Abstract
The heme-containing peroxidase family comprises eight members in humans. The physiological and pathophysiological roles of heme-containing peroxidases are not well understood. Phagocyte-derived myeloperoxidase (MPO) utilizes chloride and bromide, in the presence of hydrogen peroxide (H2O2), to generate hypochlorous acid and hypobromous acid, potent oxidizing species that are known to kill invading pathogens. Vascular peroxidase 1 (VPO1) is a new member of the heme-containing peroxidase family; VPO1 is highly expressed in the cardiovascular system, lung, liver, pancreas, and spleen. However, functional roles of VPO1 have not been defined. In this report, we demonstrate the capacity for VPO1 to catalyze the formation of hypohalous acids, and characterize its enzymatic properties. VPO1, like MPO but unlike lactoperoxidase, is able to generate hypochlorous acid, hypobromous acid, and hypothiocyanous acid in the presence of H2O2. Under physiological pH and concentrations of halides (100 µM KBr, 100 µM KSCN, and 100 mM NaCl), VPO1 utilizes approximately 45% of H2O2 for the generation of hypobromous acid, 35% for hypothiocyanous acid, and 18% for hypochlorous acid. The specific activity of VPO1 is ~10- to 70-fold lower than that of MPO, depending on the specific substrate. These studies demonstrate that the enzymatic properties and substrate specificity of VPO1 are similar to MPO; however, significantly lower catalytic rate constants of VPO1 relative to MPO suggest the possibility of other physiologic roles for this novel heme-containing peroxidase.
Keywords: Heme-containing peroxidase, Vascular peroxidase 1, Hypohalous acid, Hypothiocyanous acid, Kinetics
Introduction
Heme-containing peroxidases (hPxes) participate in host defense, biosynthesis of thyroid hormone, and in pathological conditions by the generation of hypohalous acids which may induce cell/tissue injury. It is widely accepted that generation of hypohalous acids by hPxes is via a two-electron oxidation of halides and thiocyanate (wherein the latter is considered a “pseudohalide”). hPxes first react with hydrogen peroxide (H2O2) to form compound I; compound I then reacts with halides or thiocyanate to generate hypohalous or hypothiocyanous acids [1]. Hypohalous or hypothiocyanous acids have the capacity to oxidize proteins, lipids, and DNA, resulting in damage to cells and tissues. Generation of hypohalous acids is the primary biochemical basis for both the physiological and the pathological roles of hPxes.
Members of the hPx family have distinct cell/tissue distributions [2]. Myeloperoxidase (MPO), the proto-enzyme of this family, has been extensively studied and is proposed to play an important role in innate immunity and inflammatory responses. MPO expression is restricted to neutrophils and monocytes; MPO oxidizes chloride, bromide, iodide, and thiocyanate to generate hypohalous and hypothiocyanous acids. The products generated are largely dependent on the concentration of halides at sites of enzymatic activity. Generally, chloride is considered the major physiological substrate of MPO, as it is present at relatively high concentrations in plasma and the phagosome of neutrophils (100–140 mM) [1]. It has been reported that, at 100 mM chloride and 100 µM thiocyanate, MPO utilizes ~60% of H2O2 to oxidize chloride to hypochlorous acid (HOCl) and ~40% of H2O2 to hypothiocyanous acid (HOSCN) [3]. Eosinophil peroxidase (EPO) is expressed in eosinophils. EPO oxidizes bromide, iodide, and thiocyanate. The major physiological substrates of EPO are bromide (20–120 µM in plasma) [4] and thiocyanate (19–203 µM in blood) [5]. However, at acidic pH (≤ 6.5), EPO may also oxidize chloride to HOCl [4]. Lactoperoxidase (LPO) is secreted into some body fluids, including milk, saliva, and airway secretions. LPO can oxidize bromide, iodide, and thiocyanate, but not chloride. Its physiological role is to prevent bacterial growth at these sites. Thiocyanate is the major substrate of LPO in cow’s milk (87.5 ± 52.5 µM) [6] and saliva (542 ± 406 µM) [5]. Thyroid peroxidase (TPO) is produced in the thyroid; it oxidizes iodide and thiocyanate, but not chloride or bromide [7]. The physiological role of TPO is to catalyze the iodination of tyrosine residues in thyroglobulin and to synthesize thyroid hormone [8]. There is no evidence demonstrating the capacity of hPxes to oxidize fluoride in the presence of H2O2. A common physiological role for hPxes (with the exception of TPO) is in host defense [1].
