/ff) however the prevalence increased to 0.58 in carriers of both major homozygotes (CC/FF). In contrast, individuals who were not carriers of either major homozygote (cc/ff) were resilient to the effects of childhood trauma; the prevalence of suicide attempt was the same in these individuals (0.24) as in all other genotype groups who had not experienced childhood trauma (0.16–0.22).
3.3. Secondary analyses
3.3.1. CRHR1
As shown in Fig. 3, one haplotype block extended distally across the gene and included seven SNPs from rs110402 to rs17689918 (listed in Table 1). There were five haplotypes with ≥0.05 frequency accounting for 85% of the haplotype diversity (Fig. S1). There were no haplotype or diplotype associations with suicidality, no G × E interactive effects and no SNP associations.
Two SNPs, rs9900679 and rs4792887, were not in LD with any SNPs (Fig. 3, Fig. S1). Carriers of the rs9900679 variant allele that is unique to individuals of African ancestry (Table 1) were more likely not to have attempted suicide (0.07 vs 0.03; L-R χ2 = 7.4, 3 df, p = 0.007). This result survived Bonferroni correction.
3.3.2. CRH
All four SNPs were in strong LD (Fig. 3). There were four haplotypes with ≥0.05 frequency that accounted for 99% of the haplotype diversity (Fig. S1). There were no haplotype or SNP associations or G × E interactive effects on suicidality.
3.3.3. CRHR2
There was no LD across the gene (Fig. 3). There were no main or G × E effects.
4. Discussion
In this study we investigated main effects of the CRHBP gene and G × E interactive effects on suicidal behavior in a sample of African American participants with substance dependence. We did indeed find nominally significant G × E effects for CRHBP SNPs rs7728378, rs10474485 and rs6453267 although after FDR correction only the results for rs7728378 remained significant. Other than rs6453267, which has a protective allele that is common in African Americans but is rare or absent in other ethnic groups, we did not detect any main effects of CRHBP variation on suicide risk.
The CRHBP SNPs rs7728378 and rs10474485 that were implicated in suicidal behavior showed significant or trend associations with anxiety disorders in Plains Indians and AUDs in U.S. Caucasians and were included in one distal haplotype block in these two samples (Enoch et al., 2008). The SNP rs7728378 that showed the strongest effect in our earlier study also showed the strongest interactive effect with childhood trauma in our current study. It is also noteworthy that the same rs7728378 allele was implicated in suicidal behavior in the African Americans and AUD in the U.S. Caucasians however the allele frequencies were very different: 0.77 in African Americans and 0.38 in U.S. Caucasians. The results from four other CRHBP studies (see Fig. 3) also lend support to our findings. In a study of schizophrenics, rs1875999 heterozygotes had a greater risk of attempting suicide (De Luca et al., 2010). Rs10474485 was associated with remission of depressive symptoms, predominantly in individuals with anxious depression (Binder et al., 2010) and with depressive symptoms in alcoholics in a study that genotyped the same SNPs as in the current study, using the same platform (Kertes et al., 2010). Finally, rs10055255, located within the distal region of CRHBP (Fig. 3), has been shown to moderate stress-induced craving in heavy drinkers (Ray, 2011). Thus the results of our and other studies provide mounting evidence that CRHBP variation may play a role in stress-related disorders.
Since 65–90% of total CRH is thought to be tightly bound to CRHBP (Behan et al., 1997), the effectiveness of CRH in stimulating ACTH to release cortisol might be influenced by variation in CRHBP expression. The binding of cortisol to the GR and the translocation of the GR into the nucleus resulting in altered expression of stress-response genes is moderated by a large molecular complex. Of the genes encoding these proteins, only FKBP5 has been shown to be associated with stress-related phenotypes (Binder et al., 2004). In an earlier study of this dataset we showed that the interaction between childhood trauma and FKBP5 haplotypes and SNPs influenced suicidal behavior (Roy et al., 2010). The current study shows that an additive effect between CRHBP and FKBP5 variation in the interaction with childhood trauma predicts risk for suicide attempt. Amongst individuals who had been exposed to high childhood trauma, carriers of either the CRHBP rs7728378 major homozygote or the FKBP5 rs3800373 major homozygote had suicide attempt rates of 0.31–0.35 respectively but this rate was almost double (0.58) in individuals who carried both major homozygotes. In contrast, trauma-exposed individuals who carried neither major homozygote had the same suicide rate (0.24) as individuals not exposed to childhood trauma.
