Abstract
Objectives
Attention-deficit hyperactivity disorder (ADHD) is prevalent in patients with bipolar disorder (BP), but very few studies consider this when interpreting magnetic resonance imaging findings. No studies, to our knowledge, have screened for or controlled for the presence of ADHD when examining cortical thickness in patients with BP. We used a 2 × 2 design to evaluate the joint effects of BP and ADHD on cortical thickness and uncover the importance of ADHD comorbidity in BP subjects.
Methods
The study included 85 subjects: 31 healthy controls, 17 BP-only, 19 ADHD-only, and 18 BP/ADHD. All patients with BP were subtype I, were euthymic, and were not taking lithium. Groups did not differ significantly in age or sex distribution. We used cortical thickness measuring tools combined with cortical pattern matching methods to align sulcal/gyral anatomy across participants. Significance maps were used to check for both main effects of BP and ADHD and their interaction. Post-hoc comparisons assessed how the effects of BP on cortical thickness varied as a function of the presence or absence of ADHD.
Results
Interactions of BP and ADHD diagnoses were found in the left subgenual cingulate and right orbitofrontal cortex, demonstrating that the effect of BP on cortical thickness depends on ADHD status.
Conclusions
Some brain abnormalities attributed to BP may result from the presence of ADHD. Diagnostic interactions were found in regions previously implicated in the pathophysiology of BP, making it vital to control for an ADHD comorbid diagnosis when attempting to isolate neural or genetic abnormalities specific to BP.
Keywords: bipolar disorder, attention-deficit hyperactivity disorder, comorbidity, magnetic resonance imaging, cortical thickness, cortical pattern matching, prefrontal cortex, anterior cingulate cortex, subgenual cingulate cortex, orbitofrontal cortex
Attention-deficit hyperactivity disorder (ADHD) is prevalent in adults with bipolar disorder (BP) (1-4), with a reported comorbidity percentage ranging from 9.5% (5) to 19.4% (6). Very few neuroimaging studies of patients with BP, however, consider the impact of ADHD comorbidity on brain structure or function. Efforts are underway in the imaging genetics field to identify brain measures which are typical of patients with a specific diagnosis but amenable to large scale genetic analysis via genome-wide association (7). If such endophenotypes are to serve as reliable proxies for diagnostic classifications, it is vital that the patient groups from which they are derived are not confounded by comorbid conditions, especially if those conditions may alter the brain phenotype. Unfortunately, the Structured Clinical Interview for DSM-IV (SCID-IV) (8), which remains the most commonly used diagnostic assessment tool in research studies of adults with psychiatric disorders, does not include a specific assessment module for the presence of ADHD. Failing to assess or control for the presence of ADHD in brain research of adults with BP could confound findings that are attributed to a BP diagnosis, when they are in fact due to the effects of ADHD.
Existing studies have used structural imaging tools to compare patients with BP to healthy controls across brain regions using cortical thickness or volumetric techniques. These studies have reported gray matter deficits in the prefrontal cortex (PFC) and anterior cingulate cortex (ACC), including the orbital frontal cortex (OFC) (9, 10), left dorsomedial cortex (9, 10), left ventrolateral prefrontal cortex (VLPFC) (10, 11), left frontopolar cortex (10, 12), and bilateral paracingulate cortex (13). Findings in the ACC are inconsistent, with some studies indicating decreased gray matter in BP relative to controls (9-11, 14-16), others showing increased gray matter in BP (17), and still others finding no detectable difference between patients with BP and healthy controls (17-19). Of these structural studies, only one controlled for ADHD (9).
Studies on adults with ADHD have used similar structural analysis tools, and have revealed abnormalities in similar brain regions to those implicated in BP. Specifically, gray matter deficits have been reported in the ACC (20-22) and the left OFC (23). One recent study comparing adults with ADHD to healthy controls found ADHD to be associated with increased cortical thickness in some regions of the PFC [specifically the OFC and right dorsolateral prefrontal cortex (DLPFC)], and reduced cortical thickness in others [specifically the VLPFC and portions of the bilateral DLPFC (24)]. Other studies have also reported abnormalities in the DLPFC (20, 21, 25), superior frontal gyrus (26) and inferior parietal lobule (25).
Despite this overlap of structural abnormalities, few studies have examined the effect of comorbid ADHD on cortical thickness in patients with BP. To our knowledge, only one structural imaging study has examined the effects of ADHD comorbidity on gray matter volume abnormalities in adults with BP. This study reported that ADHD and BP contributed additively to volumetric abnormalities in comorbid patients (27). Patients with BP had lower volume (compared to controls) in the left OFC, regardless of an ADHD comorbidity, while an ADHD diagnosis was associated with reduced gray matter in the overall frontal lobe, superior PFC, right ACC and cerebellum, regardless of a BP comorbidity. These ADHD main effects imply differences between BP patients with and without ADHD comorbidity and suggest that it may be unwise to combine these subgroups when comparing BP patients to healthy controls. Only two functional magnetic resonance imaging (fMRI) studies have examined how ADHD affects measures of brain function in patients with BP (28, 29). Of these, only one study was carried out in adults. That study revealed a significant interaction between the two diagnoses, meaning that comorbid (BP + ADHD) patients had neural activation patterns that did not reflect the sum of BP-only and ADHD-only activation maps, but rather had their own unique pattern of activation (29). The findings from these structural and functional imaging studies emphasize the importance of controlling for ADHD comorbidity when assessing neural abnormalities in patients with BP.
With only these few studies examining how ADHD affects brain structure or function in patients with BP, it remains unclear (i) whether brain volume in patients with BP differs in those with or without an ADHD comorbidity and (ii) whether brain differences seen in BP patients comorbid for ADHD represent (i) an additive effect of the ADHD and BP neural phenotypes or, (ii) an interaction between the ADHD and BP diagnoses, whereby the effect of each of the two disorders on the neural phenotype depends on the presence of the other. Either of these patterns would make it problematic to combine BP patients with and without comorbid ADHD when trying to identify abnormalities attributable to BP. In this study, we compared patients with BP alone, ADHD alone, a combined diagnosis (of BP and ADHD) and healthy controls using cortical thickness measures. Specifically, we sought to determine the nature of the joint effect (i.e., none, additive or interactive) of ADHD and BP on cortical thickness.
We employed the cortical pattern matching (CPM) technique, which improves on traditional registration approaches by using sulcal features to align corresponding anatomy across participants, thereby localizing subtle differences in cortical brain structure across groups (30). This technique has been previously used by our groups in studies of cortical thickness in both patients with BP (10) and ADHD (31). Whole brain data were collected and analyzed, with a focus on a priori regions of interest (ROIs) within the PFC and ACC. Given that specific subregions within these broad ROIs carry significance for different endophenotypes of both BP and ADHD, we extended our analysis to the more focal Brodmann's areas (BA) within both the PFC and ACC. For example, both the function and structure of the orbitofrontal cortex (consisting of BA 10, 11, and 47) has been of particular interest to both our group (10, 32, 33) and others researching BP (34, 35). Thus we sought to determine whether cortical thickness abnormalities in patients with BP varied according to ADHD comorbidity status in the PFC and ACC, employing a detailed investigation into each area's anatomically defined subregions. We hypothesized that there would be differences resulting from either additive or interactive effects of the two diagnoses, between comorbid BP/ADHD patients and BP-only patients. Specifically, we hypothesized there would be differences between the BP and BP/ADHD groups in focal regions of the PFC and ACC where neuroimaging studies have found structural abnormalities separately in both disorders.