Vascular peroxidase 1 (VPO1) was identified by our group as a novel member of the hPx family; it contains a catalytic domain at its C-terminus and a large N-terminal domain that includes five leucine-rich repeats (LRRs) and four immunoglobulin-like domains [9]. Unlike other members of the family, VPO1 is highly expressed in the cardiovascular system, lung, pancreas, and spleen [9]. VPO1 is secreted into plasma at high concentrations, approximately three orders of magnitude higher than that of MPO [10]. VPO1 is induced by lipopolysaccharides and TNF-α [10], and mediates proliferation of vascular smooth muscle cells [11]. We previously reported that VPO1, in the presence of H2O2, catalyzes the oxidation of chloride to generate HOCl under physiological conditions [12]. This observation changed the prevailing view that the sole enzymatic source of HOCl generation is MPO. However, it is unclear whether VPO1 catalyzes the oxidation of other halides and “pseudohalides,” given plasma concentrations of chloride (100–140 mM), bromide (20–120 µM), and thiocyanate (19–203 µM) in the presence of H2O2 (1–7 µM) [13,14]. In present study, we demonstrate that VPO1, like MPO, catalyzes the oxidation of chloride, bromide, and thiocyanate. In a phosphate buffer (pH 7.4) containing chloride (100 mM), bromide (100 µM), and thiocyanate (100 µM) in the presence of H2O2 (10 µM), VPO1 utilizes ~18, ~45, and ~37% of H2O2 to oxidize chloride, bromide, and thiocyanate, respectively. Thus, bromide, thiocyanate, and chloride are all the physiological substrates of VPO1. In addition, the specific activity of VPO1 is ~10- to 70-fold lower than that of MPO, depending on substrate. The significantly lower catalytic rate constants of VPO1 (relative to MPO) suggest the possibility of alternative physiologic roles of this novel hPx. We propose that VPO1 plays a role in H2O2 homeostasis and in maintaining plasma sterility. The enzymatic properties of VPO1 reported here may be useful for distinguishing this novel hPx from other members of the family.
Materials and methods
Materials
All reagents were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise noted. Recombinant VPO1 (rVPO1) was used for this study; it was produced from cells stably expressing VPO1 and purified as described previously [10]. MPO was purchased from Elastin Product Company (Cat. No. MY167, Owensville, MO). H2O2 was purchased from Sigma-Aldrich as 30% solution and its molar concentration was determined by measuring its absorbance at 240 nm (ε240 = 43.6 M−1 cm−1). 5-Thio-2-nitrobenzoic acid (TNB) was prepared from 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) as described in [12]. Preparation of reagent HSCN was as previously described [15]. Reagent HOCl was synthesized and its concentration determined, as previously described [16]. Reagent HOBr was prepared by mixing NaOCl with KBr, as previously described [17]. Chlorotaurine (Cl-tau) was prepared as previously reported [18]. The concentration of Cl-tau was determined by absorbance at 252 nm (ε252 = 415 M−1 cm−1). Bromotaurine (Br-tau) was prepared by mixing reagent taurine and HOBr, as previously described [19].
Measurement of heme concentration
All peroxidase concentrations are expressed as nM per heme. The concentrations of MPO, bLPO, and rVPO1 were determined by using the extinction coefficients 91, 114 and 112 mM−1 cm−1 at 428, 412, and 410 nm, respectively [12].
Taurine-TMB oxidation assay
HOBr and HOCl were detected by utilizing taurine in combination with subsequent 3,3′,5,5′-tetramethylbenzidine (TMB) oxidation in the presence of iodide, as previously reported [12,16] or in the absence of iodide [19] with slight modifications. Cl-tau is unable to directly oxidize TMB. Herein, iodide catalyzes TMB oxidation by Cl-tau. The reactions were proposed previously [16]. In brief, H2O2 was added to 100 µl of 20 mM phosphate buffer (pH 7.4) containing140 mM NaCl or 100 µM KBr, 5 mM taurine, and 100 nM rVPO1 to 50 µM to start the reaction. The reaction was incubated at 37 °C for 30 min and stopped by adding catalase (25 µg/ml). This reaction mixture was then mixed with freshly made developing agent. The developing agent consisted of 400 mM acetate buffer, pH 5.4, 1 mM TMB (predissolved in 100% dimethylformamide), and 100 µM sodium iodide. After 5 min, absorbance at 650 mm was recorded. In some experiments, peroxidase inhibitors [4-aminobenzoic acid hydrazide (ABAH) and NaN3], H2O2 scavenger (catalase), or HOCl scavenger (methionine) was added. In some experiments, TMB was directly oxidized by indicated amounts of Cl-tau and/or Br-tau. In pH-dependent or dose-dependent experiments, the pH values of buffer, substrate concentrations, or peroxidase concentrations were indicated.
TNB oxidation assay for detection of VPO1-mediated generation of HOSCN
HOSCN generation was detected by utilizing TNB oxidation assay, as previously reported [16], with slight modifications. TNB is yellow, whereas the oxidized product, DTNB, is colorless. By monitoring absorbance at 412 nm, the activity of hPx is measured. In the presence of hPx, the decrease of absorbance at 412 nm is proportionally (linearly) enhanced. In brief, 100 µl of 20 mM phosphate buffer, pH 5.5, contains 500 nM rVPO1, 100 µM KSCN, and 100 µM TNB. The reaction was initiated by adding H2O2 (100 µM, final concentration) to the reaction mixture. Absorbance at 412 nm was recorded at 37 °C every 30 s for 30 min by a VersaMax microplate reader (Molecular Devices, Sunnyvale, CA). In pH-dependent or dose-dependent experiments, the pH values of buffer, substrate concentrations, or peroxidase concentrations were varied as indicated.
