Abstract
G protein–coupled receptor (GPCR) oligomers have been proposed to play critical roles in cell signaling, but confirmation of their existence in a native context remains elusive, as no direct interactions between receptors have been reported. To demonstrate their presence in native tissues, we developed a time-resolved FRET strategy that is based on receptor labeling with selective fluorescent ligands. Specific FRET signals were observed with four different receptors expressed in cell lines, consistent with their dimeric or oligomeric nature in these transfected cells. More notably, the comparison between FRET signals measured with sets of fluorescent agonists and antagonists was consistent with an asymmetric relationship of the two protomers in an activated GPCR dimer. Finally, we applied the strategy to native tissues and succeeded in demonstrating the presence of oxytocin receptor dimers and/or oligomers in mammary gland.
G protein–coupled receptors (GPCRs) represent the largest protein family and play key roles in cell-cell communication. Their implication in dimeric or higher-order oligomeric GPCR complexes1 in living cells is the subject of intense research that has yielded new insights into the precise regulation of cell signaling. However, despite extensive evidence regarding the formation and the dynamics of GPCR dimers in transfected cells, their actual physiological role will remain elusive until more is known about these signaling complexes in their native context. Performing analyses of GPCR interactions in a native environment has so far proven a significant challenge2.
To investigate the existence of GPCR complexes in native tissues, various approaches such as atomic force microscopy3, co-immuno-precipitation4 and binding or functional assays5–8 have been used. The most convenient methods to monitor interactions are based on resonance energy transfer performed with labeled proteins. In contrast to GPCRs expressed in heterologous expression systems and fused with peptide tags or fluorescent proteins, the sequence and the expression level of receptors in native context cannot be modified except by knock-in strategies, making the molecular engineering of labeling approaches more difficult. However, the labeling can be performed by selective probes2 such as antibodies or fluorescent ligands9,10. Because various types of ligands (agonists, antagonists) can be used, fluorescent ligands can also provide information about the active versus inactive states of GPCR species within the oligomeric complexes.
Such an approach has never been applied to native tissues because of insufficient sensitivity and signal-to-noise ratio. Here we succeeded in developing a time-resolved fluorescence resonance energytransfer(TR-FRET)–based approach that offers a much higher signal-to-noise ratio11. We validated the strategy using various sets of fluorescent ligands in transfected cells expressing receptors that have been shown to dimerize: the peptide vasopressin (AVP) V1a, V2 and oxytocin receptors12–14 as well as a biogenic amine receptor, the dopamine D2 receptor15–18. Because most FRET methods do not strictly distinguish between dimers and higher-order oligo mers, we will refer throughout to the term ‘dimer’, as it represents the minimal oligomeric arrangement. On the basis of the cooperative binding of various ligands15,16,18,19, we demonstrated that the ligand binding– dependent FRET signal results from receptor dimerization. We also carried out experiments on mammary gland native tissues, either on membrane preparations or on patches of organs that express oxytocin receptors, and demonstrated, for the first time, GPCR dimerization in native tissues. The existence of GPCR dimers and their asymmetric organization in an activated signaling unit may provide an explanation for the difference in FRET efficacies that we observed between antagonists and agonists.
RESULTS
FRET between antagonists on AVP and oxytocin receptors
Much effort has been devoted to trying to use ligands derivatized with short-life fluorophores9,10,20,21 to detect receptor dimers through FRET between ligands (Fig. 1a). However, this approach has been mostly unsuccessful and does not allow quantitative assessment of the interactions because of poor signal-to-noise ratios. Therefore, we decided to use fluorophores compatible with homogeneous time-resolved FRET (HTRF), which have numerous advantages. (i) Homogeneous time-resolved FRET (HTRF) is based on an energy transfer between a lanthanide (europium or terbium) and a compatible fluorophore. Because of lanthanide’s long-lasting fluorescence (>800 μs), the fluorescence of acceptors engaged in a FRET with lanthanides can be measured after a time delay. By contrast, short-life fluorescence resulting from the direct excitation of acceptors or any fluorophores present in the medium or bio logical preparation is not measurable after this time delay. Therefore, with HTRF, experiments can be performed in homogeneous conditions. (ii) The Förster distances of the fluorophore pairs, which consist of a lanthanide cryptate (either europium pyridine bisbipyridine (Eu-PBBP) or Lumi4-Tb) and Alexa Fluor 647 (Alexa-647) or Alexa-488, are between 65 Å and 46 Å and thus are compatible with FRET between two ligands bound to a dimer (Fig. 1a). (iii) HTRF provides an increased signal-to-noise ratio because of the time- resolved condition and its excellent spectral compatibilities11,22. (iv) Finally, there is minimal orientation constraint of the fluoro-phores engaged in FRET23.
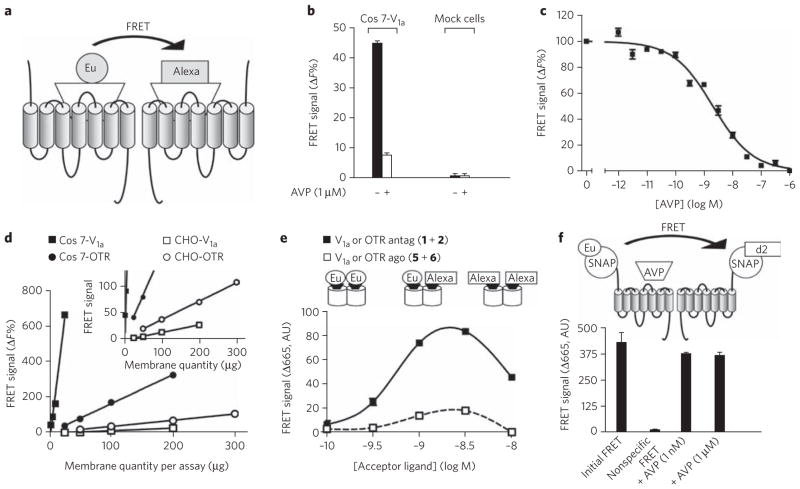
Figure 1. FRET signal between antagonists bound to V1a or oxytocin receptors expressed in heterologous expression systems.