Methods
Participants
The study was approved by the University of California, Los Angeles (UCLA) and Department of Veterans Affairs (VA) Greater Los Angeles Healthcare System Institutional Review Boards. All participants provided written informed consent. Patients with a bipolar I disorder diagnosis were recruited through the UCLA Mood Disorders Outpatient Clinic, the Bipolar Disorders Outpatient Clinic of the VA Greater Los Angeles Healthcare System in West Los Angeles, and through local advertising. Participants with an ADHD diagnosis were recruited through UCLA's Adult ADHD outpatient clinic, local ADHD support groups, and local advertising. Healthy control participants were recruited by local advertising in newspapers and campus flyers.
A total of 85 participants were included in the study, consisting of 31 healthy controls, 17 BP-only, 19 ADHD-only, and 18 comorbid BP/ADHD patients. The groups were balanced on age and gender (Table 1). All participants were assessed using the SCID-IV (8) to confirm a BP diagnosis. All subjects were screened for ADHD using the behavioral disorders module of the Kiddie Schedule of Affective Disorders (K-SADS) (36), which had been modified for adult populations.
Table 1.
Demographic and clinical characteristics of study participants
| Controls (n = 31) | BP (n = 17) | ADHD (n = 19) | BP + ADHD (n = 18) | Test statistic (df) | p-value | |
|---|---|---|---|---|---|---|
| Age, years, mean (SD) | 37.81 (13.09) | 39.29 (10.80) | 37.00 (10.64) | 36.11 (13.30) | F(81,3) = 0.22 | 0.88 |
| Education, years, mean (SD) | 15.84 (1.90) | 14.76 (1.82) | 14.50 (1.20) | 14.18 (2.24) | F(79,3) = 3.83 | 0.01 |
| Female n (%) | 13 (42) | 7 (41) | 7 (37) | 7 (39) | χ2(3) = 0.15 | 0.99 |
| Race, n (%) | χ2(12) = 17.40 | 0.14 | ||||
| Caucasian | 20 (65) | 10 (59) | 14 (74) | 16 (89) | ||
| Asian | 7 (22) | 1 (5.9) | 3 (12) | 1 (5.6) | ||
| African American | 4 (13) | 5 (29) | 1 (5.3) | 1 (5.6) | ||
| Hispanic | 0 | 1 (5.9) | 0 | 0 | ||
| Other | 0 | 0 | 1 (5.3) | 0 | ||
| Age of onset, years, mean (SD) | NA | 19.88 (6.85) | NA | 16 (7.94) | F(31,1) = 0.71 | 0.41b,c |
| Months in current mood state, mean (SD) | NA | 20.12 (23.39) | NA | 16 (21.73) | F(32,1) = 0.28 | 0.60b,c |
| HAM-D-21 score, mean (SD) | 1.00 (1.20) | 3.25 (1.95) | 3.21 (2.12) | 3.88 (2.09) | F(78,3) = 12.32 | < 0.001 |
| F(31,1) = 0.18 | 0.68b,c | |||||
| F(34,1) = 0.02 | 0.88c,d | |||||
| YMRS score, mean (SD) | 0.55 (1.26) | 0.94 (1.60) | 1.00 (1.53) | 2.17 (2.48) | F(81,3) = 3.50 | 0.02 |
| F(33,1) = 3.39 | 0.07b | |||||
| F(35,1) = 3.67 | 0.06d | |||||
| K-SADS total score, mean (SD) | NA | NA | 11.84 (3.35) | 9.22 (3.17) | F(35,1) = 0.82 | 0.02d |
| Inattentive | NA | NA | 7.32 (1.38) | 5.39 (2.30) | F(35,1) = 0.04 | < 0.01d |
| Hyperactive | NA | NA | 4.53 (2.39) | 3.83 (2.15) | F(35,1) = 0.67 | 0.36d |
| Medications, n (%)a | ||||||
| Unmedicated | 31 (100) | 5 (30) | 16 (84) | 5 (28) | χ2(3) = 39.47 | < 0.001 |
| Lithium | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Anticonvulsants | 0 (0) | 9 (53) | 0 (0) | 8 (44) | ||
| Antipsychotics | 0 (0) | 9 (53) | 0 (0) | 10 (56) | ||
| Antidepressants | 0 (0) | 4 (24) | 0 (0) | 7 (39) | ||
| Benzodiazapines | 0 (0) | 0 (0) | 0 (0) | 1 (5.5) | ||
| Stimulants | 0 (0) | 0 (0) | 3 (16) | 1 (5.5) | ||
| ADHD subtype, n (%) | ||||||
| Inattentive | NA | NA | 12 (63) | 4 (22) | ||
| Hyperactive | NA | NA | 1 (5) | 2 (11) | ||
| Combined | NA | NA | 6 (32) | 3 (17) | ||
| NOS | NA | NA | 0 (0) | 4 (22) |
SD = standard deviation; HAM-D-21 = 21-item Hamilton Depression Rating Scale; YMRS = Young Mania Rating Scale; BP = bipolar disorder; ADHD = attention-deficit hyperactivity disorder; NOS = not otherwise specified; NA = not applicable; CM = comorbid for ADHD and BP.
Anticonvulsants included valproic acid (BP = 3, CM = 1), lamotrigine (BP = 5, CM = 5), carbamazepine (BP = 1, CM = 1), and oxcarbazepine (CM = 1). Antipsychotics included aripiprazole (BP = 4, CM = 6), ziprasidone (BP = 1), risperidone (BP = 1), quetiapine (BP = 1, CM = 4), and olanzapine (BP = 2). Antidepressants included citalopram (CM = 1), escitalopram (BP = 1), paroxetine (BP = 1), fluoxetine (BP = 1, CM = 1), trazodone (BP = 1, CM = 1), venlafaxine (CM = 1), and buproprion (CM = 3). Benzodiazepines included temazepam (CM = 1). Stimulants included dextroamphetamine (CM = 1, ADHD = 3).
Test statistic was determined based on differences between BP and BP + ADHD comorbid group.
Some subjects excluded due to missing data.
Test statistic was determined based on differences between ADHD and BP + ADHD comorbid group.
Exclusion criteria for patients with BP included current use of lithium as lithium has been demonstrated to affect gray matter structure (10, 37-39). Additionally, as mood state has been reported to affect gray matter measurements (40-42), we excluded BP patients with a current depression or mania, operationalized as having (i) a current mood episode based on the SCID-IV, (ii) a 21-item Hamilton Depression Rating Scale (HAM-D-21) score > 7, or (iii) a total Young Mania Rating Scale (YMRS) (43) score > 7. Additional exclusion criteria for ADHD, BP, and comorbid patients included other current Axis I disorders. Healthy control participants were excluded if they met criteria for any current or past psychiatric diagnosis, had a history of substance abuse, or were currently receiving medications for psychiatric reasons. Additional exclusion criteria for all groups included left-handedness, untreated hypertension, neurological illness, metal implants, and a history of skull fracture or head trauma with a loss of consciousness exceeding five minutes.
Data acquisition
All participants were scanned using a 1.5 Tesla (T) Siemens Sonata MRI scanner to obtain contiguous sagittal high-resolution three-dimensional (3-D) magnetization-prepared rapid gradient echo (MP-RAGE) T1-weighted images (160 slices; field of view = 256 mm; isotropic voxel size = 1mm3; repetition time = 1.900 msec; echo time = 4.38 msec, flip angle = 15 degrees; averages 4; total scan time = 8.14 min).