VPO1-mediated H2O2 decomposition in the presence of halides
Reactions were carried out at 22 °C in 20 mM potassium phosphate, pH 7.4, containing 10 µM H2O2, 100 mM NaCl, 100 µM KBr or 100 µM KSCN, and 100 nM rVPO1 or 10 nM bLPO. The reactions were started by adding hPx. H2O2 concentrations were continuously monitored by using an Apollo 4000 Free Radical Analyzer (World Precision Instruments, Inc., Sarasota, FL). The H2O2 electrode was calibrated by known amounts of H2O2. The current measured by the Free Radical Analyzer was following the amount of H2O2 added. It was linearly proportional to the amount of H2O2 in the solution. To observe the effects of substrate concentration, substrate concentrations were varied as indicated, and initial rates of H2O2 loss were determined over the first 10 s of the reaction.
Results
VPO1 mediates HOBr generation
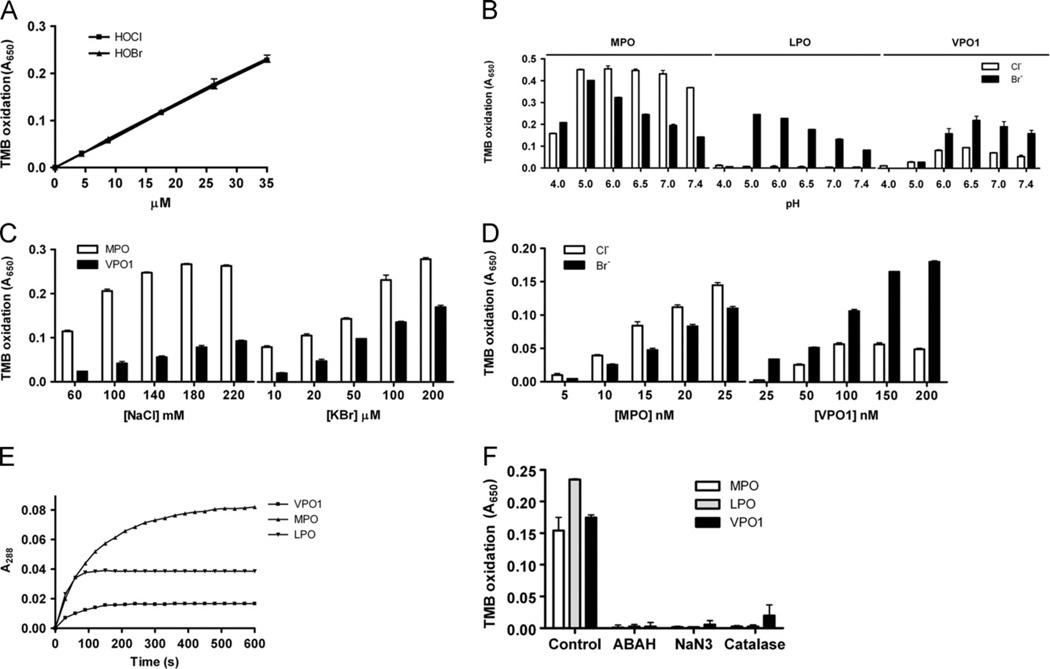
Our previous report demonstrated that VPO1 is able to catalyze chloride oxidation to generate HOCl under physiological conditions [12]. The redox potential of bromide oxidation (2Br− ↔ Br2 + 2e, 1.07) is less than that of chloride (2Cl− ↔ Cl2 + 2e, 1.36) [2,7,20]; therefore, we hypothesized that VPO1 catalyzes bromide oxidation to generate HOBr. HOBr generation by VPO1 was measured by an assay that combines bromination of taurine and TMB oxidation in the presence of iodide ion (I−) [16]. The original assay was used to detect HOCl generation by the MPO/H2O2/Cl− system, and was recently demonstrated to be suitable for measurement of HOBr [19]. We first confirmed the suitability of this assay to detect HOCl and HOBr in the presence of I−. We found that bromination of taurine and subsequent oxidation of TMB correlate in a dose-dependent manner with HOBr and HOCl concentrations (Fig. 1A). Both HOCl and HOBr have similar oxidation rates for taurine (Fig. 1A). We then measured HOBr generation by the VPO1/H2O2/Br− system. The dependency of HOBr generation on pH was evaluated. At pH 6.5, VPO1 generated the largest amount of HOBr (Fig. 1B). At physiological pH, HOBr generation by VPO1 was ~75% of that at pH 6.5. HOBr generation by VPO1 was dependent on bromide concentration (Fig. 1C), similar to that by MPO. In addition, HOBr generation was dependent on VPO1 concentration (Fig. 1D). The amount of VPO1-mediated HOBr generation is approximately one-tenth that of MPO-mediated HOBr generation. VPO1-mediated bromination of taurine is time-dependent, similar to MPO and LPO (Fig. 1E). Taurine bromination by VPO1, as well as by MPO or bLPO, was blocked by a variety of peroxidase inhibitors (ABAH and NaN3), and H2O2 scavenger (catalase) (Fig. 1F). These data indicate that VPO1 is capable of catalyzing the production of HOBr from H2O2 and bromide.
