Abstract
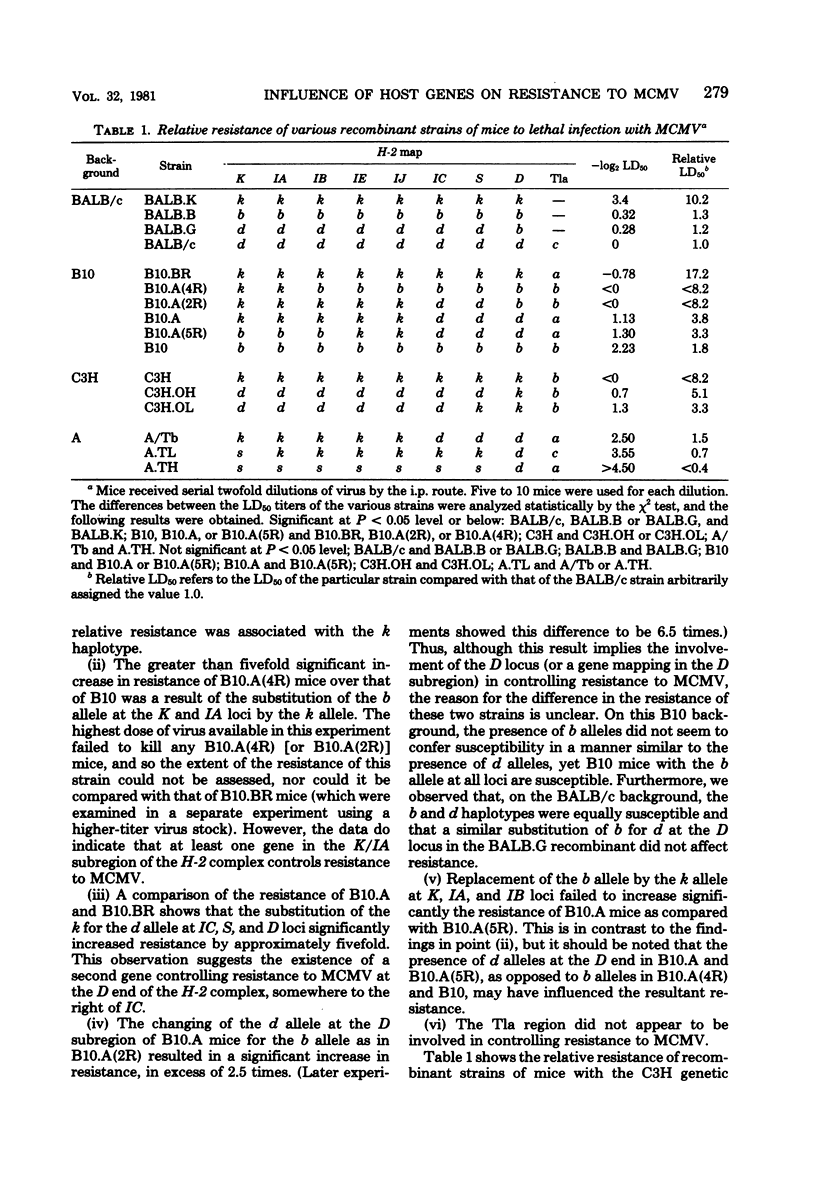
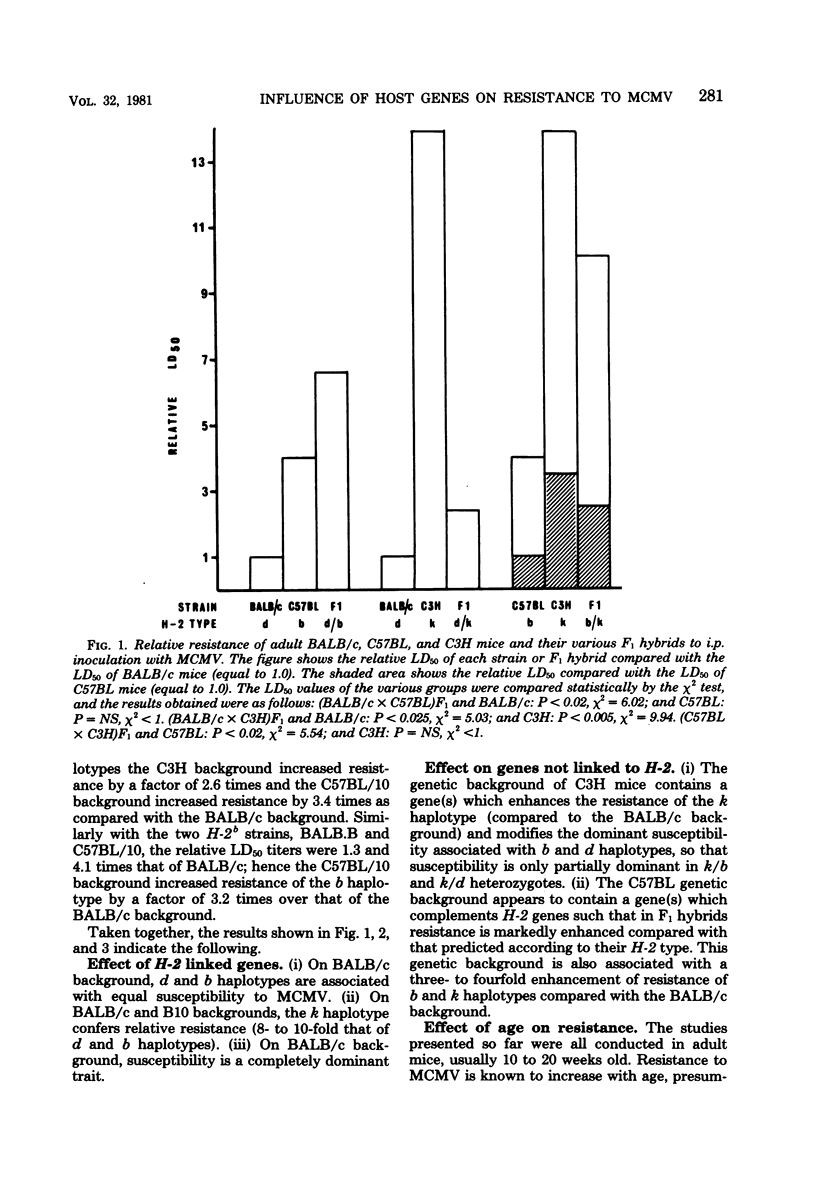
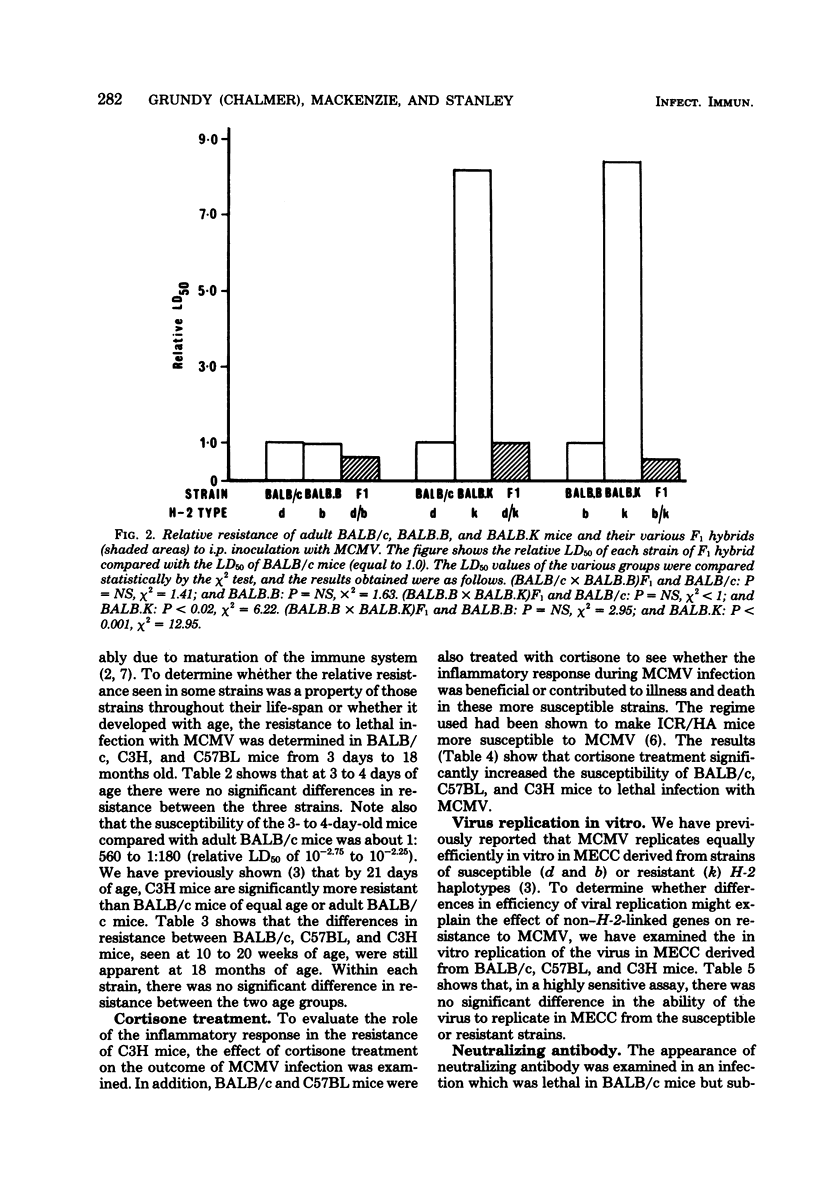
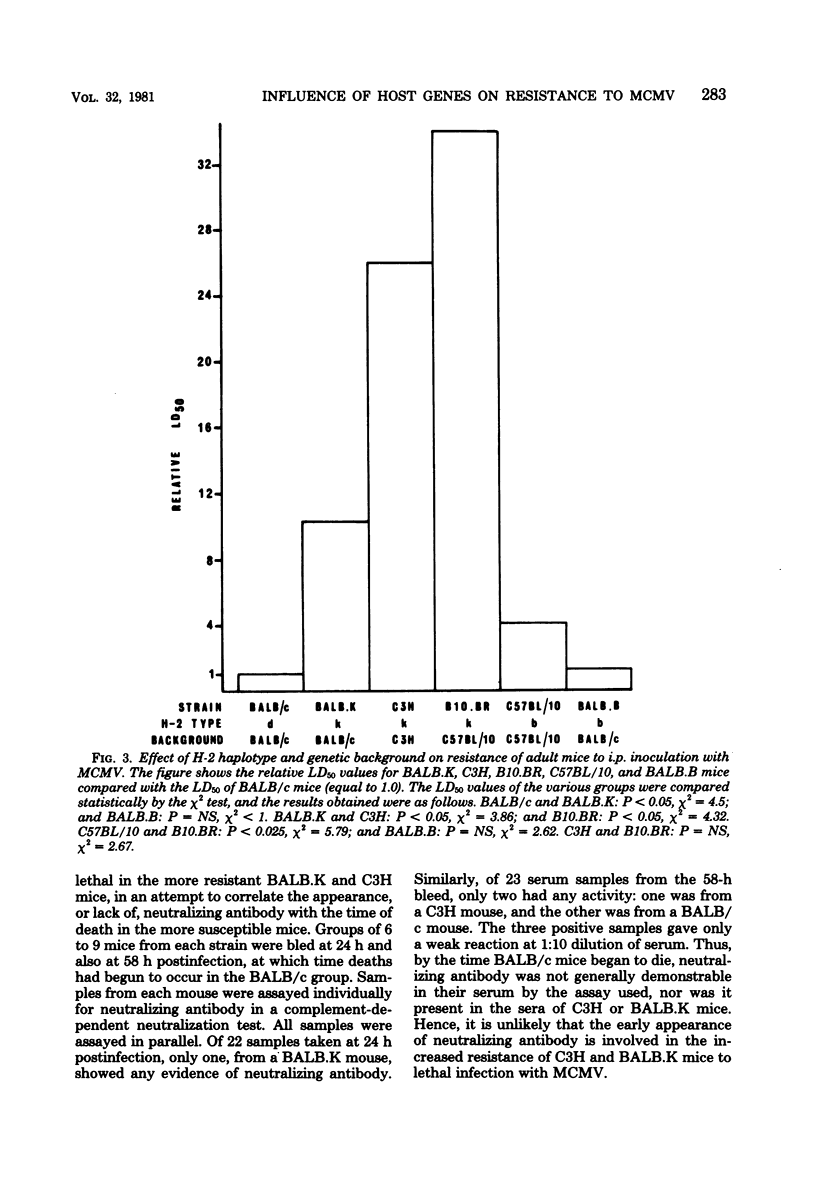
The resistance of adult mice to acute lethal infection with murine cytomegalovirus is controlled by genes linked to the H-2 complex. The k haplotype is approximately 10 times more resistant than the b or d haplotypes. Susceptibility is inherited as a completely dominant trait. At least two genes within the H-2 complex are involved, one mapping to the K/IA subregion and the other to the D subregion. The data suggest that interactions may occur between these K- and D-end genes which further affect resistance to the virus. The precise mechanism of H-2 gene control of resistance to murine cytomegalovirus remains to be elucidated. Non-H-2 linked genes also affect resistance to the virus, particularly in the C57BL genetic background, which is associated with an increased resistance to murine cytomegalovirus. Newborn mice