(a) Diagram illustrating the principle of FRET between ligands bound to GPCRs. (b) FRET signal observed on Cos7 cells transiently transfected with V1a receptors or on mock cells and labeled with [Lys8(Eu–PBBP)]PVA (1) (1.4 nM) and [Lys8(Alexa-647)]PVA (2) (0.7 nM) in the absence or presence of an excess of AVP (1 μM). (c) Inhibition of the FRET signal by increased concentrations of AVP (IC50 = 2.10 ± 0.2 nM). (d) Influence of the amount of receptor on the FRET signal. Experiments were performed with membrane preparation of Cos7 cells or on stable CHO cell lines expressing V1a or oxytocin receptors. Inset: same data at a different scale. (e) Variations in the FRET signal on membranes from oxytocin receptor (OTR)–expressing CHO cells as a function of ligand concentration. Donor ligands [Lys8(Eu–PBBP)]PVA (1) and HO-[Thr4,Orn8(Eu–PBBP)]VT (5) were used at 1.5 nM, with increasing concentrations of acceptor ligands ([Lys8(Alexa-647)]PVA (2) (black symbols) and HO-[Thr4,Orn8(Alexa-647)]VT (6) (white symbols)). (f) Effect of AVP (1 nM or 1 μM) on the FRET signal measured on Snap-tag–vasopressin V1a fusion receptors. Cells were incubated in the presence of benzyl guanine conjugated with europium cryptate (BG-K) (0.3 μM) and d2 (0.5 μM). Negative control has been provided by incubation of membranes with BG-K (0.3 μM) and unlabeled benzyl guanine (0.5 μM). Illustrated data are representative of at least three independent experiments performed in triplicate. Values correspond to the mean ± s.e.m. AU, arbitrary units.
We first chose to develop this strategy using vasopressin and oxytocin receptors, as various high-affinity ligands for these receptors have been derivatized with classic fluorophores and characterized24. We have previously reported that, depending on the antagonist considered, either positive cooperative binding processes or no cooperative binding can be observed, in both cases indicating that the antagonist is able to bind two sites within a dimer19. Therefore, we derivatized one of them, phenylpropionyl linear vasopressin ([Lys8]PVA), and synthesized HTRF-compatible fluorescent antagonists20: [Lys8(Eu-PBBP)] PVA (1) and [Lys8(Alexa-647)]PVA (2) (Supplementary Table 1 and Supplementary Methods for structures).
FRET signals could easily be detected with the fluorescent antagonists (with concentrations up to 3 × Kd for 1 and 2, corresponding to about 86% of receptor occupancy) on cells expressing vasopressin V1a or oxytocin receptors (Fig. 1). It was clear that the FRET signal resulted from the interactions of these ligands with receptors because (i) it was fully inhibited by an excess of unlabeled competitor and was not observable on mock cells (Fig. 1b); (ii) inhibition with an increasing concentration of vasopressin led to a sigmoid curve with a half-maximal inhibitory concentration (IC50) 2.1 ± 0.2 nM, corresponding to its known affinity for the V1a receptor25 (Fig. 1c); (iii) the FRET signal was proportional to the amount of receptor over a wide range of receptor amount per well (Fig. 1d); and (iv) the FRET signal followed a bell-shaped curve as the 1/2 concentration ratio was increased (Fig. 1e). Indeed, in the presence of an excess of acceptor- or donor-labeled ligand, most receptor dimers are expected to be labeled with the same ligand species, leading to a very low FRET signal. By contrast, a correct ratio of both types of ligands, leading to the binding of both ligands to a receptor dimer, resulted in a maximal FRET signal. Altogether, these data demonstrate a close proximity between binding sites in the cases of vasopressin and oxytocin.
Similar results were obtained with the V2 receptor expressed in Cos7 cells using another set of fluorescent antagonists created using a second modified vasopressin, d(CH2)5[DTyr(Et)2,Ile4, Eda)9]VP, with Lumi4-Tb as donor (d(CH2)5[DTyr(Et)2,Ile4,Eda (Lumi4-Tb)9]VP (3) and Alexa-488 as acceptor (d(CH2)5[DTyr (Et)2,Ile4,Eda(Alexa-488)9]VP (4)) (see Supplementary Fig. 1, Supplementary Table 2 and Supplementary Methods for structures).
Less FRET with agonists on AVP and oxytocin receptors
We have reported that, in contrast to the above data consistent with two antagonists being able to bind on the V1a or oxytocin receptor dimer, agonists bind with a stoichiometry of one agonist per dimer, independently of the coupling to a G protein19. Moreover, the maximal binding measured by satu ration experiments with tritiated vasopressin was about half of that measured with the tritiated antagonist26, consistent with negative cooperativity of agonist binding. We therefore compared the FRET signals obtained with fluorescent agonists and antagonists. From an analog of vasotocin (VT), new fluorescent agonists HO-[Thr4,Orn8(Eu-PBBP)]VT (5) and HO-[Thr4,Orn8(Alexa-647)]VT (6) were synthesized, and both showed high affinities for oxytocin receptors (Supplementary Table 1 and Supplementary Methods for structures). In contrast to antagonists, the fluorescent agonists (up to 5 × Kd for 5 and 10 × Kd for 6) led only to very weak FRET signals when membrane preparations from transfected cells were used (Fig. 1e). The lower signal obtained with agonists did not result from an agonist-induced dissociation of the dimers into monomers. Indeed, unlabeled agonist, AVP, did not affect the dimeric state of the receptors, as shown by TR-FRET signal measured between two receptors carrying fluorescent Snap-tags at their extracellular ends27 (Fig. 1f).