Data analysis
Demographic variables
Statistical analysis of demographic variables was performed using PASW Statistics v.18.0.3 (www.spss.com). Group differences in categorical and continuous variables were evaluated using chi-squared and analysis of variance models (ANOVA), respectively. Alpha was set at 0.05. As age and gender are factors that impact cortical thickness, they were controlled for as covariates in subsequent analyses to account for their contribution to the variance even though they did not differ by group.
Image preprocessing
Image preprocessing consisted of (i) adjustment for head position and transformation of data into a common stereotaxic coordinate system without scaling (http://www.bic.mni.mcgill.ca/software); (ii) automated exclusion of non-brain tissue and cerebellum (44); (iii) correction for magnetic field inhomogeneity artifacts (45); (iv) resampling using isotropic voxels of size 0.33 mm to estimate cortical thickness with subvoxel accuracy, and (v) use of a partial volume classification method to classify voxels as gray matter, white matter, CSF, or non-brain (46).
Measurement of cortical gray matter thickness
Cortical thickness was computed separately for each participant from preprocessed magnetic resonance images. Thickness was defined as the shortest 3-D distance from the cortical white-gray matter boundary to the hemispheric surface without crossing voxels classified as CSF. Specifically, an implementation of the Eikonal equation was applied to voxels segmenting as cortical gray matter to compute these distances (in millimeters) in a fully automated manner at each point along the cortical surface (30). A uniform spatial filter of a radius of 15 mm was applied which additionally served to ensure that thickness values for gyri not separated by CSF could not be misattributed. These methods produce thickness measurements that agree with those in postmortem samples (47, 48) and are stable over time in validation studies using short-interval repeat scans of multiple subjects (47).
Cortical pattern matching procedure
To allow for the comparison of cortical thickness across subjects, cortical pattern matching methods were applied. Each participant's T1 image was processed to create a 3-D surface model of the cortex using automated software that deforms a spherical mesh surface to fit cortical surface tissue using a threshold intensity value that differentiates extracortical CSF from brain tissue (49). Thirty-one separate sulci per hemisphere were manually delineated on each participant's surface model. Sulcal tracing was performed by a trained researcher, blind to participant characteristics, using the MNI-Display software package (http://www.bic.mni.mcgill.ca/software) in conjunction with a previously validated surface-based anatomical protocol (47). Tracer reliability was confirmed using the 3-D root mean square difference (in millimeters) between sulci in a set of six test brains and those of a gold standard set. A disparity of less than 2 mm between the test and gold standard brains was used as the reliability threshold for all landmarks.
To align sulcal/gyral anatomy, warping algorithms were used to compute the amount of shift in the x, y, and z directions needed to explicitly match each sulcus in every participant to that of the average anatomical study template (generated from patients and healthy comparison subjects, combined) (30). Cortical pattern matching algorithms were then used to associate the same parameter space coordinates across participants without actually changing the dimensions of the cortical surface models. This process reparameterized individual cortical models so that corresponding anatomy across participants bore the same coordinate locations.
Statistical analyses of cortical thickness
We used the R-programming language (http://www.r-project.org/) to fit, at each cortical surface point, a general linear model (GLM) with main effects of ADHD and BP diagnoses and their interaction, controlling for age and sex. This allowed us to produce uncorrected thresholded significance maps, projected onto a 3-D group averaged hemispheric surface model, showing the joint effects of BP and ADHD diagnoses on cortical thickness. Our primary objective was to determine whether there were significant differences between BP alone and BP/ADHD patients, which could occur if there were either (i) an interaction between BP and ADHD status or (ii) a main effect of ADHD.
After obtaining the uncorrected two-tailed probability maps described above, regional corrected p-values for both interactions and main effects were obtained via permutation testing, both on the overall PFC and ACC and on individual BAs within these regions (BA 44–47 and 8–11 in the PFC; BA 24, 25, 32, and 33 in the ACC) which were anatomically defined using a deformable BA Atlas (50) previously employed by our group (10). Permutation testing works by randomly shuffling group memberships a large number of times (in this study, 10,000 times) to measure the distribution of diagnostic features in the statistical maps that would be observed by accident. It yields p-values for each region that are corrected for multiple comparisons across the surface points within that area. We included the overall tests for the PFC and ACC in our analyses for completeness since these were our global regions of interest. However, our previous work has suggested that effects are often localized to discrete areas within these regions. Moreover, interaction effects tend to be smaller than main effects and are thus more likely, if they are localized, to be washed out in an overall regional test. Since our primary interest was in these localized effects, we performed the tests in the individual BAs regardless of whether the overall tests for the PFC and ACC were significant.
Tests for interactions were run first. In regions with significant interactions, we performed post-hoc pair-wise comparisons to better understand the how the magnitude and direction of the effects of BP on cortical thickness vary depending on ADHD status, and in particular to determine whether or not there was a significant difference between BP subjects with and without the comorbidity. In regions without significant interactions we tested for main effects of the disorders since even an additive effect of ADHD would imply a difference between BP subjects with and without the comorbidity. The reported p-values are unadjusted except for the permutation correction for multiple comparisons across voxels. We used a two-tailed significance level of α = .05 as the threshold for identifying effects of interest but clearly specify which subregions survived a Bonferroni correction for the number of BAs within each global region of interest (as defined above, totaling eight BAs for the PFC and four for the ACC.) Results for BAs that do not survive this correction should be considered preliminary, although we note that the Bonferroni correction is probably substantially over-conservative as tests within the same overall region will not be independent. Comparisons of cortical thickness outside our a priori regions of the PFC and ACC were also treated as exploratory and thus should be further investigated in future studies.
Results
Participants
Groups did not differ significantly in age [F(81,3) = 0.23, p = 0.88] or sex [χ2(3) = 0.15, p = 0.99]. Additionally, BP subgroups (BP-only, comorbid BP/ADHD) did not differ significantly in YMRS [F(33,1) = 3.39, p = 0.07], or HAM-D-21 scores [F(31,1) = 0.18, p = 0.68]. ADHD subgroups (ADHD-only, comorbid BP/ADHD) also did not differ significantly in YMRS [F(35,1) = 3.67, p = 0.06] or HAM-D-21 scores [F(34,1) = 0.02, p = 0.88]. This and other clinical and demographic information on subjects are presented in Table 1.
Cortical thickness analyses
Interaction analyses
As anticipated, we did not find significant interactions between ADHD and BP diagnoses for the PFC and ACC overall. However, localized interactions were found within both these ROIs, specifically, in the right orbitofrontal cortex (BA47, p = 0.037) in the PFC and in the left subgenual cortex (BA25, p = 0.013) of the ACC (Table 2, Fig. 1A). As discussed in our methods, these p-values, as well as any subsequent p-values reported, are corrected for comparisons across surface points within the given region. This latter interaction survives a Bonferonni correction for multiple comparisons for the four subregions examined within the ACC (BA 24, 25, 32, and 33). The interaction in right BA47 does not survive a Bonferroni correction. However, the PFC is an extremely broad region, and previous work from our group (10, 32, 33) and others (34, 35) has specifically implicated BA47 as an area with functional and structural abnormalities in patients with BP. We therefore consider this finding to be noteworthy.
Table 2.