Fig. 1.
HOBr generation by VPO1. (A) Verification of detection of HOBr by taurine-TMB oxidation assay. One hundred microliters of reaction containing 20 mM phosphate buffer, pH 7.4, 5 mM taurine, and HOCl or HOBr as indicated was carried out at 37 °C for 30 min. The reaction was stopped by adding catalase (25 µg/ml). The stopped reaction was then mixed with developing agent. After 5 min, absorbance at 650 nm was recorded. (B) pH-dependent HOBr generation by VPO1. One hundred microliters of reaction containing 20 mM phosphate buffer, pH as indicated, 100 µM KBr, 50 µM H2O2, 5 mM taurine, and 100 nM rVPO1, 20 nM MPO, or 100 nM bLPO was carried out at 37 °C for 30 min. The reaction was stopped by adding catalase (25 µg/ml). The stopped reaction was then mixed with developing agent. After 5 min, absorbance at 650 mm was recorded. (C) Dose-dependent HOBr generation by VPO1, compared with that by MPO. The experiments were carried out as in B at pH 7.4 containing 100 nM rVPO1 or 20 nM MPO, Concentrations of KBr and NaCl are indicated. (D) VPO1-dependent HOBr generation. The experiments were carried out as in B at pH 7.4, 100 µM Br−. The concentrations of rVPO1 and MPO are indicated. (E) Time course of rVPO1, MPO, and bLPO-mediated Br-tau formation. The experiments were carried out as in B at pH 7.4 with 100 µM Br−. Reactions started by adding hPx. Absorbance at 288 nm was continuously monitored. (F) Inhibition of HOBr generation. The experiments were carried out as in C with 100 µM Br−. In the reaction mixture, ABAH (200 µM), NaN3 (1 mM), and catalase (25 µg/ml) were added, respectively. The data are from three independent experiments. Error bars, SD.
VPO1 mediates generation of hypohalous acids under physiological concentrations of chloride and bromide
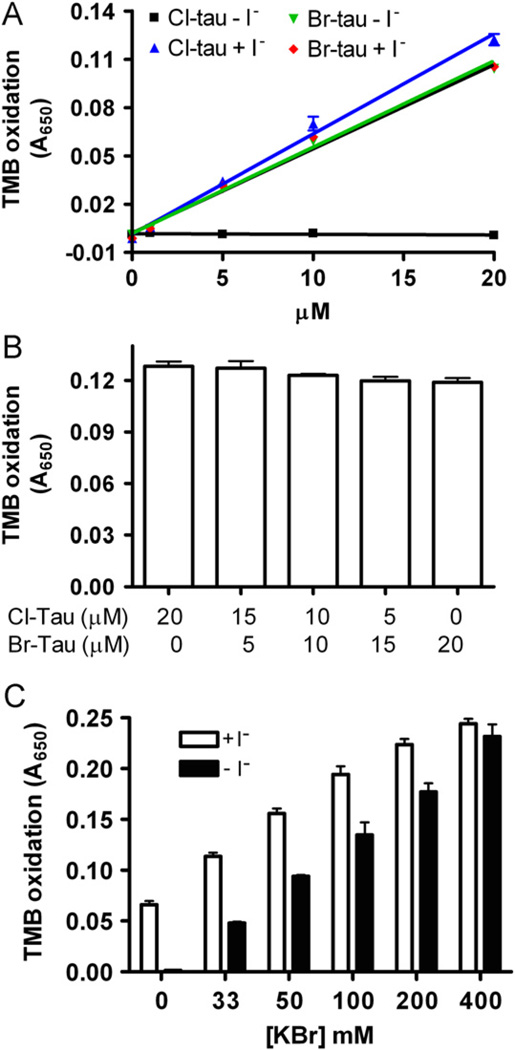
VPO1 is secreted into human plasma at a high concentration of ~1.1 µM [10]. Circulating plasma also contains 1–7 µM H2O2 [13,14], 100–140 mM chloride, and 20–120 µM bromide. Therefore, we investigated the potential generation of HOCl and HOBr in plasma by VPO1. To distinguish the bromination from chlorination, we carried out the assay reported by Senthilmohan et al. [19], with slight modifications. In this assay, taurine is oxidized by HOBr and/or HOCl to produce Br-tau and Cl-tau, respectively. Br-tau oxidizes TMB in the presence or absence of iodide, while Cl-tau only oxidizes TMB in the presence of iodide (Fig. 2A). Cl-tau mediated TMB oxidation revealed rates similar to that of Br-tau (Fig. 2A), consistent with the observations using reagent HOCl or HOBr (Fig. 1A). These data were further verified by detection of TMB oxidation by a series of mixtures of Cl-tau and Br-tau (the total concentration of oxidants was maintained at 20 µM) in the presence of iodide (Fig. 2B). We then evaluated VPO1-mediated bromination and chlorination of taurine using this strategy. In the presence of 140 mM NaCl and varying concentrations of bromide (from 0 to 400 µM), VPO1-mediated Cl-tau decreased with increasing bromide concentrations. At 140 mM chloride and 100 µM bromide, VPO1-mediated generation of HOCl was approximately 30% that of HOBr (Fig. 2C). We assumed that, under these conditions, ~30 and ~70% of H2O2 were used for the generation of HOCl and HOBr, respectively.