FRET between ligands on the dopamine D2 receptors
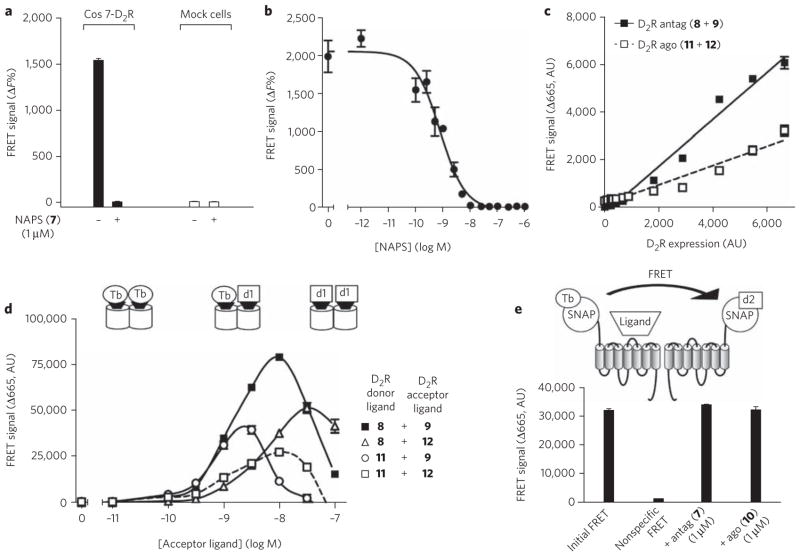
As other receptors have ligand-cooperative binding and dimerization processes, similar FRET results should be observed for other GPCR models. We focused on the dopamine D2 receptor, for which cooperative binding properties independent of G-protein coupling and related to receptor dimerization have been reported15–18. We deri-vatized two sets of fluorescent ligands with terbium (Lumi4-Tb) as a donor and the fluorophore d1 as an acceptor (see Supplementary Methods for the spectral properties of the fluorophore). Fluorescent antagonists were obtained from N-(p-aminophenethyl)spiperone (NAPS) (7), leading to the synthesis of NAPS(Lumi4-Tb) (8) and NAPS(d1) (9), and fluorescent agonists were obtained from (±)-4′-amino-2-(N-phenethyl-N-propyl)-amino-5-hydroxytetralin (PPHT) (10), leading to PPHT(Lumi4-Tb) (11) and PPHT(d1) (12) (see Supplementary Methods for structures and Supplementary Table 3 for their binding properties). It is noteworthy that Lumi4-Tb, which is brighter than europium cryptates, leads to a larger FRET signal. As for the vasopressin receptor, incubation of cells expressing the D2 receptor with both labeled antagonists (8 and 9) led to a FRET signal sensitive to the presence of unlabeled ligand (Fig. 2a, b). The IC50 of the sigmoid competition curve (1.9 nM ± 0.9) correlates well with the affinity of the ligand reported previously. FRET efficacy was constant over a wide range of receptor densities, as the FRET signal changed linearly with receptor expression (Fig. 2c). Finally, variation of the 8/9 antagonist ratio led to a bell-shaped curve (Fig. 2d). Notably, a smaller FRET signal was observed with the fluorescent agonists (11 and 12), whatever the concentration used. The weaker signal did not result from an inability of agonists to enter into a FRET, as incubation with 8 and 12 or with 9 and 11 resulted in a significant FRET. Finally, we also performed TR-FRET experiments using the Snap-tag technology27. The addition of an excess of agonists or antagonists did not significantly modify the FRET signal, suggesting that agonists do not destabilize dimers and that antagonists do not promote dimer formation (Fig. 2e). The difference in FRET efficacy between agonists and antagonists for the dopamine D2 receptor is very similar to the one observed for the vasopressin receptor, demonstrating that our results are not restricted to a single GPCR family.
Figure 2. FRET signal between antagonists bound to dopamine D2 receptors expressed in heterologous expression systems.
(a) FRET signal observed on Cos7 cells transiently transfected with D2 receptors or on mock cells and labeled with NAPS(Lumi4-Tb) (8) (1 nM) and NAPS(d1) (9) (5 nM) in the absence or presence of an excess of NAPS (7) (1 μM). (b) Inhibition of the FRET signal by increasing concentrations of NAPS (IC50 = 1.9 ± 0.9 nM). (c) Effect of the variation of receptor density in Cos7 cells on the FRET signal between either the fluorescent antagonists NAPS(Lumi4-Tb) (8) (1 nM) and NAPS(d1) (9) (5 nM) (black squares) or the fluorescent agonists PPHT(Lumi4-Tb) (11) (7 nM) and PPHT(d1) (12) (5 nM) (white squares). (d) Comparison of FRET signals obtained with the various pairs of dopamine D2 receptor ligands. Donor ligands, NAPS(Lumi4-Tb) (8) and PPHT(Lumi4-Tb) (11), were used at 1 nM and 7 nM, respectively. Acceptor ligand corresponds either to NAPS(d1) (9) or PPHT(d1) (12). (e) Effect of NAPS (1 μM) or PPHT (1 μM) on the stability of the FRET signal measured on dopamine D2 receptor fused at its N terminus to the Snap-tag sequence. Cells were incubated in the presence of benzyl guanine conjugated with Lumi4-Tb (BG-Lumi4-Tb) (100 nM) and d2 (BG-d2) (250 nM). Negative control has been provided by incubation of cells in the absence of benzyl guanine conjugated with acceptor. Illustrated data are representative of at least three independent experiments performed in triplicate. Values correspond to the mean ± s.e.m. AU, arbitrary units.
Detection of FRET signals in native tissues
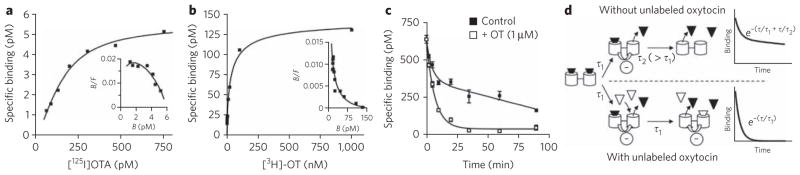
To investigate the presence of dimers in native tissues, we carried out our study on an organ with high expression levels of a GPCR and large quantities of tissue, allowing binding and FRET studies. We focused on the mammary glands of lactating rats, which express high densities of oxytocin receptors (1–3 pmol mg−1 protein) and, to our knowledge, no vasopressin receptor. Similarly to vasopres-sin and oxytocin receptors expressed in a heterologous expression system, oxytocin receptors expressed in native tissues show cooperative binding. This is illustrated by saturation experiments performed with the antagonist [125I]OTA, which showed saturation curves with a Hill coefficient greater than 1 (1.19 ± 0.08) (Fig. 3a) and convex Scatchard curves (Fig. 3a, inset), consistent with positive cooperative binding19,28,29. By contrast, saturation experiments (Fig. 3b) performed with tritiated oxytocin ([3H]OT), the natural agonist, yielded a concave Scatchard plot with a Hill coefficient of 0.53 ± 0.01 (Fig. 3b, inset). Dissociation kinetics experiments indicated that the apparent heterogeneity is likely due to negative receptor cooperativity rather than to the coexistence of multiple independent binding sites7,19,30. Indeed, an excess of unlabeled oxytocin (1 μM) changed the [3H]OT dissociation kinetics (Fig. 3c) from a two-phase exponential decay to a one-phase exponential decay. The impact of an excess of oxytocin on the second dissociation phase is illustrated in Figure 3d.
Figure 3. Binding experiments on the oxytocin receptor expressed in membrane preparations from native tissue.
(a, b) Saturation curves and the associated Scatchard plot (insets) obtained with the antagonist [125I]OTA and [3H]OT. The best fits with the Hill equation gave a Hill coefficient of 1.4 (a) and 0.54 (b). Experiments were performed on native tissues expressing oxytocin receptors (preparation of fetal membrane (a) and mammary gland preparation (b)). (c) Dissociation kinetics in the absence or presence of an excess of oxytocin; the fits correspond to a two-phase exponential decay (2.7 ± 0.3 min and 71 ± 16 min) and to a one-phase exponential decay (5.48 ± 0.59 min), respectively. (d) Diagram illustrating the influence of an excess of unlabeled oxytocin (white triangle) on the dissociation kinetics of [3H]OT (black triangle). Dissociation of receptor dimer–ligand complex follows a two-phase exponential decay with the time constants τ1 and τ2. In the absence of unlabeled ligand, owing to the negative cooperativity, the dissociation of the first ligand is faster than that of the second ligand (τ1 < τ2). The addition of an excess of unlabeled ligand does not affect the dissociation constant of the first ligand (time constant τ1) but promotes the dissociation of the second labeled ligand. Indeed, because of the negative cooperativity, the binding of unlabeled ligand on the second protomer increases the dissociation of the first labeled ligand (τ2 = τ1). Illustrated data are representative of at least three independent experiments performed in triplicate. Values correspond to the mean ± s.e.m.