Frontal and temporal lobe subregions demonstrating an interaction between bipolar disorder and attention-deficit hyperactivity disorder
| Cortical region | Hemisphere | BA | p-valuea |
|---|---|---|---|
| Anterior cingulate cortex | |||
| Subgenual cingulate cortex | Left | 25 | 0.01b |
| Prefrontal cortex | |||
| Lateral orbital cortex | Right | 47 | 0.04 |
| Ventrolateral cortex | Right | 45 | 0.09 |
| Inferior temporal cortex | |||
| Superolateral cortex | Left | 20 | 0.02 |
| Right | 20 | 0.003 | |
| Fusiform gyrus | Left | 37 | 0.04 |
BA = Brodmann's area.
Two-tailed significance levels obtained from permutation testing after controlling for age and gender, corrected for comparisons across voxels within the BA.
Indicates survival of Bonferroni correction for multiple comparisons.
Fig. 1.
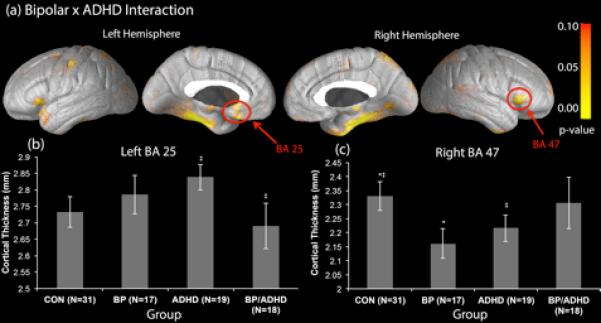
Regions of interaction between bipolar disorder (BP) and attention-deficit hyperactivity disorder (ADHD) diagnoses. BA = Brodmann's area; CON = controls. *Indicates significant differences (p < 0.05) in cortical thickness between groups. **Indicates trend level differences (p < 0.10) in cortical thickness between groups.
To better understand the nature of these interactions, and in particular to determine whether abnormalities in cortical thickness in these regions differ between BP individuals with and without comorbid ADHD, we conducted post hoc subgroup comparisons in both BA25 and BA47. Post-hoc subgroup comparisons of BA25 revealed that in the absence of an ADHD comorbidity, there was no significant effect of a BP diagnosis on cortical thickness (p > 0.10). However, when comorbid with ADHD, a BP diagnosis was associated with trend level cortical thinning, relative to BP patients without ADHD, in BA25 (p = 0.07) (Fig. 1B). For BA47 our results indicated that BP diagnosis was associated with significant cortical thinning (p = 0.05) (Fig. 1C) in the absence of an ADHD comorbidity. However, when comorbid with ADHD, a BP diagnosis was not associated with thinning in this region (p > 0.10) (Fig. 1C). A non-comorbid diagnosis of ADHD was not associated with abnormalities in BA47 cortical thickness.
Main effects analyses
Next we examined main effects of BP and ADHD (Fig. 2). Main effects analyses examine the effects of the two diagnoses separately, comparing all subjects with a BP diagnosis to all subjects without a BP diagnosis (Table 3, Fig. 2A) or comparing all subjects with an ADHD diagnosis to all subjects without an ADHD diagnosis (Table 4, Fig. 2B). These analyses were performed in the overall PFC and ACC and in BAs without evidence of a significant interaction. They were not performed for BA47 and BA25 since the significant interactions in those regions imply the effects of BP and ADHD are interdependent and cannot be assessed separately. This analysis was important because, even without an interaction, a main effect of ADHD would mean that there were significant differences between comorbid BP/ADHD and BP-only patients, making it unwise to combine these two subgroups for comparisons with healthy controls.
Fig. 2.
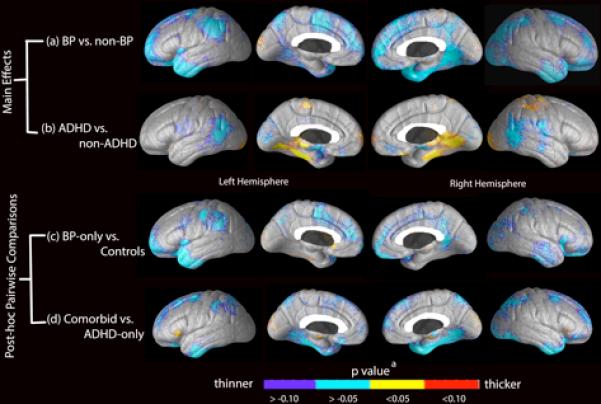
Significance maps of cortical thickness associated with main effects and subgroup comparisons.
aThe color bar reflects the p-value associated with comparisons using the general linear model performed at each cortical surface point. Main effects of (A) groups with bipolar disorder (BP) [BP-only and BP/attention-deficit hyperactivity disorder (ADHD)] versus those without BP (ADHD-only and controls), where p < 0 (blue/purple) signifies regions in which the presence of BP is associated with cortical thinning, and (B) groups with ADHD (ADHD-only and BP/ADHD) versus those without ADHD (BP-only and controls), where p < 0 signifies regions in which the presence of ADHD is associated with cortical thinning, and p > 0 (red/yellow) is associated with increased cortical thickness. Pairwise comparisons of (C) BP-only versus healthy controls, where p < 0 indicates regions in which BP-only was associated with cortical thinning relative to controls, and (D) ADHD-only versus BP/ADHD, where p < 0 indicate regions in which BP/ADHD is associated with cortical thinning relative to ADHD-only.
Table 3.
Cortical regions for which a main effect of bipolar disorder was associated with cortical thinning
| Cortical region | Hemisphere | BA | p-valuea |
|---|---|---|---|
| Prefrontal cortex | |||
| Orbital cortex | Left | 11 | 0.003b |
| Right | 11 | 0.002b | |
| Ventrolateral cortex | Left | 44 | 0.05 |
| Frontopolar cortex | Left | 10 | < 0.01 |
| Right | 10 | 0.04 | |
| Dorsomedial cortex | Left | 8 | 0.02 |
| Right | 8 | 0.02 | |
| Left | 9 | 0.00 | |
| Superolateral cortex | Left | 6 | 0.03 |
| Right | 6 | 0.03 | |
| Anterior cingulate cortex | |||
| Left | 24 | 0.03 | |
| Left | 32 | 0.04 | |
| Inferior temporal cortex | |||
| Superolateral cortex | Left | 20 | 0.003 |
| Right | 20 | < 0.01 | |
| Superior temporal cortex | Left | 38 | < 0.01 |
| Right | 38 | 0.002 | |
| Medial temporal cortex | Left | 21 | 0.02 |
| Right | 21 | 0.02 | |
| Inferior parietal cortex | |||
| Angular gyrus | Left | 39 | 0.04 |
| Supramarginal gyrus | Left | 40 | 0.01 |
| Occipital lobe | Right | 18 | 0.01 |
| Right | 19 | < 0.01 |
BA = Brodmann's area.
Two-tailed significance levels obtained from permutation testing after controlling for age and gender, corrected for comparisons across surface points within the BA.
Indicates survival of Bonferroni correction for multiple comparisons.
Table 4.
Cortical regions for which a main effect of attention-deficit hyperactivity disorder (ADHD) was associated with either increased or decreased cortical thickness
| Cortical region | Hemisphere | BA | p-valuea |
|---|---|---|---|
|
Main effect of ADHD associated with increased cortical thickness
| |||
| Inferior temporal cortex | |||
| Perirhinal cortex | Left | 35 | 0.04 |
|
Main effect of ADHD associated with decreased cortical thickness | |||
| Superolateral parietal cortex | |||
| Angular gyrus | Left | 39 | < 0.01 |
| Right | 39 | 0.02 | |
| Occipital lobe | Left | 19 | 0.04 |
| Right | 19 | 0.04 | |
BA = Brodmann's area.