Some studies have shown interactive effects between childhood trauma (measured by the CTQ) and CRHR1 variation, including SNPs genotyped in our study: rs110402 and rs242924, on adult depression/depressive effects (Bradley et al., 2008; Heim et al., 2009; Polanczyk et al., 2009), and cortisol response (Tyrka et al., 2009); and rs4792887 on suicidality in individuals with low stress exposure (Wasserman et al., 2008). The one finding of the current study was that the non-ancestral allele of CRHR1 rs9900679, unique to African ancestry, was protective against suicide attempt. Since no studies have associated CRH or CRHR2 variation with suicidality other than one study indicating a CRHR2 microsatellite association with severity of suicidal behavior in bipolar disorder (De Luca et al., 2007), our negative findings for CRH and CRHR2 were as predicted.
CRHBP is highly conserved across vertebrates and in humans encodes a 322 amino acid protein that contains 5 disulphide bonds that are essential for CRH binding (Westphal and Seasholtz, 2006). The CRHBP SNPs that have been associated with stress-related phenotypes in our and other studies are located at the 3′ end of the gene. At the present time, no evidence has been provided that any of the SNPs in this region are or are not functional although Binder et al. (2010) did show that the variant allele of a 3′ SNP rs10473984 was associated with higher ACTH concentrations and speculated that this might be due to reduced CRHBP expression. It is noteworthy that a second CRHBP isoform has been identified in brain in which the terminal exon is spliced out in favor of two alternative exons that lie 3′ to the gene resulting in a truncation of the protein at the C-terminus with the normal 52 amino acids being replaced by 18 novel amino acids (Enoch et al., 2008). This change in peptide sequence might affect protein folding and stability that might alter CRH binding affinity.
Studies in rats have established that poor maternal contact in early life, comparable to emotional neglect of children, results in HPA axis dysregulation and reduced expression of hippocampal GR gene NR3C1 resulting from hypermethylation of the promoter region (Weaver et al., 2004). Likewise, a study of postmortem hippocampus showed that suicide victims exposed to childhood maltreatment had increased methylation of an NR3C1 promoter and decreased NR3C1 expression (McGowan et al., 2009). Similarly, it is conceivable (but speculative) that our CRHBP G × E findings might be explained by the effects of childhood trauma on epigenetic modifications that influence the expression of the alternative exons and 3′UTR that is the location of the SNP cluster identified in our study (Tilgner et al., 2009).
There are a few limitations to this study. The CTQ is a self-report questionnaire although it has been shown to have high reliability and validity (Bernstein et al., 1994, 1997, 2003; Bernstein and Fink, 1998; Scher et al., 2001; Thombs et al., 2007). The nature and severity of each suicide attempt was not systematically recorded. Since the complete SCID was not administered, data about possible comorbid disorders such as depression, anxiety disorders and PTSD was not available. Although the sample size decreased to 474 (112 suicide attempters) when examining G × E interactions, it is important to note that the participants in this study had for the most part been exposed to considerable childhood trauma and this could increase the strength of G × E interactions. For example, using the dichotomous CTQ clinical cut-off scores that differentiate between the presence or absence of significant abuse/neglect (Walker et al., 1999) we found that only 27% of individuals did not meet criteria for significant abuse/neglect. Finally this study was performed in African Americans predominantly with substance dependence and it is not clear that the results can be generalized to other populations or other patient groups.