Fig. 2.
VPO1-mediated HOBr and HOCl generation at physiological concentrations of chloride and bromide. (A) Oxidation of TMB by Cl-tau and Br-tau in the absence or presence of I−. The experiments were carried out by adding Cl-tau and Br-tau (as indicated) into TMB solution ± I− (5 mM). After 5 min, absorbance at 650 nm was recorded. (B) Oxidation of TMB by mixture of Cl-tau and Br-tau in the presence of I−. The concentrations of Cl-tau and/or Br-tau were sustained at 20 µM. (C) Oxidation of TMB by VPO1-mediated HOCl and HOBr at physiological concentrations. One hundred microliters of reaction containing 20 mM phosphate buffer, pH 7.4, 50 µM H2O2, 5 mM taurine, and 100 nM rVPO1, 140 mM NaCl, and 0–400 µM KBr was carried out at 37 °C for 30 min. The reaction was stopped by adding catalase (25 µg/ml). The stopped reaction was then mixed with developing agent in the presence or absence of I−. After 5 min, absorbance at 650 mm was recorded. The data are from three independent experiments. Error bars, SD.
VPO1 mediates HOSCN generation
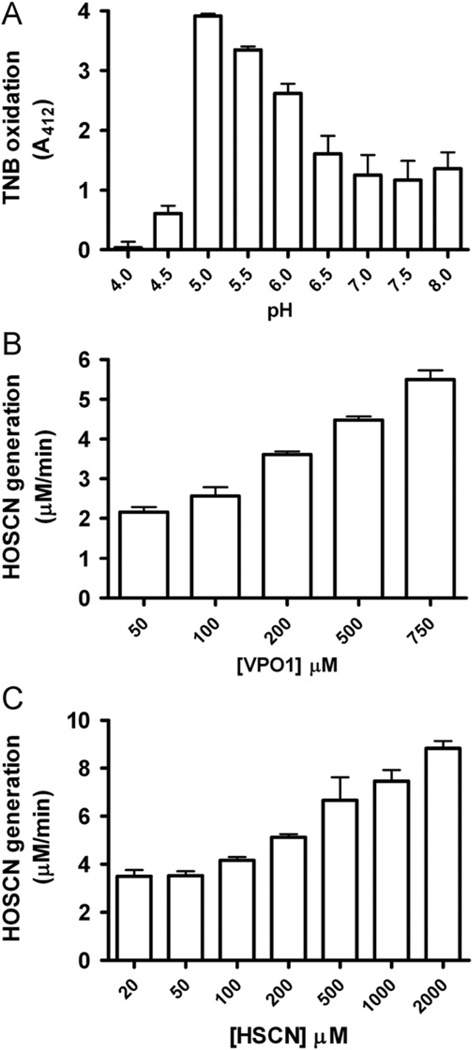
Human plasma also contains 19–203 µM thiocyanate [5]. It has been reported that the oxidation of thiocyanate and the generation of HOSCN may be mediated by MPO and EPO in the presence of H2O2 [3,21]. During inflammatory responses, increased HOSCN generation may induce damage to cells/tissues (e.g., oxidation of thiol groups on proteins), and activate transcriptional factors that regulate proinflammatory pathways [22]. Since VPO1 concentration is ~1000-fold higher than that of MPO in plasma, we further determined if VPO1 is able to catalyze thiocyanate oxidation and characterized its enzymatic features. The ability of VPO1 to mediate HOSCN generation was evaluated by the TNB oxidation assay. We carried out the assay at pH ranging from 4.0 to 8.0. TMB oxidation by VPO1 was highest at pH 5.0 (Fig. 3A). The rate of TMB oxidation by VPO1-mediated HOSCN was dependent on SCN− concentration (Fig. 3B), as well as on VPO1 concentrations (Fig. 3C). These data strongly support the ability of VPO1 to catalyze HOSCN generation.
Fig. 3.
VPO1-mediated HOSCN generation. (A) pH-dependent HOSCN generation by VPO1. One hundred microliters of reaction containing 20 mM phosphate buffer, pH range from 4 to 8 as indicated, 500 nM rVPO1, 100 µM H2O2, 100 µM KSCN, and 100 µM TNB was carried out at 37 °C for 30 min. The absorbance at 412 nm was recorded. (B) VPO1 dose-dependent HOSCN generation. Experiments were carried out as in A at pH 5.5 and VPO1 concentration as indicated. (C) HOSCN dose-dependent generation by VPO1. Experiments were carried out as in A at pH 5.5. rVPO1 concentration was 500 nM and KSCN concentrations are indicated. The data are from three independent experiments. Error bars, SD.