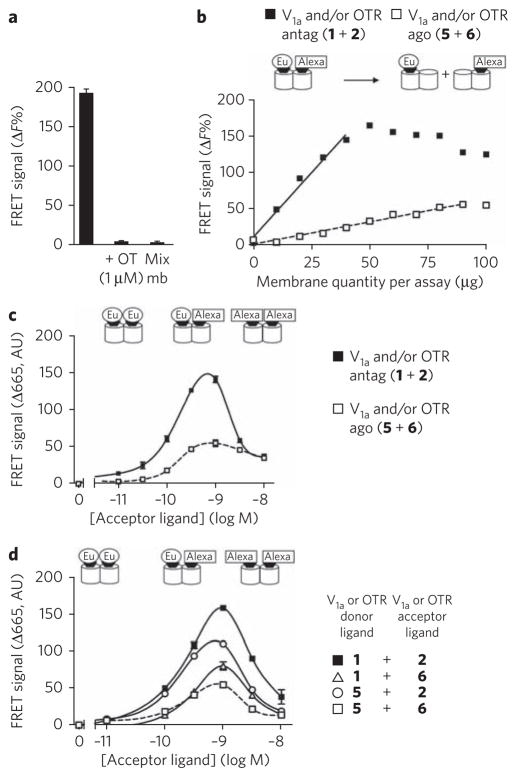
FRET signals could easily be detected with the fluorescent antagonists 1 and 2 on mammary gland membranes (Fig. 4). As in cell lines, it was clear that these FRET signals resulted from the interactions of these ligands with receptors because (i) the ligands were sensitive to unlabeled competitor and not observed on a mixture of membrane samples labeled with either 1 or 2 (Fig. 4a and Supplementary Fig. 2), and (ii) the FRET signal is proportional to the receptor amount over a wide range (Fig. 4b) and then decreases when the receptor amount is too high to be occupied by both ligands. Moreover, the FRET signal followed a bell-shaped curve when the 1/2 concentration ratio increased (Fig. 4c, black squares), indicating that FRET is observable when both ligands bind on a receptor dimer. Altogether, these data demonstrate close proximity between oxytocin binding sites in native tissues.
Figure 4. FRET signals between fluorescent antagonists or agonists bound to native oxytocin receptors expressed in rat mammary glands.
(a) Membrane preparations were labeled with [Lys8(Eu-PBBP)]PVA (1) (1 nM) and [Lys8(Alexa-647)]PVA (2) (1 nM). To produce negative controls, an excess of oxytocin (1 μM) was added or membrane samples labeled with either ligand 1 or 2 were mixed (Mix mb). Notably, membrane samples were mixed just before the reading of the fluorescent signal. (b) Effect of membrane quantity of lactating rat mammary gland on the FRET signals measured between fluorescent agonists (5 and 6) (1 nM) (white squares) compared to those obtained with antagonists (1 and 2) (1 nM) (black squares). (c) Variation in the FRET signal as a function of antagonist (black squares) or agonist (white squares) concentration. Donor ligands [Lys8(Eu-PBBP)]PVA (1) and HO-[Thr4,Orn8(Eu-PBBP)]VT (5) were used at 1.2 nM and 1.5 nM, respectively. (d) Comparison of FRET signals obtained with the various pairs of ligands. Donor ligands were used at 1 nM. Acceptor ligand corresponds either to ([Lys8(Alexa-647)]PVA (2); black squares and white circles) or (HO-[Thr4,Orn8(Alexa-647)]VT (6); white squares or triangles). Illustrated data are representative of at least three independent experiments performed in triplicate. Values correspond to the mean ± s.e.m. AU, arbitrary units.
In contrast to antagonists, and in agreement with negative cooperative binding observed for agonists, the fluorescent agonists 5 and 6 (up to 5 × Kd for the donor and 10 × Kd for the acceptor) led only to very weak FRET signals on membranes from mammary gland (Fig. 4c, white squares). We observed that the linear dependence of the FRET signal on native tissue membrane concentration was steeper with antagonists than with agonists, indicating a much lower FRET efficacy with the latter (Fig. 4b). Finally, the absence of a high FRET signal between agonists did not result from quenching, as 5 and 6 were able to generate FRET signals when associated with 2 and 1, respectively (Fig. 4d). All these data suggest a lower tendency for two agonists to bind simultaneously to the same receptor dimer.
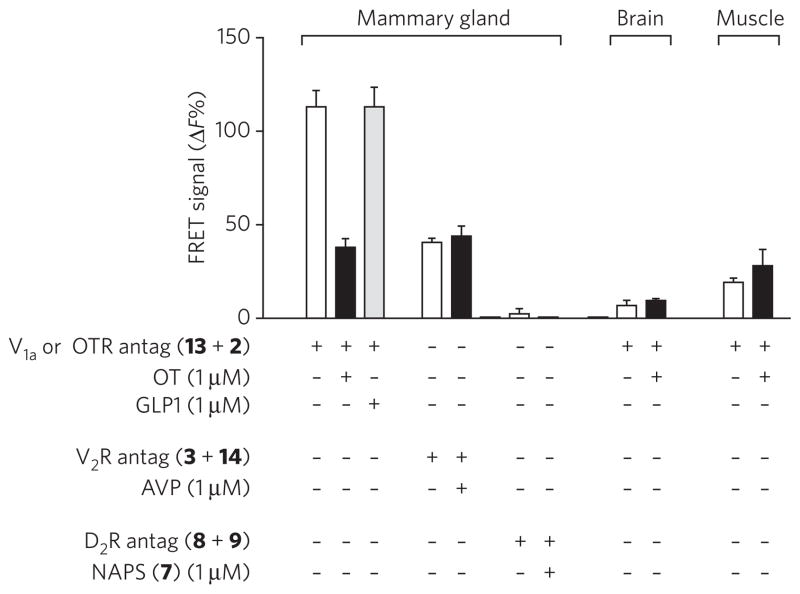
Although it is unlikely, we cannot exclude the possibility that the FRET signal we observed on membrane preparations results from receptor complexes in intracellular compartments, such as endoplasmic reticulum or Golgi membranes, rather than receptors in the plasma membrane at the cell surface. To address this, we performed FRET experiments on patches of organs (Fig. 5), which preserve the tissue organization. In these conditions, the preparation autofluorescence was so high that we had to use Lumi4-Tb as a fluorescent donor ([Lys8(Lumi4-Tb)]PVA (13)), as it is brighter than europium cryptate, as mentioned above. We first verified that the substitution of Lumi4-Tb for europium cryptate in the antagonist did not affect its ability to FRET after binding to recombinant receptor dimers (Supplementary Fig. 3). Incubation of patches with donor and acceptor antagonists led to a significant FRET signal. This signal is decreased in the presence of an excess of oxytocin (1 μM) and not affected by glucagon-like peptide 1 (GLP1), which shows no affinity for the oxytocin receptor. Mammary gland patches were also incubated with the same concentration of d(CH2)5[DTyr(Et)2,Ile4,Eda(Lumi4-Tb)9]VP (3) and d(CH2)5[DTyr(Et)2,Ile4,Eda(Alexa-647)9]VP (14), the latter showing a poor affinity for the oxytocin receptor. In such conditions, the FRET measured is not affected by an excess of oxytocin and is equal to the nonspecific signal previously observed. Finally, incubation of patches of brain or skeletal muscle, which do not express or only weakly express vasopressin V1a or oxytocin receptors, with fluorescent oxytocin antagonists (13 and 2) did not give any significant FRET signal. We therefore conclude that we have measured specific labeling of oxytocin receptor dimer expressed at the mammary gland cell surface.