In conclusion, the results of the present study show that interaction between childhood trauma and CRHBP variation appears to increase the risk of suicidal behavior, at least in individuals with substance dependence. We also found main effects of a CRHBP and a CRHR1 SNP on suicide attempts; both these SNPs are unique to African ancestry. Finally, there appears to be a biologically plausible additive effect between genetic variation in CRHBP and FKBP5.
Supplementary Material
Acknowledgments
Funding support
This research was supported by the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism, NIH and in part by grant RO1 DA 10336-02 to AR from the National Institute of Drug Abuse, NIH.
Footnotes
Contributors
Dr Roy designed the study, wrote the protocol, collected the patients, and participated in the writing of the paper.
Dr Enoch carried out the data analyses and participated in the writing of the paper.
Dr Goldman supervised the genetic analyses.
Dr DeLuca carried out data analysis.
Dr Hodgkinson was involved in the genetic analysis.
Appendix. Supplementary material
Supplementary data associated with this article can be found, in the online version, at doi: 10.1016/j.jpsychires.2011.09.009.
Disclosure/conflict of interest
None of the authors have any disclosures or conflicts of interest to declare.
References
- Arató M, Bánki CM, Bissette G, Nemeroff CB. Elevated CSF CRF in suicide victims. Biological Psychiatry. 1989;25:355–9. doi: 10.1016/0006-3223(89)90183-2. [DOI] [PubMed] [Google Scholar]
- Behan DP, Khongsaly O, Owens MJ, Chung HD, Nemeroff CB, De Souza EB. Corticotropin-releasing factor (CRF), CRF-binding protein (CRF-BP), and CRF/CRF-BP complex in Alzheimer’s disease and control postmortem human brain. Journal of Neurochemistry. 1997;68:2053–60. doi: 10.1046/j.1471-4159.1997.68052053.x. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behavioural Brain Research. 2001;125:279–84. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- Bernstein D, Fink L. Childhood trauma questionnaire: a retrospective self-report manual. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. American Journal of Psychiatry. 1994;151:1132–6. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bernstein D, Ahluvalia T, Handelsman L. Validity of the childhood trauma questionnaire in an adolescent psychiatric population. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:340–8. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the childhood trauma questionnaire. Child Abuse and Neglect. 2003;27:169–90. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl. 1):S186–95. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Pütz B, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nature Genetics. 2004;36:1319–25. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- Binder EB, Owens MJ, Liu W, Deveau TC, Rush AJ, Trivedi MH, et al. Association of polymorphisms in genes regulating the corticotropin-releasing factor system with antidepressant treatment response. Archives of General Psychiatry. 2010;67:369–79. doi: 10.1001/archgenpsychiatry.2010.18. [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Archives of General Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell W, Schlesser M. The dexamethasone suppression test and suicide prediction. American Journal of Psychiatry. 2001;158:748–53. doi: 10.1176/appi.ajp.158.5.748. [DOI] [PubMed] [Google Scholar]
- Coryell W, Young E, Carroll B. Hyperactivity of the hypothalamic–pituitary–adrenalaxis and mortality in major depressive disorder. Psychiatry Research. 2006;142:99–104. doi: 10.1016/j.psychres.2005.08.009. [DOI] [PubMed] [Google Scholar]
- De Luca V, Tharmalingam S, Kennedy JL. Association study between the corticotropin-releasing hormone receptor 2 gene and suicidality in bipolar disorder. European Psychiatry. 2007;22:282–7. doi: 10.1016/j.eurpsy.2006.12.001. [DOI] [PubMed] [Google Scholar]
- De Luca V, Tharmalingam S, Zai C, Potapova N, Strauss J, Vincent J, et al. Association of HPA axis genes with suicidal behaviour in schizophrenia. Journal of Psychopharmacology. 2010;24:677–82. doi: 10.1177/0269881108097817. [DOI] [PubMed] [Google Scholar]
