Abstract
Depression, anxiety, and impairments in learning and memory are all associated with traumatic brain injury (TBI). Because of the strong link between zinc deficiency, depression, and anxiety, in both humans and rodent models, we hypothesized that dietary zinc supplementation prior to injury could provide behavioral resiliency to lessen the severity of these outcomes after TBI. Rats were fed a marginal zinc deficient (5 ppm), zinc adequate (30 ppm), or zinc supplemented (180 ppm) diet for 4 weeks followed by a moderately-severe TBI using the well-established model of controlled cortical impact (CCI). Following CCI, rats displayed depression-like behaviors as measured by the 2-bottle saccharin preference test for anhedonia. Injury also resulted in evidence of stress and impairments in Morris water maze (MWM) performance compared to sham-injured controls. While moderate zinc deficiency did not worsen outcomes following TBI, rats that were fed the zinc supplemented diet for 4 weeks showed significantly attenuated increases in adrenal weight (p<0.05) as well as reduced depression-like behaviors (p<0.001). Supplementation prior to injury improved resilience such that there was not only significant improvements in cognitive behavior compared to injured rats fed an adequate diet (p<0.01), there were no significant differences between supplemented and sham-operated rats in MWM performance at any point in the 10-day trial. These data suggest a role for supplemental zinc in preventing cognitive and behavioral deficits associated with TBI.
Keywords: zinc, TBI, depression, anxiety, anhedonia, spatial memory
1. Introduction
Over 1.5 million Americans sustain a traumatic brain injury (TBI) every year. Additionally, it has been estimated that over 20% of military personnel returning from the current conflicts in Iraq and Afghanistan have suffered TBI [1]. Although the mechanisms are not fully understood, many TBI patients experience long-term cognitive and behavioral deficits that include memory loss, depression, and anxiety [2]. In fact, as many as 40% of patients hospitalized with TBI develop major depression, making this the most common long-term complication of TBI [3]. Even mild TBI has been recognized as a risk factor for mood disorders [4].
Treatment options for the cognitive and behavioral consequences of TBI are limited. While antidepressant drugs are commonly prescribed, a recent review of the efficacy of this approach reveals that it is difficult to draw conclusions from the available reports because of small sample sizes and study designs that vary widely [5]. The lack of clear and effective treatment options have led to the search for factors that may improve resiliency in the event of a TBI. This approach would be particularly useful in populations at risk for TBI.
Interestingly, there is a well-documented association between low serum zinc levels and mood disorders. Not only do patients with major depression appear to have reduced serum zinc levels, there is an inverse relationship between serum zinc levels and the severity of the symptoms of depression [6–8]. Additionally, animal models of zinc deficiency have shown increased depression- and anxiety-like behaviors [9,10]. These data are particularly striking because we have known for some time that TBI results in increased urinary zinc excretion and significantly depressed serum zinc levels. In fact, an early report showed that urinary zinc excretion was proportional to the severity of the brain injury with the most severely injured patients having mean urinary zinc levels that were as high as 14 times normal values, resulting in rapid zinc depletion [11]. Other work has shown that following TBI both severe and moderate zinc deficiency result in increased cell death [12,13].
While supplemental zinc has successfully been employed as a treatment following TBI to improve visceral protein status and neurologic outcomes [14], no work to date has tested the use of zinc to improve resiliency to TBI in humans or experimental models of brain injury. Thus, the work reported here was designed to test the hypothesis that 4 weeks of dietary zinc supplementation would improve resiliency in a rodent model of TBI known to induce depression- and anxiety-like behaviors and produce deficits in spatial memory.
2. Material and Methods
2.1 Animal care
Young adult 6 wk old male Sprague-Dawley rats (Charles Rivers Laboratories, Wilmington, MA) were individually housed in temperature-controlled rooms with a 12-hour light-dark cycle. Animals were fed a commercially prepared semi-purified diet using zinc carbonate as the zinc source (Research Diets Inc., New Brunswick, NJ). Rats were fed a zinc supplemented (ZS, 180 ppm), zinc adequate (ZA, 30 ppm), or marginal zinc deficient (ZD, 5 ppm) diet for a period of 4 weeks. Body weight and food intakes were measured weekly. All procedures were approved by the Florida State University Animal Care and Use Committee (ACUC).
2.2 Traumatic brain injury
At the end of the 4 week dietary period, TBI was induced by a controlled cortical impact (CCI) to the medial frontal cortex. Prior to CCI, rats (n=10/group) were anesthetized using isoflurane gas. Aseptic procedures were used throughout the surgical procedures and body temperatures were maintained using a homeothermic blanket. Following stereotaxic placement and a midline incision, a 6 mm diameter mid-sagittal bilateral craniotomy 3 mm rostral to bregma was performed. A 5 mm diameter pneumatic cortical contusion device (MyNeuroLab, Inc., Richmond, IL) was used to produce a 3.0 mm deep contusion using an impact velocity of 2.25 m/s and an impact time of 500 ms [15]. Following TBI, the incision was immediately sutured. Additional animals (n=5/group) which were anesthetized and received the midline incision, but not the craniotomy or impact, were used as sham-operated controls for ZS, ZA and ZD dietary groups. After TBI or sham surgery, animals were maintained on their assigned diets throughout the behavioral testing period. Behavioral testing began 1 week after TBI.