Two-tailed significance levels obtained from permutation testing after controlling for age and gender, corrected for comparisons across surface points within the BA.
Patients with BP demonstrated overall cortical thinning in the PFC (left: p = 0.007, right: p = 0.02) and left ACC (p = 0.03). Permutation analysis of the BA's within these broader ROI's revealed that the effect in the PFC was driven by thinning in left BA9 (p = 0.04) as well as bilaterally in BA10 (left: p = 0.007; right: p = 0.04) and BA11 (left: p = 0.003; right: p = 0.002). BA24 (p = 0.03) and BA32 (p = 0.04) drove the main effect in the left ACC. The significant effects on the overall a priori ROIs protect against the need for multiple comparisons in the follow-up tests of the individual subregions. However, we feel it is worth noting that the effect seen in BA11 was particularly robust and would in fact survive a Bonferroni correction for the eight BAs in the PFC, which reduced the significance threshold to 0.006 (0.05/8) (see Table 3). We did not observe any main effects of ADHD in either the PFC or ACC or their respective subregions. However, additional, exploratory analyses outside of these regions showed cortical thickening in some areas and cortical thinning in others (Table 4, Fig. 2B).
Discussion
In this study, we examined how cortical thickness abnormalities in patients with BP vary according to the presence or absence of ADHD. Existing structural and functional brain imaging studies of adults with BP have, for the most part, neglected the potential importance of accounting for ADHD comorbidity when examining neural abnormalities. Several of our key findings support the importance of accounting for comorbid ADHD in structural neuroimaging studies of patients with BP. Interaction and pairwise analyses between groups revealed that the effect of BP on cortical thickness was different in patients with and without ADHD comorbidity in the right lateral OFC (BA47) and the left subgenual cingulate (BA25). The effects of BP and ADHD in these regions were found to be not additive, but rather interdependent, resulting in a unique phenotypic signature for the comorbid diagnostic group. However, in other subregions of the PFC (BA 8, 9, 10, and 11) and ACC (BA 24, 32, and 33), the effect of a BP diagnosis on cortical thickness was not changed by ADHD comorbidity status.
In the right lateral OFC (BA47), BP was associated with significant cortical thinning only in the absence of an ADHD diagnosis; however, in the presence of ADHD no such cortical thinning was detected. Results from prior studies of BP examining cortical thickness of the lateral OFC of the prefrontal cortex are variable (9, 10, 51, 52). Our findings suggest the possibility that a varying proportion of subjects with a comorbid ADHD diagnosis in each of these studies could account for this inconsistency. One recent study showed increases in cortical thickness in adults with ADHD in the PFC (24) which, when combined with the cortical thinning associated with BP, may explain in our study the non-significant difference in the comorbid BP-ADHD relative to controls.
The interaction findings in left BA25 showed that BP-only patients did not differ from healthy controls in this region, but that the presence of ADHD and BP together was associated with cortical thinning relative to the ADHD-only group. Similar to the findings in BA47, BA25 has been previously reported to be anatomically and functionally abnormal in some studies of patients with BP (14, 17, 53, 54), but not in others. The interaction effect in BA25, and subsequent comparisons demonstrating no significant difference between the BP-only group and healthy controls in this region, again suggests that the discrepancy in results from prior studies could be due to differences in the proportion of patients in each study with an ADHD comorbidity. We would like to note, however, that the thinning associated with BP in the presence of ADHD was only trend-level significance, a finding that may or may not remain significant with larger population samples. Other studies using larger samples report reduced gray matter volume in BA25 in patients with BP (14, 33), suggesting that the trend level significance in our findings may simply be due to a lack of power. Interestingly, a follow-up analysis that included K-SADS scores for ADHD and comorbid patients (calculated by combining the hyperactive and inattentive scores) revealed that the initial trend level (p = 0.07) thinning of comorbid patients relative to ADHD-only patients reached significance (p = 0.04) when ADHD severity was accounted for in the model. Regressing the values out of the model gave us the important comparison of cortical thickness measurements in ADHD and comorbid patients with the same symptoms.
Our findings of significant interactions show that ADHD has non-uniform effects on the cortical thickness of BP patients. In BA47, the presence of ADHD eliminates the cortical thinning associated with BP relative to controls. On the other hand, BP is only associated with cortical thinning in BA25 when ADHD is present. This again suggests that a comorbid diagnosis is not merely represented by the overlaying of the cortical abnormalities associated with BP and ADHD separately, but rather that individual brain regions are differentially affected by the comorbid diagnosis.
Mood disorders are typically associated with dysfunction in a network such as the corticolimbic circuit, which includes both the subgenual cingulate and the lateral orbitofrontal cortex. It is striking that both regions of interaction are found in the corticolimbic circuit. Future research is necessary to further investigate the nature of these interactions. It's is possible that the interaction of BP and ADHD in BA25 disrupts the corticolimbic circuitry in a way such that cortical thinning in BA47 associated with BP is strengthened. For example, given that both regions are involved in inhibition, perhaps the weakening of the corticolimbic projections from one region strengthens those from another as a compensatory mechanism. We acknowledge that this is speculative and clearly more work needs to be done, with larger populations samples, to investigate this relationship. In addition, the differential interaction of ADHD and BP on brain structure raises questions about the problems associated with strictly phenotypically defined diagnoses. Although outside of the scope of this paper, the non-uniform effect of ADHD on the cortical structure of patients with BP may be representative of the diagnostic classification problems currently facing the scientific community.
Main effects analyses revealed cortical thinning associated with a BP diagnosis, regardless of ADHD comorbidity, in certain PFC regions (BA10, BA11, and left BA9) and the left ACC (BA24 and BA32). Our finding of cortical thinning in these areas is consistent with previous studies in BP (9, 10, 13, 55, 56). Of particular interest is the robust main effect found in the medial OFC (BA11) since a recent fMRI study by our group examining the effects of a comorbid diagnosis on BOLD signal also revealed a BP main effect (in this case, of hypoactivation) in BA11 (29). This may serve as evidence that functional and structural abnormalities in BA11 are hallmark features of BP.
To our knowledge, only one other study has examined how brain structure in adults with BP varies in the presence of ADHD. That study found that ADHD and BP contributed additively and selectively to brain structure, resulting in a comorbid phenotype comprised of the abnormalities found separately in each individual disorder (27). Our maps extend these prior findings by suggesting that, in some regions, the impact of a BP/ADHD comorbidity is not simply additive but may be reflective of an interactive effect, resulting in a distinct neural signature. Two previous fMRI studies evaluating BP patients with ADHD comorbidity similarly have found differences in neural signatures between ADHD/BP patients and patients with BP-only (28, 29). Adler et al. reported that a BP/ADHD diagnosis in adolescents is associated with hyperactivation of the posterior parietal cortex and middle temporal gyrus, as well as hypoactivation of the PFC and ACC, when compared to BP-only patients (28). In another study by our group, Townsend et al. (currently under review) found interaction effects in the anterior and posterior cingulate, left medial and middle frontal gyri, left inferior parietal lobule, precuneous and striatum, suggesting that the neural effects of BP vary in relation to the presence or absence of ADHD (29). These studies together suggest that ADHD comorbidity must be considered in neuroimaging analyses.