Figure 5. FRET experiments performed on patches of native tissues.
Mammary gland, brain and skeletal muscle patches were incubated in the presence of ligands as indicated. Illustrated data are representative of at least three independent experiments performed in dodecaplicate (mammary gland) or triplicate. Values correspond to the mean ± s.e.m.
DISCUSSION
Proving the existence of GPCR dimers in native tissues remains a major challenge. Here we have succeeded in demonstrating that ligands conjugated to TR-FRET–compatible fluorophores can be used to validate the existence of GPCR dimers. As mentioned above, various criteria such as the binding of fluorescent ligands on the receptors, the competition with unlabeled ligand and the competition between fluorescent ligands have been used by the authors to demonstrate the specificity of the signal through experiments performed on four different receptors in transfected cells.
We considered different hypotheses before concluding that a specific FRET signal was due to receptor dimerization. However, (i) the difference between FRET signal obtained with agonists and with antagonists strongly supports the idea that the signal does not result from collision of a monomeric receptor moving into the membrane but from receptor dimerization; indeed, the same level of occupancy of the receptor by agonists and antagonists should lead, in the collision hypothesis, to the same FRET signal amplitude, which is obviously not the case. (ii) We have also shown that the weak FRET signal obtained with agonists does not result from dimer dissociation subsequent to agonist binding, at least for receptors expressed in cell lines. (iii) Owing to the encaging of lanthanides, the donor dipole is not constrained, suggesting that the low agonist time-resolved FRET is not due to incompatible fluorophore orientation23. (iv) The decreased FRET efficacy is not due to a longer distance between the fluorophores, as, in regard to vasopressin and oxytocin receptors, evidence suggests that agonists and antagonists dock in the receptor binding pocket in a very similar manner. For example, peptide antagonists and agonists of vasopressin and oxytocin receptors can easily be derivatized with bulky groups on residue 8 or, to a lesser extent, residue 9, whereas derivatization on any other position results in a loss of affinity21. This indicates that the side chains of residues 8 and 9 point to the extracellular domain, whereas the other residues are more deeply embedded in the binding pocket. Moreover, computational modeling validated by mutagenesis, binding and site-specific photolabel-ing experiments has shown a similar docking of peptide agonists or antagonists31–34. A decrease in agonist FRET signal due to a variation in distance between the fluorophores is therefore unlikely. (v) Although the difference between agonists and antagonists could result from the quenching of the fluorescence of the bound agonists, this is unlikely, as the difference has been observed for the three receptors, one of which (the D2 receptor) greatly diverges in its sequence from the other two (V1a and oxytocin receptors), with two sets of agonists and antagonists and both peptidic and non-peptidic ligands. In such conditions, it seems very improbable that similar quenching phenomena would be systematically observed with agonists and not with antagonists. (vi) Finally, the weak FRET signal with agonists does not result from receptor internalization, as all the experiments have been carried out at 4 °C and, as shown by confocal microscopy, internalization of the oxytocin receptor labeled with the same agonist derivatized with tetramethylrhodamine, which occurs normally at 37 °C, is blocked at 4 °C35.
Our FRET data are, however, consistent with a variation in the ligand–receptor dimer stoichiometry, with two antagonists per dimer leading to a strong FRET signal and preferential binding of one agonist per dimer (or a ligand–binding site stoichiometry of less than 1, if we consider higher-order oligomers) resulting in a weak FRET signal. Notably, this hypothesis is in accordance with the binding data of the vasopressin and the dopamine D2 receptor models: positive or no cooperative binding for some antagonists19 and negative cooperative binding for agonists18,36 that are insensitive to G-protein coupling. In theory, increasing the concentration of agonist should lead to the saturation of all the binding sites and therefore should result in a FRET signal equivalent to that with antagonists. However, increasing the concentration of fluorescent agonists above 10 nM generates a high, nonspecific, dynamic FRET signal preventing the observation of any specific FRET signal (see Methods). Moreover, as some antagonists have negative binding cooperativity15,18, it would be interesting to test their fluorescent derivatives in FRET experiments.
Although the generalization of our conclusions to other GPCRs remains to be established, our results should be related to those obtained in other heterologous expression systems that strongly suggest (i) that one agonist is sufficient to trigger G-protein activation18,36–38; (ii) that there exists a conformational switch between protomers within a dimer39,40; (iii) that negative cooperative binding of agonists on other GPCRs occurs7,41; and (iv) that the GPCR dimer active state is asymmetric18,36,42,43.
The strength of the FRET strategy in the context of fluorescent ligands is in its applicability to native tissues without any modification of the receptor sequence. The results obtained on mammary glands are very similar to those obtained in cell lines: that is to say, the specific FRET signal measured is dependent on the oxytocin receptor expression, is abolished in the presence of unlabeled oxytocin, and possesses intensity consistent with the positive and the negative binding cooperativity observed for the antagonists and the agonists, respectively. Finally, as for oxytocin receptors expressed in a cell line, FRET experiments carried out on patches of native tissues demonstrated the specificity of the signal. All these data have led to our conclusion that oxytocin dimers exist in native mammary gland tissue.
Various data obtained on different GPCR families support receptor dimerization: (i) cooperative ligand binding has been reported, for example, for cardiac muscarinic5,6 and chemokine receptors41,44; (ii) synergism between ligands to activate different receptors has been observed45,46; (iii) the effects of bifunctional ligands for opioid receptors in vivo have been reported47; and (iv) the targeting of α1D adrenergic receptor to the cell surface, dependent on α1B receptor expression48, correlated to a decrease of α1D receptor–ligand affinity in α1B receptor knockout mice. All these data are consistent with GPCR dimerization, but no previous evidence for direct physical interaction of GPCRs in native tissue has been reported, and alternative hypotheses have been proposed to explain some of these effects49,50. Here we succeeded in demonstrating the interaction between oxytocin receptors, establishing the existence of dimers in native tissues.