2.3 Elevated plus maze
Anxiety-like behaviors were measured using standard protocols for elevated plus maze (MedAssociates, Inc., Georgia, Vermont) in rodents following 4 weeks of diet and again post-TBI (day 20). Rats (n=8–9/group) were placed in the center of the maze facing the closed arms and allowed to explore the maze for a period of 5 min (the reduced number of rats from n=10 in the dietary groups to n=8–9 reflects the small mortality rate after TBI). The number of explorations into the open arms and the amount of time spent in the open arms was recorded for each animal.
2.4 Anhedonia
As a measure of depression-like behavior, the standard two-bottle choice paradigm for saccharin preference was used to examine the effect of injury and diet on the development of anhedonia [10]. Anhedonia was first measured 4 weeks of the zinc diets, but prior to injury, and then again after TBI. To eliminate preference for saccharin due to novelty seeking behavior, rats were first exposed to two bottles of 0.025% saccharin for 2 days. They were then given a choice between saccharin and deionized water for a 4 day period; two days at 0.025% saccharin followed by 2 days at 0.05%. Intake of water and saccharin were measured daily. To avoid preferences associated with bottle placement, the position of the bottles was changed daily.
2.5 Morris water maze
Morris water maze (MWM) performance was tested using a circular tank (diameter 114 cm, height 58 cm) of water filled to a depth of 30 cm and rendered opaque by the addition of non-toxic water-based white paint. A square, plexiglass escape platform was submerged 2 cm below the surface of the opaque water and located in the north quadrant 10 cm from the edge of the wall. The platform remained in the same position throughout testing. Spatial visual cues, which stayed in the same location throughout the testing period, were placed on the north, east, and west walls of the tank. Acquisition trials consisted of two trials per day for 10 days starting postsurgery day 8 and began with a single day of training on day 0 [16]. Rats (n=8–10/group) were placed in two different locations on each trial (south and east quadrants) and required to find the hidden platform. If a rat did not find the hidden platform during the 90-sec trial period, it was then physically guided to platform and placed on it for a period of 20 sec. Following completion of the second trial each day, the rat was dried and returned to its home cage. Time to find the hidden platform was recorded for both trials and averaged for analysis.
2.6 Brain Zinc
Seventy-two hours after brain injury, rats (n=3/group) were killed by CO2 asphyxiation followed by decapitation. Tissue was collected from both the hippocampus and site of cortical injury and weighed (wet weight). Tissue samples and blanks were digested in a concentrated trace metal free nitric acid and diluted in 4% nitric acid and diluted for measurement of total zinc concentrations by inductively coupled plasma mass spectrometry (ICP-MS) on a Finnigan Element-1 ICP-MS at the National High Magnetic Field Laboratory [17].
2.7 Adrenal gland weight
Three days after the completion of behavioral testing, rats were killed with CO2 asphyxiation followed by decapitation and bilateral adrenal glands removed and weighed.
2.8 Statistical Analysis
All data were expressed as the mean ± SEM and statistical significance was set at p<0.05 with 95% confidence (Prism; GraphPad, San Diego, CA). All behavioral data was analyzed by one-way ANOVA with a Bonferroni post-hoc test except MWM which was a two-way ANOVA repeated measures. A two-way ANOVA was used to analyze brain zinc concentrations followed by a Bonferroni post-hoc test.
3. Results
3.1 Body Weight and Food Intake
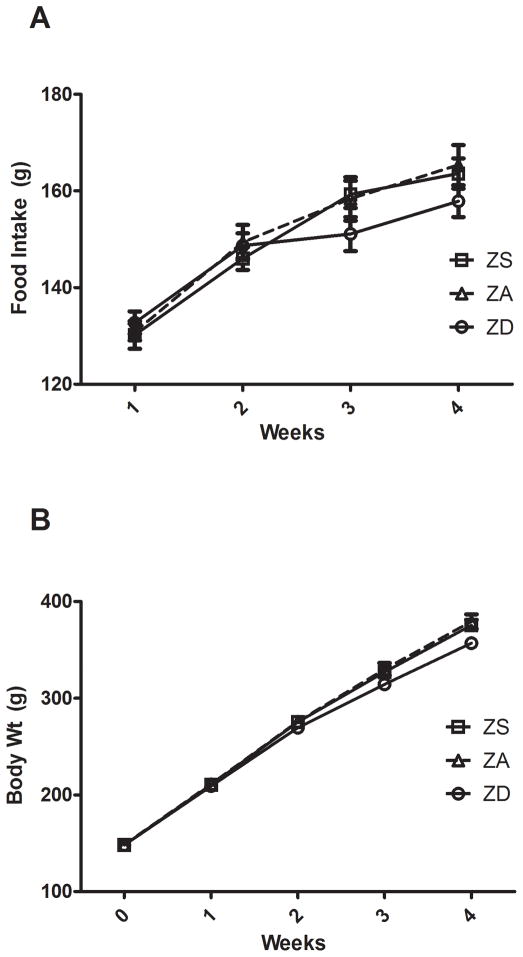
Examination of longitudinal food intake revealed that dietary zinc (ZS, ZA, or ZD) did not alter food intake during the 4 week dietary period (Fig 1A). At weeks 3 and 4, mean body weight of ZD animals was approximately 5% lower than ZA controls (p<0.05, Fig 1B). However, at no point during the dietary treatment were mean body weights of ZS animals significantly different from ZA controls.