The etiology of cortical thinning in patients with BP remains to be determined. It may reflect fewer neurons, a reduction of glia without a loss of neurons, or an increase in white matter myelination rather than gray matter reduction (57, 58). Any of these structural abnormalities may affect the function of not only the structurally abnormal brain region (59), but also those regions to which it projects (60). Many brain regions implicated in our interaction and main effects analyses are part of an anterior limbic network. Functional deficits in portions of this network are associated with the emotional dysregulation characteristic of BP (32, 61-66). For example, the lateral OFC (BA47) and medial OFC (BA11) (where abnormalities were associated with BP in our analyses) have direct and indirect reciprocal projections to regions within the limbic circuit, including the amygdala, anterior temporal cortex (BA38/20), ACC (BA24/32) and subgenual cingulate (BA25) (61, 62).
Although not included in our a priori hypothesis, the left fusiform gyrus (BA37) and the bilateral superolateral cortex (BA20) in the inferior temporal lobe also showed an interaction between BP and ADHD (Table 2). Strakowski et al. (64) recently found blunted fusiform gyrus responses in manic patients with BP during an emotional response task when compared to healthy control subjects. Nomura et al. (67) reported a negative correlation between activation in the fusiform gyrus and amygdala. To our knowledge, there are no studies directly examining the relationship between the fusiform gyrus and amygdala in BP, although this may be useful to evaluate in future studies. In further analysis of the interaction effect found in BA20, we found that the presence of ADHD was associated with an increase in the magnitude of the BP effect in this region. BA20 projects to the lateral OFC (BA47), suggesting that abnormalities BA20 may contribute to the dysfunction of the anterior limbic network. The effect of BP on cortical thinning in this region may explain why in this study the presence of ADHD was not associated with any BA47 cortical thinning in patients with BP relative to controls.
The current study has several strengths in its design. First, no participants were currently taking lithium. Second, all BP patients were euthymic at the time of scanning. In prior structural studies of BP, only four of these studies controlled for patient use of lithium (10, 15-17), and only two of these studies explicitly controlled for mood state and BP subtype (10, 68). Gray matter volume may increase in as little as four weeks post lithium treatment (69). Further, significant differences have been found in gray matter volume between patients with BP treated with lithium and those who were not (37). The impact of mood state on gray matter measures has also been reported, with a depressed mood state being associated with gray matter deficits relative to patients in a euthymic state (40-42).
There are some limitations, however, in the current study. First, while none of the patients in this study were currently taking lithium, many were on other medications, including anticonvulsants, antipsychotics, and stimulants. The effects of these medications on brain structure are not clearly known. One study has reported that antipsychotics do not affect cortical thickness (70), although others have found a medication effect (71). Some studies have reported that stimulants have no effect (72, 73) while others have reported an association with a deficit in gray matter (74-76). Second, it has been suggested that reductions in gray matter may occur as a consequence of affective episodes rather than as a result of aging (77). As the previous number of episodes correlates with illness duration and age, we could not disentangle these effects. However, the number of episodes did not differ significantly between BP patients with and without ADHD and therefore could not by itself account for our findings. In addition, there has been some evidence that gray matter volume scales with brain size. We find it unlikely that brain size significantly contributes to our findings since the aforementioned study reported the exponent factor of cortical thickness scaling to brain size as less than one third, identifying a much stronger relationship between cortical surface area and brain size (78). In addition, a postmortem study found no correlation between brain size and cortical thickness, again reporting that increases in gray matter volume with brain size are attributable to cortical surface area (79). However, as an extra precaution, we re-examined regions identified as having either a significant interaction or main effect and found that while controlling for total brain volume could shift the p-value slightly in either direction (depending on the region in question), it did not affect the statistical significance of any findings. Last, a larger subject pool for each of our patient groups would have been ideal; it is possible that there are other regions of interaction or main effects that may not have been detected here due to insufficient power. None-the-less, the findings here underscore the importance of properly distinguishing between BP and ADHD diagnoses, and we hope that they will be expanded upon with larger subgroups in future studies.
In conclusion, this is one of the first studies, to assess interactions of BP and ADHD diagnoses on brain structure. Interactions, which were present in the left subgenual cingulate and right orbitofrontal cortex, suggest that the effect of BP on cortical thickness in these regions varies according to the presence or absence of ADHD. An accurate depiction of the underlying neural phenotype of BP, as opposed to that of a combined BP/ADHD diagnosis, is essential for the understanding of the pathophysiology of BP and developing targeted treatment approaches.
Acknowledgements
The authors gratefully acknowledge the Furlotti Family Foundation, (LLA), and the National Institute of Mental Health (K24 MH001848, R21 MH075944) (LLA), 5F31MH078556) (LCF), (EB008432, EB008281, EB007813, HD050735, and RR013642) (PMT) for their financial support of this study. For generous support the authors also wish to thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones–Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family, and Northstar Fund. The project described was supported by grant #RR12169, #RR13642, and #RR00865 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). The contents of the paper are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Footnotes
Disclosures
The authors of this paper do not have any commercial associations that might pose a conflict of interest in connection with this manuscript.
Reference
- 1.Kent L, Craddock N. Is there a relationship between attention deficit hyperactivity disorder and bipolar disorder? J Affect Disord. 2003;73:211–221. doi: 10.1016/s0165-0327(02)00092-7. [DOI] [PubMed] [Google Scholar]
- 2.Wingo AP, Ghaemi SN. A systematic review of rates and diagnostic validity of comorbid adult attention-deficit/hyperactivity disorder and bipolar disorder. J Clin Psychiatry. 2007;68:1776–1784. doi: 10.4088/jcp.v68n1118. [DOI] [PubMed] [Google Scholar]
- 3.Tamam L, Karakus G, Ozpoyraz N. Comorbidity of adult attention-deficit hyperactivity disorder and bipolar disorder: prevalence and clinical correlates. Eur Arch Psychiatry Clin Neurosci. 2008;258:385–393. doi: 10.1007/s00406-008-0807-x. [DOI] [PubMed] [Google Scholar]