Kinetic measurements of VPO1-catalyzed generation of HOCl, HOBr, and HOSCN
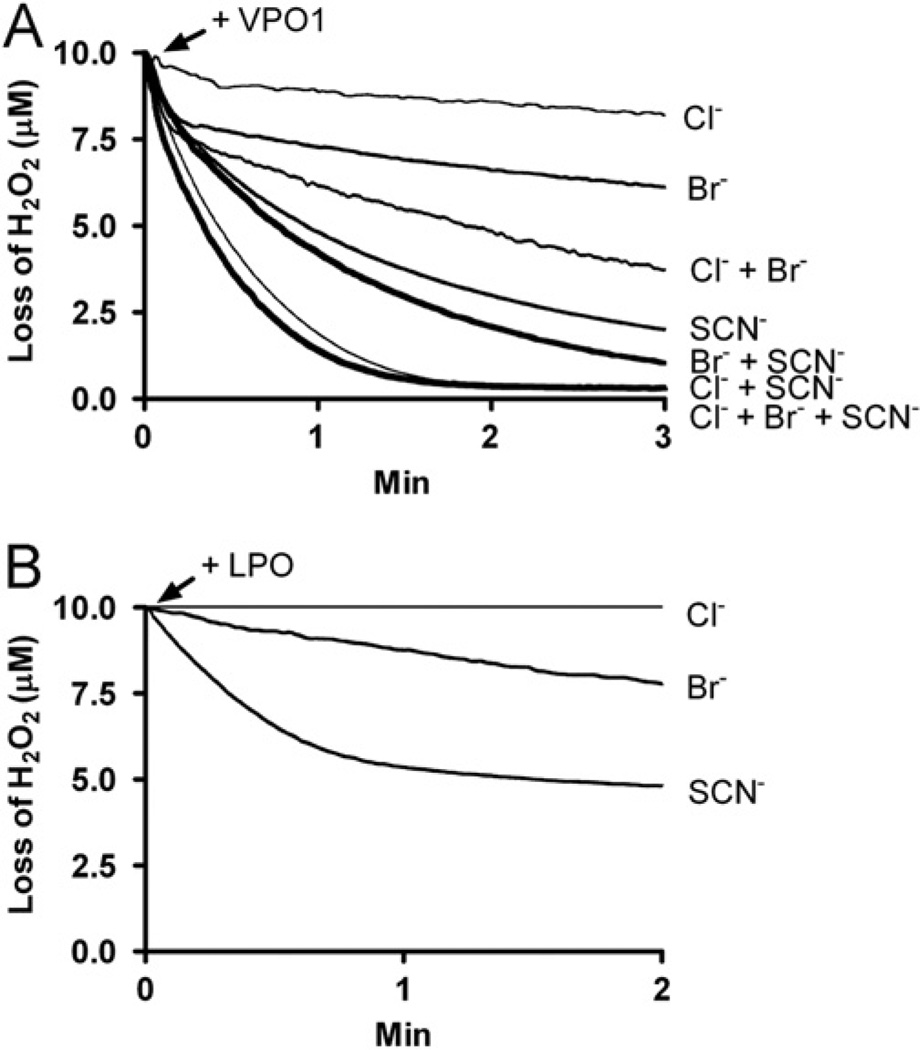
We further evaluated VPO1-catalyzed HOBr, HOCl, and HOSCN generation using the H2O2 electrode. Reactions were initiated by adding VPO1 and decomposition rates of H2O2 were continuously monitored. As shown in Fig. 4A, VPO1 catalyzed H2O2 decomposition in the presence of Cl−, Br−, and SCN− at physiological pH. Loss of H2O2 was not observed when omitting either VPO1 or the halide from the reaction mixture (data not shown). bLPO mediated H2O2 decomposition in the presence of SCN− or Br−, but not Cl− (Fig. 4B). These data confirm the results from the taurine oxidation assay and the TNB oxidation assay, supporting VPO1-catalyzed formation of HOCl, HOBr, and HOSCN (Figs. 1 and 3); this is also consistent with our previous report [12]. Interestingly, the rate of H2O2 decomposition is greater in the presence of multiple halides than with any single halide alone (Fig. 4A).
Fig. 4.
VPO1-catalyzed H2O2 decomposition in the presence of Cl−, Br−, and/or SCN−. (A) Reactions were started by adding 100 nM rVPO1 (A) or 10 nM bLPO (B) to 20 mM phosphate buffer, pH 7.4, containing 10 µM H2O2, 100 mM NaCl, 100 µM KBr, and/or 100 µM KSCN at 22 °C. Loss of H2O2 was continuously monitored with H2O2 electrode. Traces are representative of three independent experiments.