Figure 1.
Effect of diet on food intake and body weight. Rats were fed a zinc supplemented (ZS, 180 ppm), zinc adequate (ZA, 30 ppm), or marginal zinc deficient (ZD, 5 ppm) diet for 4 weeks. (A) Food intake (g) was measured weekly. Bars indicate mean ± SEM. (B) Body weight (g) was measured weekly. Bars indicate mean ± SD.
3.2 Anxiety
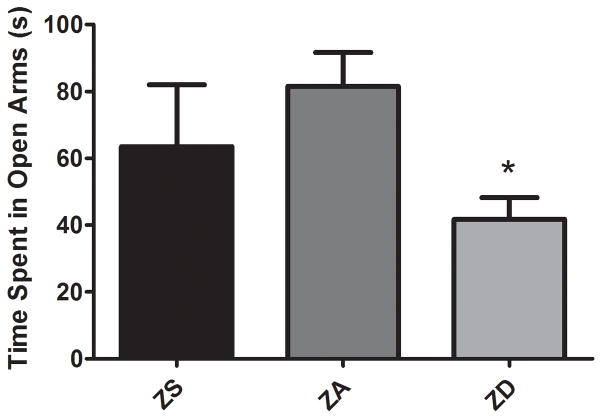
Marginal zinc deficiency significantly increased anxiety-like behaviors in uninjured rats (p<0.05, Fig. 2). Rats fed the ZD diet showed an approximately 50% reduction in the time spent in the open arms compared to ZA controls. Four weeks of zinc supplementation did not change the amount of time spent in the arms.
Figure 2.
Marginal zinc deficiency induces anxiety-like behavior. Rats were fed a zinc supplemented (ZS), zinc adequate (ZA), or marginal zinc deficient (ZD) diet for 4 weeks. Anxiety-like behavior was quantified using an elevated plus maze by measuring the amount of time (s, seconds) spent in the open arms during a 5 min trial. Bars indicate the time spent in the open arms (mean ± SEM). *Significantly different from zinc adequate controls at p<0.05.
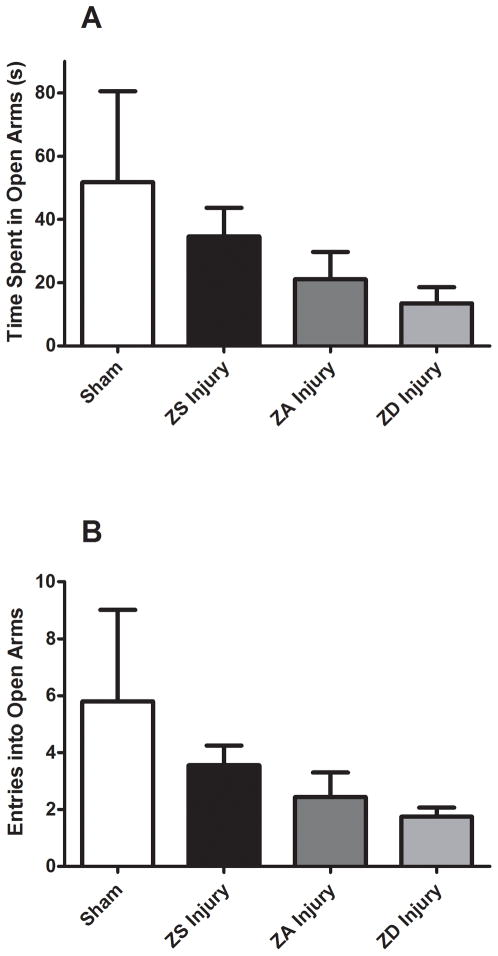
Injury resulted in increased anxiety, with ZA injured animals spending 41% less time in the open arms (p<0.05) and 42% fewer crosses into the open arms (p<0.05) compared to ZA sham-operated controls. Figure 3 shows that while not statistically significant, there were trends toward reduced time in open arms and number of entries in zinc deficiency TBI animals, while supplemented animals showed a trend toward increased time and crosses (Fig. 3).
Figure 3.