- 4.McIntyre RS, Kennedy SH, Soczynska JK, et al. Attention-deficit/hyperactivity disorder in adults with bipolar disorder or major depressive disorder: results from the international mood disorders collaborative project. Prim Care Companion J Clin Psychiatry. 2010;12 doi: 10.4088/PCC.09m00861gry. pii: PCC.09m00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nierenberg AA, Miyahara S, Spencer T, et al. Clinical and diagnostic implications of lifetime attention-deficit/hyperactivity disorder comorbidity in adults with bipolar disorder: data from the first 1000 STEP-BD participants. Biol Psychiatry. 2005;57:1467–1473. doi: 10.1016/j.biopsych.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 6.Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein J, Medland S, Vasquez AA, et al. Genome-Wide Association Meta-Analysis of Hippocampal Volume: Results from the ENIGMA Consortium. Organization for Human Brain Mapping; Quebec City, Canada: 2011. [Google Scholar]
- 8.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I disorders (SCID I) Biometrics Research Department, New York State Psychiatric Institute; New York: 1997. [Google Scholar]
- 9.Lyoo IK, Sung YH, Dager SR, et al. Regional cerebral cortical thinning in bipolar disorder. Bipolar Disord. 2006;8:65–74. doi: 10.1111/j.1399-5618.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- 10.Foland-Ross LC, Thompson PM, Sugar CA, et al. Investigation of cortical thickness abnormalities in lithium-free adults with bipolar I disorder using cortical pattern matching. Am J Psychiatry. 2011;168:530–539. doi: 10.1176/appi.ajp.2010.10060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nugent AC, Milham MP, Bain EE, et al. Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. Neuroimage. 2006;30:485–497. doi: 10.1016/j.neuroimage.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 12.Frangou S. The Maudsley Bipolar Disorder Project. Epilepsia. 2005;46(Suppl. 4):19–25. doi: 10.1111/j.1528-1167.2005.463005.x. [DOI] [PubMed] [Google Scholar]
- 13.Fornito A, Malhi GS, Lagopoulos J, et al. Anatomical abnormalities of the anterior cingulate and paracingulate cortex in patients with bipolar I disorder. Psychiatry Res. 2008;162:123–132. doi: 10.1016/j.pscychresns.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Drevets WC, Price JL, Simpson JR, Jr, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 15.Sassi RB, Brambilla P, Hatch JP, et al. Reduced left anterior cingulate volumes in untreated bipolar patients. Biol Psychiatry. 2004;56:467–475. doi: 10.1016/j.biopsych.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Kaur S, Sassi RB, Axelson D, et al. Cingulate cortex anatomical abnormalities in children and adolescents with bipolar disorder. Am J Psychiatry. 2005;162:1637–1643. doi: 10.1176/appi.ajp.162.9.1637. [DOI] [PubMed] [Google Scholar]
- 17.Brambilla P, Nicoletti MA, Harenski K, et al. Anatomical MRI study of subgenual prefrontal cortex in bipolar and unipolar subjects. Neuropsychopharmacology. 2002;27:792–799. doi: 10.1016/S0893-133X(02)00352-4. [DOI] [PubMed] [Google Scholar]
- 18.Sanches M, Sassi RB, Axelson D, et al. Subgenual prefrontal cortex of child and adolescent bipolar patients: a morphometric magnetic resonance imaging study. Psychiatry Res. 2005;138:43–49. doi: 10.1016/j.pscychresns.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman ME, DelBello MP, Getz GE Shear PK, Strakowski SM. Anterior cingulate subregion volumes and executive function in bipolar disorder. Bipolar Disord. 2006;8:281–288. doi: 10.1111/j.1399-5618.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- 20.Makris N, Biederman J, Valera EM, et al. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb Cortex. 2007;17:1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- 21.Seidman LJ, Valera EM, Makris N, et al. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol Psychiatry. 2006;60:1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 22.Amico F, Stauber J, Koutsouleris N, et al. Anterior cingulate cortex gray matter abnormalities in adults with attention deficit hyperactivity disorder: a voxel-based morphometry study. Psychiatry Res. 2011;191:31–35. doi: 10.1016/j.pscychresns.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Hesslinger B, Tebartz van Elst L, Thiel T, et al. Frontoorbital volume reductions in adult patients with attention deficit hyperactivity disorder. Neurosci Lett. 2002;328:319–321. doi: 10.1016/s0304-3940(02)00554-2. [DOI] [PubMed] [Google Scholar]
- 24.Seidman LJ, Biederman J, Liang L, et al. Gray matter alterations in adults with attention-deficit/hyperactivity disorder identified by voxel based morphometry. Biol Psychiatry. 2011;69:857–866. doi: 10.1016/j.biopsych.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seidman LJ, Biederman J, Liang L, et al. Gray matter alterations in adults with attention-deficit/hyperactivity disorder identified by voxel based morphometry. Biol Psychiatry. 2011;69:857–866. doi: 10.1016/j.biopsych.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almeida LG, Ricardo-Garcell J, Prado H, et al. Reduced right frontal cortical thickness in children, adolescents and adults with ADHD and its correlation to clinical variables: a cross-sectional study. J Psychiatr Res. 2010;44:1214–1223. doi: 10.1016/j.jpsychires.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 27.Biederman J, Makris N, Valera EM, et al. Towards further understanding of the comorbidity between attention deficit hyperactivity disorder and bipolar disorder: a MRI study of brain volumes. Psychol Med. 2008;38:1045–1056. doi: 10.1017/S0033291707001791. [DOI] [PubMed] [Google Scholar]
- 28.Adler CM, Delbello MP, Mills NP, Schmithorst V, Holland S, Strakowski SM. Comorbid ADHD is associated with altered patterns of neuronal activation in adolescents with bipolar disorder performing a simple attention task. Bipolar Disord. 2005;7:577–588. doi: 10.1111/j.1399-5618.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 29.Townsend J, Sugar CA, Walshaw PD, et al. Comorbid ADHD in adults with bipolar disorder impacts fMRI findings during a response inhibition task. Under review. Need citation. [Google Scholar]
- 30.Thompson PM, Hayashi KM, Sowell ER, et al. Mapping cortical change in Alzheimer's disease, brain development, and schizophrenia. Neuroimage. 2004;23(Suppl. 1):S2–S18. doi: 10.1016/j.neuroimage.2004.07.071. [DOI] [PubMed] [Google Scholar]
- 31.Sowell ER, Thompson PM, Welcome SE, et al. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003;362:1699–1707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- 32.Altshuler LL, Bookheimer SY, Townsend J, et al. Blunted activation in orbitofrontal cortex during mania: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:763–769. doi: 10.1016/j.biopsych.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Townsend JD, Bookheimer SY, Foland-Ross LC, et al. Deficits in inferior frontal cortex activation in euthymic bipolar disorder patients during a response inhibition task. Bipolar Disord. 2012;14:442–450. doi: 10.1111/j.1399-5618.2012.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nery FG, Chen HH, Hatch JP, et al. Orbitofrontal cortex gray matter volumes in bipolar disorder patients: a region-of-interest MRI study. Bipolar Disord. 2009;11:145–153. doi: 10.1111/j.1399-5618.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- 35.Stanfield AC, Moorhead TW, Job DE, et al. Structural abnormalities of ventrolateral and orbitofrontal cortex in patients with familial bipolar disorder. Bipolar Disord. 2009;11:135–144. doi: 10.1111/j.1399-5618.2009.00666.x. [DOI] [PubMed] [Google Scholar]
- 36.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 37.Sassi RB, Nicoletti M, Brambilla P, et al. Increased gray matter volume in lithium-treated bipolar disorder patients. Neurosci Lett. 2002;329:243–245. doi: 10.1016/s0304-3940(02)00615-8. [DOI] [PubMed] [Google Scholar]