This approach allows us not only to demonstrate the proximity of receptor protomers in a dimer, but also, unlike with BRET or FRET approaches with fusion proteins or antibodies to tags, to establish the functionality of these protomers. Indeed, our results revealed an asymmetry of oxytocin and dopamine protomers within a dimer as soon as one agonist binds to the receptor. The existence of receptor dimers in native tissues raises the question of their functional role. Although this hypothesis is still somewhat speculative, receptor asymmetry might be a mechanism for differential coupling and/or for the enlargement of the range of agonist concentration that generates a cellular response.
In conclusion, the specific recognition of such ligands by GPCRs, as well as their smaller size compared to antibodies or luminescent proteins, make them very attractive tools for the study of protein complexes. In association with the time-resolved FRET strategy, such ligands will offer a way to demonstrate the existence of GPCR complexes in a physiological context and to study their function. Because numerous fluorescent peptide and non-peptide ligands showing high affinities for their various cognate receptors have already been developed, the time-resolved FRET approach described here can readily be adapted to other receptors.
METHODS
Ligand syntheses, cell culture, membrane preparations and radioligand binding assays are described in Supplementary Methods.
Homogeneous time-resolved FRET assays
These experiments involve the transfer of energy from a donor europium cryptate pyridine-bipyridine (Eu-PBBP) or Lumi4-Tb to an acceptor Alexa-647 or d1. Antagonists and agonists were labeled with Eu-PBBP or Lumi4-Tb or with Alexa-647 or d1 (see Supplementary Methods). HTRF experiments were performed on transiently transfected Cos7 cells distributed into a black 96-well assay plate (Costar) at a density of 100,000 cells per well, on membrane preparations (20 to 300 μg per assay) from Cos7 or CHO cells expressing vasopressin or oxytocin receptors, and on mammary gland membrane preparations.
HTRF experiments were carried out in 100 μl for cells and 200 μl for membranes with the following medium: Tris-Krebs buffer (20 mM Tris-HCl, 118 mM NaCl, 5.6 mM glucose, 1.2 mM KH2PO4, 1.2 mM MgSO4, 4.7 mM KCl, 1.8 mM CaCl2, pH 7.4) supplemented with 0.1% (w/v) BSA and 0.1% (w/v) glucose. Cells or membranes were incubated with the fluorescent donor- and acceptor-labeled ligands. Unless otherwise stated, the ligands were used at a concentration equal to the Ki of the ligand for each receptor.
As a negative control, Cos7 cells or membranes were incubated only with the donor fluorophore–labeled ligand. The addition of an excess of unlabeled ligand or mock membrane preparations resulted in a nonspecific signal.
After an overnight incubation at 4 °C to avoid internalization processes, preparations were excited at 337 nm (the excitation wavelength of europium cryptate) and fluorescence emissions were measured at 620 nm (the emission wavelength of europium cryptate) and at 665 nm (the emission wavelength of Alexa-647) on a RUBYstar fluorometer (BMG Labtechnologies). A 400-μs reading was measured after a 50-μs delay to remove the short-life fluorescence background from the signal. The specific FRET signal was calculated using the following equation:
where R is the ratio (665 nm/620 nm) calculated for each assay and RNeg is the same ratio for the negative control (donor alone). It is noteworthy that concentrations of donor (europium cryptate or Lumi4-Tb)- or acceptor (Alexa-647 or d1)-labeled peptide cannot exceed 5 nM and 10 nM, respectively, as higher concentrations result in a large nonspecific signal.
Time-resolved FRET experiments with agonists
The signal-to-noise ratio in time-resolved FRET experiments is strongly dependent on the concentration of donor and acceptor. The higher the concentrations, the lower the signal-to-noise ratio. Indeed, high concentrations of donor and acceptor lead to dynamic FRET because of the collision between the fluorophores. Saturation of the ligand-binding sites with agonists requires high concentrations of ligands, thus leading to a very low signal-to-noise ratio in homogeneous conditions. Experiments in heterogeneous conditions have also been performed, but the time necessary for washing steps is not compatible with the dissociation kinetics of agonists from the second binding site, preventing any observation of a significant FRET signal.
Homogeneous time-resolved FRET assays on native tissue patches
The same procedure was applied on patches of tissues. After the lactating rats were killed, tissues (mammary gland, brain, kidneys and skeletal muscles) were rapidly removed and kept in cold Tris-Krebs buffer supplemented with 0.1% (w/v) BSA and 0.1% (w/v) glucose. Connective tissues, if any were present, were carefully removed, and patches consisting of 3-mm3 pieces were prepared from the various tissues. The whole dissection did not take more than 1 h. All the ligands were prepared in ice-cold medium. Patches and ligands were added together in a black, 96-well plate for overnight incubation at 4 °C. Data analysis was performed as followed:
Fluorescence of all samples was read at 620 nm (the emission wavelength of Lumi4-Tb donor) and 665 nm (the emission wavelength of the acceptors) after excitation of the samples at 340 nm (the excitation wavelength of Lumi4-Tb donor). The ratio of the fluorescence (665/620) was calculated for each sample and the ΔF parameter was calculated as described above, RNeg being the ratio determined in the presence of donor and the absence of acceptor. In these conditions, ΔF%Neg = 0.
Snap-tag labeling and TR-FRET with compatible fluorophores
Experiments were performed as previously described27. In brief, 24 h after transfection, cells (100,000 per well of a 96-well Greiner CellStar plate) were washed with DMEM and 10% (v/v) FBS and incubated in the presence of benzyl guanine conjugated with europium cryptate (BG-K) (0.3 μM) and the d2 fluorophore (BG-d2) (0.5 μM) or with Lumi4-Tb (0.1 μM) and d2 (0.25 μM) for 1 h at 37 °C, 5% CO2 in the same labeling buffer as described above. Cells were washed four times with labeling buffer. Time-resolved FRET signals were measured at 665 nm with a 50-μs delay after laser excitation at 337 nm using a RUBYstar plate reader (BMG Labtechnologies). The FRET signal is represented by the Δ665 value, which is calculated using the following formula: Δ665 = (total signal at 665 nm) – (background at 665 nm), where the background signal corresponds to that of cells labeled with the donor fluorophore in the presence of unlabeled BG (0.5 μM).