Effect of zinc supplementation on anxiety-like behavior following traumatic brain injury. Rats were fed a zinc supplemented (ZS), zinc adequate (ZA), or a marginal zinc deficient (ZD) diet for 4 weeks and then subjected to injury or sham surgery (Sham). Sham-operated controls were fed the ZA control diet. Anxiety-like behaviors were measured for 5 min using an elevated plus maze and quantified by (A) the amount of time (s, seconds) spent exploring the open arms and (B) the number of explorations into the open arms. Bars represent mean ± SEM.
Bilateral adrenal glands weights, expressed as a function of total body weight, were not significantly different in ZS, ZA, or ZD sham-operated rats (0.116±0.007, 0.091±0.008, 0.105±.006, respectively). However, injury increased relative adrenal weight by 60% compared to ZA control shams (p<0.05, Fig. 4). Zinc supplementation prevented the injury-induced increases in adrenal weight such that ZS adrenal weights were not significantly different from sham-operated controls (Fig. 4).
Figure 4.
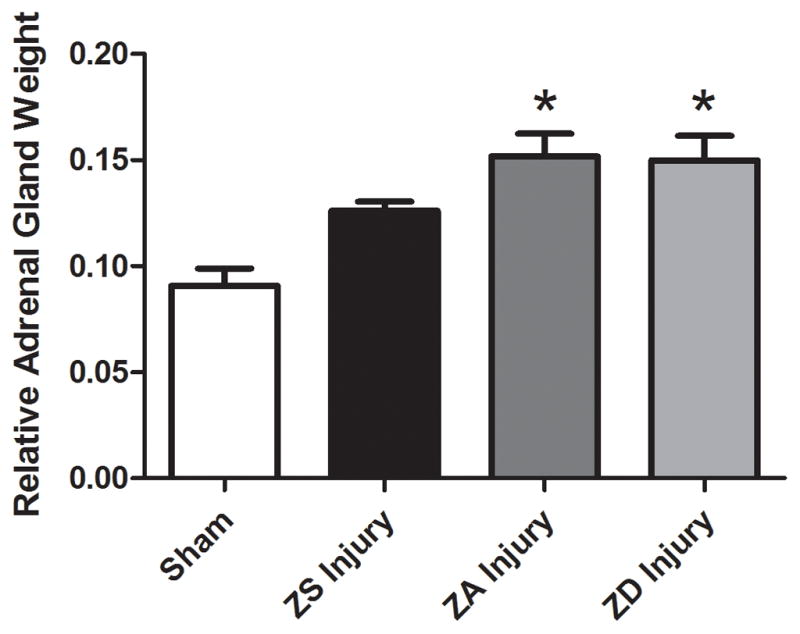
Effect of zinc supplementation on relative adrenal gland weight in traumatic brain injury. Rats were fed a zinc supplemented (ZS), zinc adequate (ZA), or a marginal zinc deficient (ZD) diet for 4 weeks followed by either injury or sham surgery (Sham). Sham-operated controls were fed the ZA control diet. Bilateral adrenal gland weight was measured for each group and normalized to body weight. Bars indicate relative bilateral adrenal gland weight (mean ± SEM). *Significantly different from sham-operated rats at p<0.05.
3.3 Anhedonia
Prior to TBI or sham surgery, 4 weeks of dietary treatment resulted in no significant changes in the preference for saccharin in the two-bottle test. Similarly, in sham-operated controls, mean saccharin:water intake ratios were not different (ZA Sham vs. ZS Sham vs. ZD Sham). However, the preference test revealed that TBI significantly increased depression-like behavior in ZA animals (p<0.0001, Fig. 5). While the ZD diet did not result in a worsening of this behavior, 4 weeks of zinc supplementation prior to TBI completely abolished the appearance of anhedonia after injury (Fig. 5).
Figure 5.
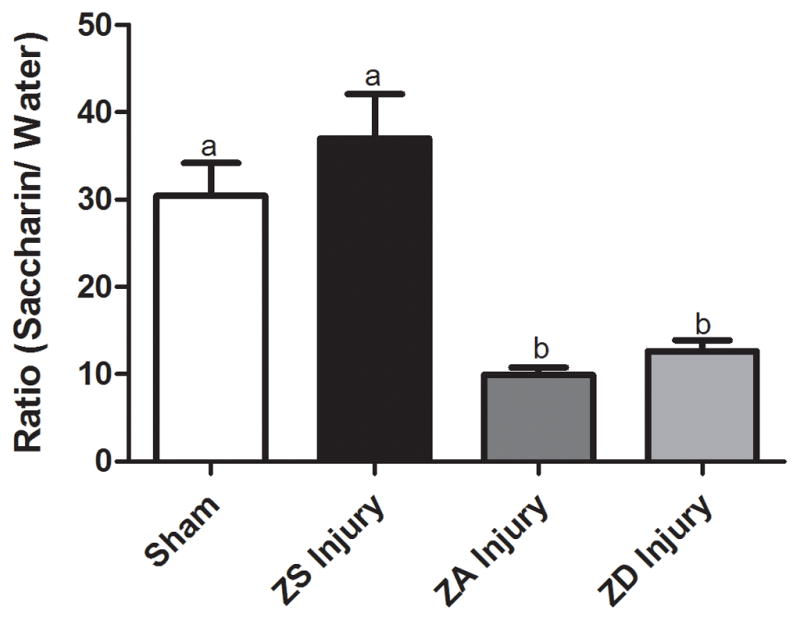
Zinc supplementation improves depression-like symptom anhedonia following traumatic brain injury. Rats were fed a zinc-supplemented (ZS), zinc adequate (ZA), or marginal zinc deficient (ZD) diet for 4 weeks and then subjected to either an injury or sham surgery (Sham) and intake ratios were measured. Bars represent intake ratios of saccharin to water over a 4-day period (mean ± SEM). Bars with different letters are significantly different at p<0.0001.