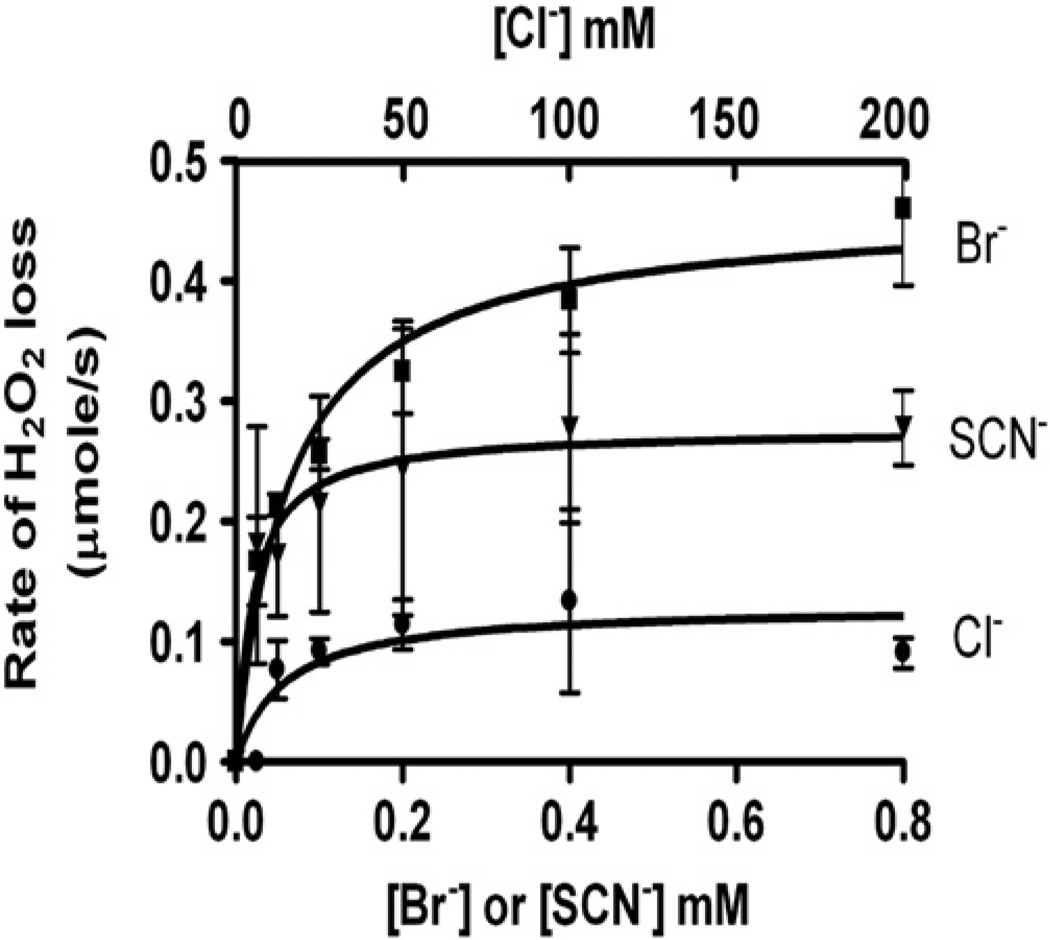
Next, we assessed the effects of substrate concentration on VPO1 activity. To minimize inhibition of VPO1 by the product, we utilized a relatively low concentration of H2O2 in our VPO1/H2O2/halide system. The plot of the rates of H2O2 loss against halide concentration is shown in Fig. 5. The kinetics of VPO1 activity was calculated from the plot (Table 1). From these results, thiocyanate and bromide were found to be more specific and preferred substrates for VPO1 than chloride. However, under physiological pH and concentrations of halides (100 mM chloride, 100 µM bromide, and 100 µM thiocyanate), VPO1 utilized approximately 18% of H2O2 to convert Cl− into HOCl, 45% into HOBr, and 35% into HOSCN. Taken together, our data demonstrate that VPO1 is capable of mediating oxidation of Cl−, Br−, and SCN− to generate HOCl, HOBr, and HOSCN, respectively, under physiological conditions and in the presence of H2O2.
Fig. 5.
Effects of substrate concentrations on VPO1 activity. Reactions were started by adding rVPO1 (100 nM, final concentration) to 20 mM phosphate buffer, pH 7.4, containing 10 µM H2O2, and varied concentrations of halides as indicated. Loss of H2O2 was continuously monitored with H2O2 electrode. The initial rates of H2O2 loss were measured over the first 10 s. The data are means and ranges of duplicate experiments.
Table 1.
Kinetic parameters of VPO1-catalyzed generation of hypohalous acids
| Substrate | Vmax (µM/min) | kcat (s−1) | km (mM) | ||||
|---|---|---|---|---|---|---|---|
| Br− | 27.6 | 4.6 | 0.063 | 7.3 × 104 | 365 | ||
| SCN− | 16.6 | 2.8 | 0.020 | 1.4 × 105 | 700 | ||
| Cl− | 7.1 | 1.2 | 5.879 | 2.0 × 102 | 1 |
The Vmax and km were determined by fitting rectangular hyperbolae to the corresponding curves in Fig. 5. The catalytic rate constants (kcat) were calculated by divided Vmax value by the concentration of VPO1. The specificity constants (kx−) were determined by dividing kcat by km value.