Supplementary Material
Acknowledgments
Thanks are due to S. Granier, P. Rondard and L. Prezeau for their critical reading of the manuscript. This work was supported by research grants from the Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, Actions Concertées Incitatives “Molécules Cibles et Thérapeutiques” (no. 240 and 355), ANR-06-BLAN-0087-03 and ANR-09-BLAN-0272) and the US National Institutes of Health (grants GM025280, DA022413, MH54137). This work was also made possible by the Plateforme de Pharmacologie-Criblage of Montpellier and the Region Languedoc-Roussillon. NAPS and PPHT amines were synthesized by the National Institute of Mental Health Chemical Synthesis and Drug Supply Program and provided by A.N. and J.J.
Footnotes
Competing financial interests
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/naturechemicalbiology/.
Author contributions
L.A. and T.D. originated the project. L.A., M.C. and T.D. executed most of the experiments and wrote the manuscript; S.S. and M.M. performed the peptide agonist synthesis; R.S. synthesized the antagonist peptide; I.B. performed experiments with SNAP-tag receptors; M.-L.R., M.K., A.N. and J.J. contributed to the development of the fluorescent dopamine receptor ligands and assay system; T.R. characterized the dopamine ligands; H.B., E.B. and L.L. labeled the ligands with fluorophores; C.B. performed saturation experiments with [125I]OTA; E.T., B.M. and J.-P.P. supported the project and participated in the writing of the manuscript.
Additional information
Supplementary information and chemical compound information is available online at http://www.nature.com/naturechemicalbiology/. Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/.
References
- 1.Terrillon S, Bouvier M. Roles of G-protein-coupled receptor dimerization. EMBO Rep. 2004;5:30–34. doi: 10.1038/sj.embor.7400052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferré S, et al. Building a new conceptual framework for receptor heteromers. Nat Chem Biol. 2009;5:131–134. doi: 10.1038/nchembio0309-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fotiadis D, et al. Structure of the rhodopsin dimer: a working model for G-protein-coupled receptors. Curr Opin Struct Biol. 2006;16:252–259. doi: 10.1016/j.sbi.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Gomes I, et al. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci USA. 2004;101:5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wreggett KA, Wells JW. Cooperativity manifest in the binding properties of purified cardiac muscarinic receptors. J Biol Chem. 1995;270:22488–22499. doi: 10.1074/jbc.270.38.22488. [DOI] [PubMed] [Google Scholar]
- 6.Chidiac P, Green MA, Pawagi AB, Wells JW. Cardiac muscarinic receptors. Cooperativity as the basis for multiple states of affinity. Biochemistry. 1997;36:7361–7379. doi: 10.1021/bi961939t. [DOI] [PubMed] [Google Scholar]
- 7.Urizar E, et al. Glycoprotein hormone receptors: link between receptor homodimerization and negative cooperativity. EMBO J. 2005;24:1954–1964. doi: 10.1038/sj.emboj.7600686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waldhoer M, et al. A heterodimer-selective agonist shows in vivo relevance of G protein–coupled receptor dimers. Proc Natl Acad Sci USA. 2005;102:9050–9055. doi: 10.1073/pnas.0501112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roess DA, Horvat RD, Munnelly H, Barisas BG. Luteinizing hormone receptors are self-associated in the plasma membrane. Endocrinology. 2000;141:4518–4523. doi: 10.1210/endo.141.12.7802. [DOI] [PubMed] [Google Scholar]
- 10.Patel RC, et al. Ligand binding to somatostatin receptors induces receptorspecific oligomer formation in live cells. Proc Natl Acad Sci USA. 2002;99:3294–3299. doi: 10.1073/pnas.042705099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bazin H, Trinquet E, Mathis G. Time resolved amplification of cryptate emission: a versatile technology to trace biomolecular interactions. J Biotechnol. 2002;82:233–250. doi: 10.1016/s1389-0352(01)00040-x. [DOI] [PubMed] [Google Scholar]
- 12.Terrillon S, et al. Oxytocin and vasopressin V1a and V2 receptors form constitutive homo- and heterodimers during biosynthesis. Mol Endocrinol. 2003;17:677–691. doi: 10.1210/me.2002-0222. [DOI] [PubMed] [Google Scholar]
- 13.Devost D, Zingg HH. Identification of dimeric and oligomeric complexes of the human oxytocin receptor by co-immunoprecipitation and bioluminescence resonance energy transfer. J Mol Endocrinol. 2003;31:461–471. doi: 10.1677/jme.0.0310461. [DOI] [PubMed] [Google Scholar]
- 14.Cottet M, et al. Past, present and future of vasopressin and oxytocin receptor oligomers, prototypical GPCR models to study dimerization processes. Curr Opin Pharmacol. 2009;1:59–66. doi: 10.1016/j.coph.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong D, Strange PG. Dopamine D2 receptor dimer formation: evidence from ligand binding. J Biol Chem. 2001;276:22621–22629. doi: 10.1074/jbc.M006936200. [DOI] [PubMed] [Google Scholar]
- 16.Guo W, et al. Dopamine D2 receptors form higher-order oligomers at physiological expression levels. EMBO J. 2008;27:2293–2304. doi: 10.1038/emboj.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Javitch JA. The ants go marching two by two: oligomeric structure of G-protein-coupled receptors. Mol Pharmacol. 2004;66:1077–1082. doi: 10.1124/mol.104.006320. [DOI] [PubMed] [Google Scholar]
- 18.Vivo M, Lin H, Strange PG. Investigation of cooperativity in the binding of ligands to the D(2) dopamine receptor. Mol Pharmacol. 2006;69:226–235. doi: 10.1124/mol.105.012443. [DOI] [PubMed] [Google Scholar]
- 19.Albizu L, et al. Probing the existence of G protein–coupled receptor dimers by positive and negative ligand-dependent cooperative binding. Mol Pharmacol. 2006;70:1783–1791. doi: 10.1124/mol.106.025684. [DOI] [PubMed] [Google Scholar]
- 20.Albizu L, et al. Towards efficient drug screening by homogeneous assays based on the development of new fluorescent vasopressin and oxytocin receptor ligands. J Med Chem. 2007;50:4976–4985. doi: 10.1021/jm061404q. [DOI] [PubMed] [Google Scholar]
- 21.Durroux T, et al. Fluorescent pseudo-peptide linear vasopressin antagonists: design, synthesis, and applications. J Med Chem. 1999;42:1312–1319. doi: 10.1021/jm9804782. [DOI] [PubMed] [Google Scholar]
- 22.Mathis G. Probing molecular interactions with homogeneous techniques based on rare earth cryptates and fluorescence energy transfer. Clin Chem. 1995;41:1391–1397. [PubMed] [Google Scholar]