- 38.Bearden CE, Thompson PM, Dalwani M, et al. Greater cortical gray matter density in lithium-treated patients with bipolar disorder. Biol Psychiatry. 2007;62:7–16. doi: 10.1016/j.biopsych.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foland LC, Altshuler LL, Sugar CA, et al. Increased volume of the amygdala and hippocampus in bipolar patients treated with lithium. Neuroreport. 2008;19:221–224. doi: 10.1097/WNR.0b013e3282f48108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brooks JO, 3rd, Bonner JC, Rosen AC, et al. Dorsolateral and dorsomedial prefrontal gray matter density changes associated with bipolar depression. Psychiatry Res. 2009;172:200–204. doi: 10.1016/j.pscychresns.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nery FG, Chen HH, Hatch JP, et al. Orbitofrontal cortex gray matter volumes in bipolar disorder patients: a region-of-interest MRI study. Bipolar Disord. 2009;11:145–153. doi: 10.1111/j.1399-5618.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- 42.Brooks JO, 3rd, Foland-Ross LC, Thompson PM, et al. Preliminary evidence of withinsubject changes in grey matter density associated with remission of bipolar depression. Psychiatry Research: Neuroimaging. 2011;193:53–55. doi: 10.1016/j.pscychresns.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 44.Shattuck DW, Leahy RM. BrainSuite: an automated cortical surface identification tool. Med Image Anal. 2002;6:129–142. doi: 10.1016/s1361-8415(02)00054-3. [DOI] [PubMed] [Google Scholar]
- 45.Zijdenbos AP, Dawant BM. Brain segmentation and white matter lesion detection in MR images. Crit Rev Biomed Eng. 1994;22:401–465. [PubMed] [Google Scholar]
- 46.Shattuck DW, Sandor-Leahy SR, Schaper KA, et al. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- 47.Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Von Economo C. The Cytoarchitectonics of the Human Cerebral Cortex. Oxford Medical Publications; London: 1929. [Google Scholar]
- 49.MacDonald D. A method for identifying geometrically simple surfaces from three dimensional images (doctoral dissertation) McGill University; Montreal: 1998. [Google Scholar]
- 50.Rasser PE, Ward PB, Johnston P, Thompson PM. A deformable Brodmann area atlas.. Proceedings of the 2004 IEEE International Symposium on Biomedical Imaging: From Nano to Macro; Arlington, VA, USA. April 15-18, 2004. [Google Scholar]
- 51.López-Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, Strakowski SM. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biol Psychiatry. 2002;52:93–100. doi: 10.1016/s0006-3223(02)01350-1. [DOI] [PubMed] [Google Scholar]
- 52.Lyoo IK, Kim MJ, Stoll AL, et al. Frontal lobe gray matter density decreases in bipolar I disorder. Biol Psychiatry. 2004;55:648–651. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 53.Frangou S, Raymont V, Bettany D. The Maudsley bipolar disorder project. A survey of psychotropic prescribing patterns in bipolar I disorder. Bipolar Disord. 2002;4:378–385. doi: 10.1034/j.1399-5618.2002.01223.x. [DOI] [PubMed] [Google Scholar]
- 54.Lipsman N, McIntyre RS, Giacobbe P, Torres C, Kennedy SH, Lozano AM. Neurosurgical treatment of bipolar depression: defining treatment resistance and identifying surgical targets. Bipolar Disord. 2010;12:691–701. doi: 10.1111/j.1399-5618.2010.00868.x. [DOI] [PubMed] [Google Scholar]
- 55.Phillips ML, Drevets WC, Rauch SL, et al. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 56.Haldane M, Cunningham G, Androutsos C, et al. Structural brain correlates of response inhibition in bipolar disorder I. J Psychopharmacol. 2008;22:138–143. doi: 10.1177/0269881107082955. [DOI] [PubMed] [Google Scholar]
- 57.Bartzokis G, Lu PH, Stewart SB, et al. In vivo evidence of differential impact of typical and atypical antipsychotics on intracortical myelin in adults with schizophrenia. Schizophr Res. 2009;113:322–331. doi: 10.1016/j.schres.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sowell ER, Peterson BS, Thompson PM, et al. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 59.Lu LH, Dapretto M, O'Hare ED, et al. Relationships between brain activation and brain structure in normally developing children. Cereb Cortex. 2009;19:2595–2604. doi: 10.1093/cercor/bhp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Foland-Ross LC, Altshuler LL, Bookheimer SY, et al. Amygdala reactivity in healthy adults is correlated with prefrontal cortical thickness. J Neurosci. 2010;30:16673–16678. doi: 10.1523/JNEUROSCI.4578-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann NY Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- 62.Adler CM, DelBello MP, Strakowski SM. Brain network dysfunction in bipolar disorder. CNS Spectr. 2006;11:312–320. doi: 10.1017/s1092852900020800. [DOI] [PubMed] [Google Scholar]
- 63.Strakowski SM. Bipolar disorder and advances in neuroimaging: a progressive match. CNS Spectr. 2006;11:267–268. doi: 10.1017/s1092852900020769. [DOI] [PubMed] [Google Scholar]
- 64.Strakowski SM, Eliassen JC, Lamy M, et al. Functional magnetic resonance imaging brain activation in bipolar mania: evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol Psychiatry. 2011;69:381–388. doi: 10.1016/j.biopsych.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Altshuler L, Bookheimer S, Proenza MA, et al. Increased amygdala activation during mania: a functional magnetic resonance imaging study. Am J Psychiatry. 2005;162:1211–1213. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- 66.Altshuler L, Bookheimer S, Townsend J, et al. Regional brain changes in bipolar I depression: a functional magnetic resonance imaging study. Bipolar Disord. 2008;10:708–717. doi: 10.1111/j.1399-5618.2008.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nomura M, Ohira H, Haneda K, et al. Functional association of the amygdala and ventral prefrontal cortex during cognitive evaluation of facial expressions primed by masked angry faces: an event-related fMRI study. Neuroimage. 2004;21:352–363. doi: 10.1016/j.neuroimage.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 68.Fornito A, Yucel M, Wood SJ, et al. Anterior cingulate cortex abnormalities associated with a first psychotic episode in bipolar disorder. Br J Psychiatry. 2009;194:426–433. doi: 10.1192/bjp.bp.107.049205. [DOI] [PubMed] [Google Scholar]
- 69.Moore GJ, Cortese BM, Glitz DA, et al. A longitudinal study of the effects of lithium treatment on prefrontal and subgenual prefrontal gray matter volume in treatment-responsive bipolar disorder patients. J Clin Psychiatry. 2009;70:699–705. doi: 10.4088/JCP.07m03745. [DOI] [PubMed] [Google Scholar]
- 70.Nesvag R, Lawyer G, Varnas K, et al. Regional thinning of the cerebral cortex in schizophrenia: effects of diagnosis, age and antipsychotic medication. Schizophr Res. 2008;98:16–28. doi: 10.1016/j.schres.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 71.Thompson PM, Bartzokis G, Hayashi KM, et al. Time-lapse mapping of cortical changes in schizophrenia with different treatments. Cereb Cortex. 2009;19:1107–1123. doi: 10.1093/cercor/bhn152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lawyer G, Bjerkan PS, Hammarberg A, Jayaram-Lindström, Franck J, Agartz I. Amphetamine dependence and co-morbid alcohol abuse: associations to brain cortical thickness. BMC Pharmacol. 2010;10:5. doi: 10.1186/1471-2210-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schlaepfer TE, Lancaster E, Heidbreder R, et al. Decreased frontal white-matter volume in chronic substance abuse. Int J Neuropsychopharmacol. 2006;9:147–153. doi: 10.1017/S1461145705005705. [DOI] [PubMed] [Google Scholar]
- 74.Bartzokis G, Beckson M, Lu PH, et al. Age-related brain volume reductions in amphetamine and cocaine addicts and normal controls: implications for addiction research. Psychiatry Res. 2000;98:93–102. doi: 10.1016/s0925-4927(99)00052-9. [DOI] [PubMed] [Google Scholar]
- 75.Daumann J, Koester P, Becker B, et al. Medial prefrontal gray matter volume reductions in users of amphetamine-type stimulants revealed by combined tract-based spatial statistics and voxel-based morphometry. Neuroimage. 2011;54:794–801. doi: 10.1016/j.neuroimage.2010.08.065. [DOI] [PubMed] [Google Scholar]
- 76.Thompson PM, Hayashi KM, Simon SL, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- 78.Im K, Lee JM, Lyttelton O, et al. Brain size and cortical structure in the adult human brain. Cereb Cortex. 2008;18:2181–2191. doi: 10.1093/cercor/bhm244. [DOI] [PubMed] [Google Scholar]
- 79.Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]