3.4 Morris water maze performance
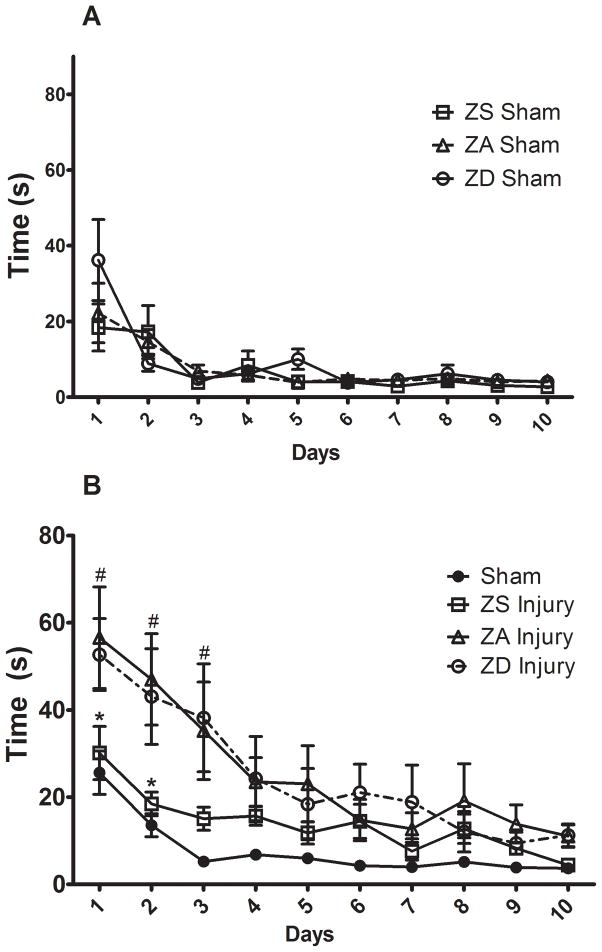
Morris water maze testing over a 10-day period revealed that the level of dietary zinc had no significant effect on the time it took sham-operated controls to find the hidden platform in the MWM task (Fig. 6A). However, there was a significant interaction between diet and injury (p<0.001, DF=27, F=2.22). Direct comparisons of ZA Sham and ZA injured rats revealed significant impairments in MWM performance on testing days 1 and 2 such that injured rats took significantly longer to find the hidden platform (p<0.05). Similarly, ZD injured rats showed a significant increase in the amount of time spent finding the hidden platform on days 2 and 3 compared to ZD sham-operated controls (p<0.05). However, when ZS Sham controls were compared to ZS injured rats, no significant differences were found between the groups during the 10 day MWM testing period.
Figure 6.
Effect of zinc supplementation on Morris Water Maze (MWM) performance following traumatic brain injury. Rats were fed a zinc supplemented (ZS), zinc adequate (ZA), or marginal zinc deficient (ZD) diet for 4 weeks and then subjected to either an injury or sham surgery (Sham). (A) Effect of zinc diets (ZS, ZA, or ZD) on MWM performance in sham-operated controls. (B) Effect of diet and injury on MWM performance. Individual points are time (sec) to hidden platform (mean ± SEM). #ZA and ZD injured rats are significantly different than sham-operated controls at p<0.001. *ZA injured rats are significantly different than ZS injured rats at p<0.01.
Because data from ZS, ZA, and ZD sham-operated controls were not different, figure 6B shows an analysis of MWM performance using pooled sham data. With only a single day of training (day 0), all sham-operated control rats were able to find the hidden platform. Traumatic brain injury impaired MWM performance (Fig. 6B). On the first day of testing, sham-operated controls were able to find the platform within 26±5 sec; whereas injured ZA rats took 57±12 sec to find the hidden platform (p<0.001). These differences were maintained through the first 3 days of MWM testing. While zinc deficiency did not significantly alter MWM performance, dietary zinc supplementation prior to injury improved resilience such that on days 1 and 2 of MWM testing ZS injured rats showed a significant reduction in the amount of time spent finding the hidden platform compared to both ZA and ZD injured rats (p<0.05). Moreover, there was no significant difference between ZS and sham-operated control rats in the amount of time to platform at any time point during the 10 d MWM testing period.