- 23.Selvin PR, Hearst JE. Luminescence energy transfer using a terbium chelate: improvements on fluorescence energy transfer. Proc Natl Acad Sci USA. 1994;91:10024–10028. doi: 10.1073/pnas.91.21.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mouillac B, Manning M, Durroux T. Fluorescent agonists and antagonists for vasopressin/oxytocin G protein–coupled receptors: usefulness in ligand screening assays and receptor studies. Mini Rev Med Chem. 2008;8:996–1005. doi: 10.2174/138955708785740607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chini B, et al. Two aromatic residues regulate the response of the human oxytocin receptor to the partial agonist arginine vasopressin. FEBS Lett. 1996;397:201–206. doi: 10.1016/s0014-5793(96)01135-0. [DOI] [PubMed] [Google Scholar]
- 26.Serradeil-Le Gal C, et al. Binding properties of a selective tritiated vasopressin V2 receptor antagonist, [H]-SR 121463. Kidney Int. 2000;58:1613–1622. doi: 10.1046/j.1523-1755.2000.00322.x. [DOI] [PubMed] [Google Scholar]
- 27.Maurel D, et al. Cell-surface protein-protein interaction analysis with time-resolved FRET and snap-tag technologies: application to GPCR oligomerization. Nat Methods. 2008;5:561–567. doi: 10.1038/nmeth.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christopoulos A, Kenakin T. G protein–coupled receptor allosterism and complexing. Pharmacol Rev. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- 29.Durroux T. Principles: a model for the allosteric interactions between ligand binding sites within a dimeric GPCR. Trends Pharmacol Sci. 2005;26:376–384. doi: 10.1016/j.tips.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Christopoulos A, Lanzafame A, Ziegler A, Mitchelson F. Kinetic studies of co-operativity at atrial muscarinic M2 receptors with an “infinite dilution” procedure. Biochem Pharmacol. 1997;53:795–800. doi: 10.1016/s0006-2952(96)00814-3. [DOI] [PubMed] [Google Scholar]
- 31.Breton C, et al. Direct identification of human oxytocin receptor-binding domains using a photoactivatable cyclic peptide antagonist: comparison with the human V1a vasopressin receptor. J Biol Chem. 2001;276:26931–26941. doi: 10.1074/jbc.M102073200. [DOI] [PubMed] [Google Scholar]
- 32.Cotte N, et al. Identification of residues responsible for the selective binding of peptide antagonists and agonists in the V2 vasopressin receptor. J Biol Chem. 1998;273:29462–29468. doi: 10.1074/jbc.273.45.29462. [DOI] [PubMed] [Google Scholar]
- 33.Mouillac B, et al. Mapping peptide antagonist binding sites of the human V1a and V2 vasopressin receptors. Adv Exp Med Biol. 1998;449:359–361. doi: 10.1007/978-1-4615-4871-3_45. [DOI] [PubMed] [Google Scholar]
- 34.Phalipou S, et al. Mapping peptide-binding domains of the human V1a vasopressin receptor with a photoactivatable linear peptide antagonist. J Biol Chem. 1997;272:26536–26544. doi: 10.1074/jbc.272.42.26536. [DOI] [PubMed] [Google Scholar]
- 35.Terrillon S, et al. Synthesis and characterization of fluorescent antagonists and agonists for human oxytocin and vasopressin V(1)(a) receptors. J Med Chem. 2002;45:2579–2588. doi: 10.1021/jm010526+. [DOI] [PubMed] [Google Scholar]
- 36.Han Y, Moreira IS, Urizar E, Weinstein H, Javitch JA. Allosteric communication between protomers of dopamine class A GPCR dimers modulates activation. Nat Chem Biol. 2009;5:688–695. doi: 10.1038/nchembio.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barki-Harrington L, Luttrell LM, Rockman HA. Dual inhibition of beta-adrenergic and angiotensin II receptors by a single antagonist: a functional role for receptor-receptor interaction in vivo. Circulation. 2003;108:1611–1618. doi: 10.1161/01.CIR.0000092166.30360.78. [DOI] [PubMed] [Google Scholar]
- 38.Damian M, Martin A, Mesnier D, Pin JP, Baneres JL. Asymmetric conformational changes in a GPCR dimer controlled by G-proteins. EMBO J. 2006;25:5693–5702. doi: 10.1038/sj.emboj.7601449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo W, Shi L, Javitch JA. The fourth transmembrane segment forms the interface of the dopamine D2 receptor homodimer. J Biol Chem. 2003;278:4385–4388. doi: 10.1074/jbc.C200679200. [DOI] [PubMed] [Google Scholar]
- 40.Vilardaga JP, et al. Conformational cross-talk between alpha2A-adrenergic and mu-opioid receptors controls cell signaling. Nat Chem Biol. 2008;4:126–131. doi: 10.1038/nchembio.64. [DOI] [PubMed] [Google Scholar]
- 41.Springael JY, et al. Allosteric modulation of binding properties between units of chemokine receptor homo- and hetero-oligomers. Mol Pharmacol. 2006;69:1652–1661. doi: 10.1124/mol.105.019414. [DOI] [PubMed] [Google Scholar]
- 42.Goudet C, et al. Asymmetric functioning of dimeric metabotropic glutamate receptors disclosed by positive allosteric modulators. J Biol Chem. 2005;280:24380–24385. doi: 10.1074/jbc.M502642200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hlavackova V, et al. Evidence for a single heptahelical domain being turned on upon activation of a dimeric GPCR. EMBO J. 2005;24:499–509. doi: 10.1038/sj.emboj.7600557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Springael JY, Urizar E, Parmentier M. Dimerization of chemokine receptors and its functional consequences. Cytokine Growth Factor Rev. 2005;16:611–623. doi: 10.1016/j.cytogfr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Ciruela F, et al. Metabotropic glutamate 1alpha and adenosine A1 receptors assemble into functionally interacting complexes. J Biol Chem. 2001;276:18345–18351. doi: 10.1074/jbc.M006960200. [DOI] [PubMed] [Google Scholar]
- 46.Sohy D, et al. Hetero-oligomerization of CCR2, CCR5, and CXCR4 and the protean effects of “selective” antagonists. J Biol Chem. 2009;284:31270–31279. doi: 10.1074/jbc.M109.054809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daniels DJ, et al. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci USA. 2005;102:19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hague C, Uberti MA, Chen Z, Hall RA, Minneman KP. Cell surface expression of alpha1D-adrenergic receptors is controlled by heterodimerization with alpha1B-adrenergic receptors. J Biol Chem. 2004;279:15541–15549. doi: 10.1074/jbc.M314014200. [DOI] [PubMed] [Google Scholar]
- 49.Chabre M, Deterre P, Antonny B. The apparent cooperativity of some GPCRs does not necessarily imply dimerization. Trends Pharmacol Sci. 2009;30:182–187. doi: 10.1016/j.tips.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 50.Rives ML, et al. Crosstalk between GABA(B) and mGlu1a receptors reveals new insight into GPCR signal integration. EMBO J. 2009;28:2195–2208. doi: 10.1038/emboj.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.