- Enoch MA. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berlin) 2011;214:17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch M-A, Shen P-H, Ducci F, Yuan Q, Liu J, White K, et al. Common genetic origins for EEG, alcoholism and anxiety: the role of CRH-BP. PLoS One. 2008;3(10):e3620. doi: 10.1371/journal.pone.0003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch M-A, Hodgkinson CA, Yuan Q, Shen PH, Goldman D, Roy A. The influence of GABRA2, childhood trauma, and their interaction on alcohol, heroin, and cocaine dependence. Biological Psychiatry. 2010;67:20–7. doi: 10.1016/j.biopsych.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Bradley B, Mletzko TC, Deveau TC, Musselman DL, Nemeroff CB, et al. Effect of childhood trauma on adult depression and neuroendocrine function: sex-specific moderation by CRH receptor 1 gene. Frontiers of Behavioral Neuroscience. 2009;3:41. doi: 10.3389/neuro.08.041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson CA, Yuan Q, Xu K, Shen PH, Heinz E, Lobos EA, et al. Addictions biology: haplotype-based analysis for 130 candidate genes on a single array. Alcohol and Alcoholism. 2008;43:505–15. doi: 10.1093/alcalc/agn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen J, Carlborg A, Martensson B, Forslund K, Nordstrom AL, Nordstrom P. DST non-suppression predicts suicide after attempted suicide. Psychiatry Research. 2007;150:297–303. doi: 10.1016/j.psychres.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Jokinen J, Nordstrom P. HPA axis hyperactivity and attempted suicide in young adult mood disorder inpatients. Journal of Affective Disorders. 2009;116:117–20. doi: 10.1016/j.jad.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Kertes DA, Kalsi G, Prescott CA, Kuo PH, Patterson DG, Walsh D, et al. Neurotransmitter and neuromodulator genes associated with a history of depressive symptoms in individuals with alcohol dependence. Alcoholism: Clinical and Experimental Research. 2010 Dec 8; doi: 10.1111/j.1530-0277.2010.01366.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Isaksson A, Traskman-Bendz L, Brundin L. Salivary cortisol and suicidal behavior – a follow up study. Psychoneuroendocrinology. 2008a;33:1061–8. doi: 10.1016/j.psyneuen.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Traskman-Bendz L, Vang F. Suicidal intent and the HPA-axis characteristics of suicide attempters with major depressive disorder and adjustment disorders. Archives of Suicide Research. 2008b;12:197–207. doi: 10.1080/13811110802100775. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Currier D. A review of prospective studies of biologic predictors of suicidal behavior in mood disorders. Archives of Suicide Research. 2007;11:3–16. doi: 10.1080/13811110600993124. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Currier DM. Stress, genetics and epigenetic effects on the neurobiology of suicidal behavior and depression. European Psychiatry. 2010;25:268–71. doi: 10.1016/j.eurpsy.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan P, Sasaki A, D’Alessio C, Dymov S, Labonte B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience. 2009;12:342–8. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Owens MJ, Bissette G, Andorn AC, Stanley M. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Archives of General Psychiatry. 1988;45:577–9. doi: 10.1001/archpsyc.1988.01800300075009. [DOI] [PubMed] [Google Scholar]
- Pfennig A, Kunzel H, Kern N, Ising M, Majer M, Fuchs B, et al. Hypothalamus–pituitary–adrenal system regulation and suicidal behavior in depression. Biological Psychiatry. 2005;57:336–42. doi: 10.1016/j.biopsych.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, et al. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Archives of General Psychiatry. 2009;66:978–85. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA. Stress-induced and cue-induced craving for alcohol in heavy drinkers: preliminary evidence of genetic moderation by the OPRM1 and CRH-BP genes. Alcoholism: Clinical and Experimental Research. 2011;35:166–74. doi: 10.1111/j.1530-0277.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- Roy A. Hypothalamic–pituitary–adrenal–adrenal axis function and suicidal behavior in depression. Biological Psychiatry. 1992;32:812–6. doi: 10.1016/0006-3223(92)90084-d. [DOI] [PubMed] [Google Scholar]