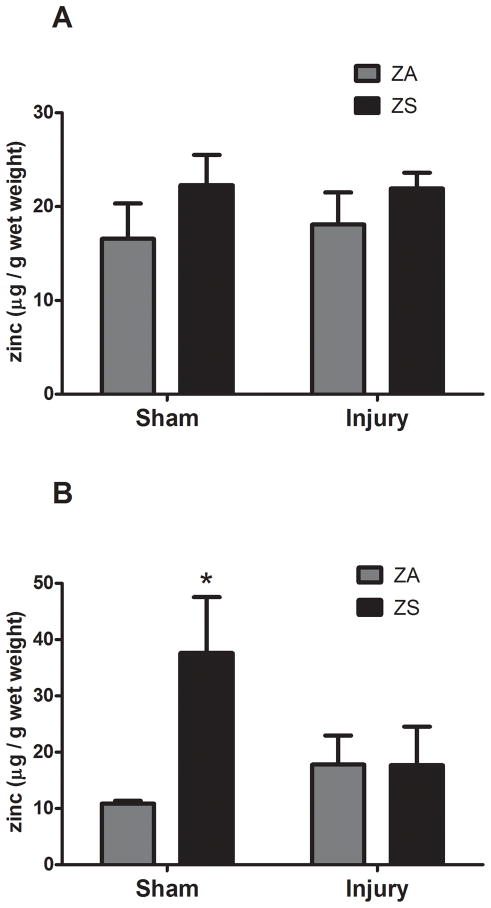
3.5 Brain Zinc
Given the behavioral changes associated with zinc supplementation, we analyzed hippocampal zinc concentrations as well as zinc levels found in the cortical region surrounding the site of injury in injured and sham-operated controls. Comparison of ZA Sham and ZA injured rats showed that there were no changes in zinc concentrations in the frontal cortex or hippocampus associated with injury (Fig. 7). However, dietary supplementation increased the zinc concentration in the hippocampus by approximately 30% (p<0.05, Fig. 7B). Curiously, this increase was eliminated after TBI in zinc supplemented rats (Fig. 7B).
Figure 7.
Effect of zinc supplementation on brain zinc concentration following traumatic brain injury. Rats were fed a zinc supplemented (ZS) or zinc adequate (ZA) diet for 4 weeks followed by TBI or sham surgery (Sham). (A) Effect of ZS on zinc levels at the site of cortical injury. (B) Effect of ZS on zinc levels in the hippocampus. Bars represent the mean ± SEM. *Significantly different than ZA sham-operated controls at p<0.05.
Discussion
There has been much debate about whether zinc is neuroprotective or neurotoxic following traumatic brain injury. Free zinc accumulation leading to neuronal death has been reported in animal models of TBI. The current work showed that zinc supplementation increased total hippocampal zinc, which appeared to be lost after injury. It is not clear if the post-injury reductions in zinc supplemented animals is the result of shifts in free zinc pools. Thus, future work will be needed to determine the significance of these findings and to examine the effect of zinc supplementation on free zinc in this region of the brain before and after injury. This work is needed because there is a significant amount of debate about the role of this form of zinc after injury. Both in vivo and in vitro models have shown that chelation of free zinc with CaEDTA resulted in reduced cell death in the dentate gyrus of the hippocampus following injury [18, 19]. However, this chelation treatment did not result in improvements in spatial memory deficits after brain injury generated by fluid percussion [20]. In contrast, others have reported zinc supplementation as beneficial following injury in both animals [13] and humans [14].
Thus, the available data suggest that behavioral outcomes are improved by zinc supplementation after TBI. These data led to the hypothesis that dietary zinc supplementation prior to injury could be used to improve behavioral resiliency to TBI. Specifically we tested the possible use of a zinc supplemented diet to improve measures of stress including anxiety-like behaviors and adrenal weight, as well as depression-like behavior, and spatial memory.
4.1 Anxiety
Within 10 days of starting a severely zinc deficient diet, rodents are known to develop anorexia that is characterized by a 4-day feeding cycle. These animals also develop anxiety–like behaviors [9, 10]. In the work reported here, marginal zinc deficiency was induced. As previously reported [13], this diet did not result in anorexia. However, it did result in the development of anxiety. Thus, these data show that marginal zinc deficiency, which is more common than severe deficiency in the general population, may contribute to the development of anxiety.
In the TBI model, significant increases in adrenal weight following injury, combined with evidence of increased anxiety-like behavior, suggest that zinc deficiency may increase stress-related anxiety. This is troubling given reports showing that at least 10% of the US population consumes less than half the Recommended Dietary Allowance (RDA) for zinc and may be at risk for moderate zinc deficiency, as reviewed by Cope and Levenson, 2010 [21]. This risk is also high in severe environmental conditions and high intensity exercise that may increase urinary zinc losses by 20–40% [22].