of all strains are equally susceptible: both the H-2- and the non-H-2-associated resistances develop in the first few weeks of life and are retained up to at least 18 months of age.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft G. J., Shellam G. R., Chalmer J. E. Genetic influences on the augmentation of natural killer (NK) cells during murine cytomegalovirus infection: correlation with patterns of resistance. J Immunol. 1981 Mar;126(3):988–994. [PubMed] [Google Scholar]
- Chalmer J. E., Mackenzie J. S., Stanley N. F. Resistance to murine cytomegalovirus linked to the major histocompatibility complex of the mouse. J Gen Virol. 1977 Oct;37(1):107–114. doi: 10.1099/0022-1317-37-1-107. [DOI] [PubMed] [Google Scholar]
- Henson D., Neapolitan C. Pathogenesis of chronic mouse cytomegalovirus infection in submaxillary glands of C3H mice. Am J Pathol. 1970 Feb;58(2):255–267. [PMC free article] [PubMed] [Google Scholar]
- Lopez C. Genetics of natural resistance to herpesvirus infections in mice. Nature. 1975 Nov 13;258(5531):152–153. doi: 10.1038/258152a0. [DOI] [PubMed] [Google Scholar]
- MANNINI A., MEDEARIS D. N., Jr Mouse salivary gland virus infections. Am J Hyg. 1961 May;73:329–343. doi: 10.1093/oxfordjournals.aje.a120192. [DOI] [PubMed] [Google Scholar]
- Nedrud J. G., Collier A. M., Pagano J. S. Cellular basis for susceptibility to mouse cytomegalovirus: evidence from tracheal organ culture. J Gen Virol. 1979 Dec;45(3):737–744. doi: 10.1099/0022-1317-45-3-737. [DOI] [PubMed] [Google Scholar]
- Neustadt P. M., Cody T. S., Monjan A. A. Failure to find H-2-associated susceptibility to LCM disease. J Immunogenet. 1978 Dec;5(6):397–400. doi: 10.1111/j.1744-313x.1978.tb00668.x. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J., Mitchell G. F., McDevitt H. O. Histocompatibility-linked genetic control of disease susceptibility. Murine lymphocytic choriomeningitis virus infection. J Exp Med. 1973 May 1;137(5):1201–1212. doi: 10.1084/jem.137.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHELL K. Studies on the innate resistance of mice to infection with mousepox. II. Route of inoculation and resistance; and some observations on the inheritance of resistance. Aust J Exp Biol Med Sci. 1960 Aug;38:289–299. doi: 10.1038/icb.1960.30. [DOI] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E., Jameson P. The neuraminidase yield-reduction bioassay of human other interferons. Proc Soc Exp Biol Med. 1975 Jun;149(2):433–438. doi: 10.3181/00379727-149-38822. [DOI] [PubMed] [Google Scholar]
- Selgrade M. K., Osborn J. E. Role of macrophages in resistance to murine cytomegalovirus. Infect Immun. 1974 Dec;10(6):1383–1390. doi: 10.1128/iai.10.6.1383-1390.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan J. W., Finlay-Jones J. J. Studies on a fractionated murine fibrosarcoma: a reproducible method for the cautious and a caution for the unwary. J Cell Physiol. 1977 Mar;90(3):535–552. doi: 10.1002/jcp.1040900316. [DOI] [PubMed] [Google Scholar]