- Roy A. Characteristics of cocaine dependent patients who attempt suicide. American Journal of Psychiatry. 2001;158:215–9. doi: 10.1176/appi.ajp.158.8.1215. [DOI] [PubMed] [Google Scholar]
- Roy A. Characteristics of opiate dependent patients who attempt suicide. Journal of Clinical Psychiatry. 2002;63:403–7. doi: 10.4088/jcp.v63n0505. [DOI] [PubMed] [Google Scholar]
- Roy A. Characteristics of drug addicts who attempt suicide. Psychiatry Research. 2003;121:99–103. doi: 10.1016/s0165-1781(03)00206-3. [DOI] [PubMed] [Google Scholar]
- Roy A, Janal MN. Risk factors for suicide attempts among alcohol dependent patients. Archives of Suicide Research. 2007;11:211–7. doi: 10.1080/13811110701250150. [DOI] [PubMed] [Google Scholar]
- Roy A, Agren H, Pickar D, Linnoila M, Doran A, Cutler N, et al. Reduced CSF concentrations of homovanillic acid and homovanillic acid to 5-hydroyindoleacetic acid ratios in depressed patients: relationship to suicidal behavior and dexamethasone nonsuppression. American Journal of Psychiatry. 1986;143:1539–45. doi: 10.1176/ajp.143.12.1539. [DOI] [PubMed] [Google Scholar]
- Roy A, Gorodetsky E, Yuan Q, Goldman D, Enoch MA. The interaction of FKBP5, a stress related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology. 2010;35:1674–83. doi: 10.1038/npp.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M. Diabetic retinopathy in African Americans with type 1 diabetes: the New Jersey 725. 1. Methodology, population, frequency of retinopathy, and visual impairment. Archives of Ophthalmology. 2000;118:97–104. doi: 10.1001/archopht.118.1.97. [DOI] [PubMed] [Google Scholar]
- Scher CD, Stein MB, Asmundson GJ, McCreary DR, Forde DR. The childhood trauma questionnaire in a community sample: psychometric properties and normative data. Journal of Traumatic Stress. 2001;14:843–57. doi: 10.1023/A:1013058625719. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M. Structured clinical interview for DSM-IV (SCID) New York: New York State Psychiatric Institute, Biometrics Research; 1995. [Google Scholar]
- Stephens M, Donnelly P. A comparison of Bayesian methods for haplotype reconstruction from population genotype data. American Journal of Human Genetics. 2003;73:1162–9. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thombs BD, Lewis C, Bernstein DP, Medrano MA, Hatch JP. An evaluation of the measurement equivalence of the childhood trauma questionnaire – short form across gender and race in a sample of drug-abusing adults. Journal of Psychosomatic Research. 2007;63:391–8. doi: 10.1016/j.jpsychores.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Tilgner H, Nikolaou C, Althammer S, Sammeth M, Beato M, Valcárcel J, et al. Nucleosome positioning as a determinant of exon recognition. Nature Structural and Molecular Biology. 2009;16:996–1001. doi: 10.1038/nsmb.1658. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: effects on hypothalamic–pituitary–adrenal axis reactivity. Biological Psychiatry. 2009;66:681–5. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EA, Unutzer J, Rutter C, Gelfand A, Saunders K, VonKorff, et al. Cost of health care use by women HMO members with a history of childhood abuse and neglect. Archives of General Psychiatry. 1999;56:609–13. doi: 10.1001/archpsyc.56.7.609. [DOI] [PubMed] [Google Scholar]
- Wasserman D, Sokolowski M, Rozanov V, Wasserman J. The CRH1 gene; a marker for suicidality in depressed males exposed to low stress. Genes Brain and Behavior. 2008;7:14–9. doi: 10.1111/j.1601-183X.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Westphal NJ, Seasholtz AF. CRH-BP: the regulation and function of a phylogenetically conserved binding protein. Frontiers in Bioscience. 2006;11:1878–91. doi: 10.2741/1931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