4.2 Depression
Numerous human studies have documented the association between low serum zinc and depression. Not only do patients with major depression have decreased serum zinc levels compared to non-depressed controls, more importantly, it appears the lower the serum zinc, the more severe the symptoms of depression [6–8]. Additionally, treatment-resistant patients appear to have even lower levels of zinc [7] and zinc supplementation has been shown to improve antidepressant drug efficacy [23].
Given the link not only between zinc deficiency and depression, but also the strong link between TBI and depression, we sought to determine the extent to which dietary zinc supplementation could prevent the development of depression-like symptoms after brain injury. The model we used to test this hypothesis produced the clear development of depression-like symptoms (significant anhedonia) that was prevented by zinc supplementation. These data suggest that in addition to the need to maintain adequate zinc after injury, there may be a protective role for zinc supplementation prior to injury in populations at risk for TBI.
4.3 Morris water maze performance
While zinc deficiency during pregnancy and lactation causes long-lasting cognitive dysfunction in offspring [24], our data confirm previous reports showing that zinc deficient diets do not significantly impair learning and memory in otherwise healthy adult rats [25]. We show that marginal dietary zinc deficiency does not exacerbate the cognitive impairment associated with TBI. Future work will be needed to determine if subjecting these animals to a more difficult task after injury will reveal an effect of zinc deficiency. What is clear, however, is that zinc supplementation prior to injury significantly improved MWM performance after TBI and enabled ZS animals to perform as well as uninjured, sham-operated controls. Because improved MWM performance has been correlated to improvements in spatial learning and memory, the mechanisms responsible for the robust effect reported here need to be elucidated.
5. Conclusions
The data presented here show for the first time that 4 weeks of dietary zinc supplementation significantly improves behavioral resiliency in a rat model of frontal cortex injury. Not only were there indicators of reduced anxiety (adrenal weight changes), there were clear reductions in depression-like behaviors and improvements in cognitive outcomes. Future work will be needed not only to determine the extent to which zinc can be used in humans populations at risk for TBI, but also to determine the optimal amounts of dietary zinc supplementation needed to improve resiliency. Furthermore, because this work used zinc supplementation before and after the injury, studies will be need to determine the extent to which zinc supplementation can be used after injury (zinc treatment) to improve behavioral outcomes.
Research Highlights.
Marginal deficiency of dietary zinc as well as cortical injury induce anxiety-like behaviors.
Zinc supplementation prior to injury reduces markers of stress that accompany TBI.
Dietary zinc supplementation prior to TBI prevents learning deficits associated with cortical injury.
Injury-induced depression-like behaviors are prevented by zinc supplementation prior to injury.
Acknowledgments
The authors would like to thank Dr. Vincent Salters at the National High Magnetic Field Laboratory (Tallahassee, FL) for his assistance with the brain zinc measurements, Shannon Gower-Winter, MS for excellent technical and editorial assistance, and the U.S. Army Medical Research and Material Command who funded this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Terrio H, Brenner LA, Ivins BJ, Cho JM, Helmick K, Schwab K, et al. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil. 2009;24:14–23. doi: 10.1097/HTR.0b013e31819581d8. [DOI] [PubMed] [Google Scholar]
- 2.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil. 1999;14:602–15. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Jorge RE, Starkstein SE. Pathophysiologic aspects of major depression following traumatic brain injury. J Head Trauma Rehabil. 2005;20:475–87. doi: 10.1097/00001199-200511000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Levin HS, McCauley SR, Josic CP, Boake C, Brown SA, Goodman HS, et al. Predicting depression following mild traumatic brain injury. Arch Gen Psychiatry. 2005;62:523–8. doi: 10.1001/archpsyc.62.5.523. [DOI] [PubMed] [Google Scholar]
- 5.Fann JR, Hart T, Schomer KG. Treatment for depression after traumatic brain injury: a systematic review. J Neurotrauma. 2009;26:2383–402. doi: 10.1089/neu.2009.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maes M, D’Haese PC, Scharpé S, D’Hondt P, Cosyns P, De Broe ME. Hypozincemia in depression. J Affect Disord. 1994;31:135–40. doi: 10.1016/0165-0327(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 7.Maes M, Vandoolaeghe E, Neels H, Demedts P, Wauters A, Melzer HY, et al. Lower serum zinc in major depression is a sensitive marker of treatment resistance and of the immune/inflammatory response in that illness. Biol Psychiatry. 1997;42:349–58. doi: 10.1016/S0006-3223(96)00365-4. [DOI] [PubMed] [Google Scholar]
- 8.Maes M, De Vos N, Demedts P, Wauters A, Neels H. Lower serum zinc in major depression in relation to changes in serum acute phase proteins. J Affect Disord. 1999;56:189–94. doi: 10.1016/s0165-0327(99)00011-7. [DOI] [PubMed] [Google Scholar]
- 9.Takeda A, Tamano H, Kan F, Itoh H, Oku N. Anxiety-like behavior of young rats after 2-week zinc deprivation. Behav Brain Res. 2007;177:1–6. doi: 10.1016/j.bbr.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Tassabehji NM, Corniola RS, Alshingiti A, Levenson CW. Zinc deficiency induces depression-like symptoms in adult rats. Physiol Behav. 2008;95(3):365–9. doi: 10.1016/j.physbeh.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 11.McClain CJ, Twyman DL, Ott LG, Rapp RP, Tibbs PA, Norton JA, et al. Serum and urine zinc response in head-injured patients. J Neurosurg. 1986;64:224–30. doi: 10.3171/jns.1986.64.2.0224. [DOI] [PubMed] [Google Scholar]
- 12.Penkowa M, Giralt M, Thomsen PS, Carrasco J, Hidalgo J. Zinc or copper deficiency-induced impaired inflammatory response to brain trauma may be caused by the concomitant metallothionein changes. J Neurotrauma. 2001;18:447–63. doi: 10.1089/089771501750171056. [DOI] [PubMed] [Google Scholar]
- 13.Yeiser EC, VanLandingham JW, Levenson CW. Moderate zinc deficiency increases cell death after brain injury in the rat. Nutr Neurosci. 2002;5:345–52. doi: 10.1080/1028415021000033811. [DOI] [PubMed] [Google Scholar]
- 14.Young B, Ott L, Kasarskis E, Rapp R, Moles K, Dempsey RJ, et al. Zinc supplementation is associated with improved neurologic recovery rate and visceral protein levels of patients with severe closed head injury. J Neurotrauma. 1996;13:25–34. doi: 10.1089/neu.1996.13.25. [DOI] [PubMed] [Google Scholar]
- 15.Cekic M, Cutler SM, VanLandingham JW, Stein DG. Vitamin D deficiency reduces the benefits of progesterone treatment after brain injury in aged rats. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rich NJ, VanLandingham JW, Figueiroa S, Seth R, Corniola RS, Levenson CW. Chronic caloric restriction reduces tissue damage and improves spatial memory in a rat model of traumatic brain injury. J Neurosci Res. 2010;88:2933–9. doi: 10.1002/jnr.22443. [DOI] [PubMed] [Google Scholar]
- 17.Salters VJM, Sachi-Kocher An ancient metasomatic source for the Walvis Ridge basalts. Chemical Geology. 2010;273:151–67. [Google Scholar]
- 18.Suh SW, Chen JW, Motamedi M, Bell B, Listiak K, Pons NF, et al. Evidence that synaptically-released zinc contributes to neuronal injury after traumatic brain injury. Brain Res. 2000;852:268–73. doi: 10.1016/s0006-8993(99)02095-8. [DOI] [PubMed] [Google Scholar]
- 19.Hellmich HL, Frederickson CJ, DeWitt DS, Saban R, Parsley MO, Stephenson R, et al. Protective effects of zinc chelation in traumatic brain injury correlate with upregulation of neuroprotective genes in rat brain. Neurosci Lett. 2004;355:221–5. doi: 10.1016/j.neulet.2003.10.074. [DOI] [PubMed] [Google Scholar]
- 20.Hellmich HL, Eidson K, Cowart J, Crookshanks J, Boone DK, Shah S, et al. Chelation of neurotoxic zinc levels does not improve neurobehavioral outcome after traumatic brain injury. Neurosci Lett. 2008;440:155–9. doi: 10.1016/j.neulet.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cope EC, Levenson CW. Role of zinc in the development and treatment of mood disorders. Curr Opin Clin Nutr Metab Care. 2010;13:685–9. doi: 10.1097/MCO.0b013e32833df61a. [DOI] [PubMed] [Google Scholar]
- 22.IOM. Mineral requirements for military personnel: Levels needed for cognitive and physical performance during garrison training. Washington, DC: The National Academies Press; 2006. [Google Scholar]
- 23.Siwek M, Dudek D, Paul IA, Sowa-Kućma, Zieba A, Popik P, et al. Zinc supplementation augments efficacy of imipramine in treatment resistant patients: a double blind, placebo-controlled study. J Affect Disord. 2009;118:187–95. doi: 10.1016/j.jad.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Tahmasebi Boroujeni S, Naghdi N, Shahbazi M, Farrokhi A, Bagherzadeh F, Kazemnejad A, et al. The effect of severe zinc deficiency and zinc supplement on spatial learning and memory. Biol Trace Elem Res. 2009;130:48–61. doi: 10.1007/s12011-008-8312-7. [DOI] [PubMed] [Google Scholar]
- 25.Chu Y, Mouat MF, Harris RB, Coffield JA, Grider A. Water maze performance and changes in serum corticosterone levels in zinc-deprived and pair-fed rats. Physiol Behav. 2003;78:569–78. doi: 10.1016/s0031-9384(03)00041-6. [DOI] [PubMed] [Google Scholar]