Discussion
In the present study, we have, for the first time, characterized VPO1-catalyzed generation of hypohalous and hypothiocyanous acids, and defined salient enzymatic properties. VPO1 is able to oxidize Cl−, Br−, and SCN− in the presence of H2O2 to generate hypochlorous, hypobromous, and hypothiocyanous acids. Under physiological conditions, VPO1 utilizes ~45% of H2O2 for the generation of HOBr, 35% for HOSCN, and ~18% for HOCl. Our data suggests that Br−, SCN−, and Cl− may serve as physiological substrates for VPO1.
VPO1 reveals distinct Kcat (s−1) for different halide substrates. The Kcat values of Br− and SCN− are ~4- and 2-fold that of Cl− (Table 1). It has been reported that Kcat values of MPO for Cl−, Br−, and SCN− are 79, 54, and 163, respectively [3]. Thus, the turnover rates of Cl−, Br−, and SCN− by MPO are 67-, 12-, and 58-fold faster than that by VPO1. This is in good agreement with our previous observation that MPO activity was ~20 higher than VPO1 for TMB oxidation [9]. Although the relative activity of VPO1 is lower than that of MPO, the total peroxidase activity of VPO1 in the bloodstream is predicted to be higher based on the ~1000-fold higher concentration of VPO1 (relative to MPO) in plasma [10]. On the other hand, the lower activity of VPO1 with relatively slow generation of hypohalous acids may suggest the possibility of alternative physiologic roles for this novel hPx. For example, it may play a homoeostatic or antioxidant function in controlling local concentrations of H2O2. Other functions for this hPx may be uncovered when other (more preferred) substrate(s) of VPO1 are discovered.
A number of factors determine the type and amount of the oxidants generated by hPx-mediated reactions, including the relative concentrations, substrate availability, and the cellular microenvironment. Although VPO1-catalyzed HOCl generation is less than HOBr and HOSCN on a molar basis, this may still be important from a pathophysiological perspective. HOCl is the strongest oxidant of the three hypohalous acids, and the net effect of VPO1-mediated halogenations to mediate oxidization of biomolecules may be similar. Our unpublished data show that 2 µM HOCl may completely kill Escherichia coli in solution in 30 min, whereas the same result requires 6 µM HOBr. Thus, VPO1-catalyzed HOCl and HOBr could each contribute to host defense under physiological conditions, as well as contribute to cell/tissue damage in pathological, inflammatory contexts.
VPO1, similar to MPO, EPO, and LPO, generates HOSCN. This finding suggests that VPO1 may contribute to inflammation, particularly in conditions of elevated SCN− levels as found with cigarette smoking and related lung disease. It has been proposed that HOSCN contributes to cell/tissue damage, in part by its ability to oxidize thiol groups on proteins. Increased generation of HOSCN is seen in inflammatory sites and induces apoptosis of murine macrophages [23]. HOSCN is up to 100-fold more potent than other oxidants in inducing tissue factor activity in human umbilical vein endothelial cells (HUVECs) [22]. HOSCN also regulates a number of cellular signaling pathways, including p65/c-Rel, ERK1/2, NFγB, and MAPK pathways [22,24,25]. As previously noted, VPO1 concentration in plasma is 1000-fold higher than that of MPO [10], and highly expressed in vessel walls [9]. The findings of the current study suggest a role for VPO1-mediated HOSCN generation in inflammatory responses at these sites of high VPO1 expression.
Our data indicate that the activity of VPO1 is greater with a combination of halides and the “pseudohalide” (thiocyanate) than either of them alone in generating hypohalous acids and HOSCN. Thus, when considering physiological and pathological roles of VPO1, one should not underestimate the synergistic effects of these substrates. Under certain pathological conditions such as excess intake of salt, cigarette smoking, and Cl− accumulation in cells (as in the case of cystic fibrosis), increases in halides and thiocyanate may enhance oxidant generation and perpetuate tissue inflammation.
Acknowledgments
This work was supported by the National Institutes of Health (NIH/NHLBI R01 HL086836) and the American Heart Association (GRNT12040409) (to G.C.). V.J.T. was supported by the National Institutes of Health (NIH/NHLBI R01 HL067967 and R01 HL094230). G.Z. was supported by the National Natural Science Foundation of China (30871052 and 30971194).
Abbreviations
- VPO1
Vascular peroxidase 1
- MPO
Myeloperoxidase
- bLPO
Bovine lactoperoxidase
- TMB
3,3′,5,5′-tetramethylbenzidine
- ABAH
Aminobenzoic acid hydrazide
- PBS
Phosphate-buffered saline
- Cl-tau
Chlorotaurine
- Br-tau
Bromotaurine
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Klebanoff SJ. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 2.Morrison M, Schonbaum GR. Peroxidase-catalyzed halogenation. Annu. Rev. Biochem. 1976;45:861–888. doi: 10.1146/annurev.bi.45.070176.004241. [DOI] [PubMed] [Google Scholar]
- 3.van Dalen CJ, Whitehouse MW, Winterbourn CC, Kettle AJ. Thiocyanate and chloride as competing substrates for myeloperoxidase. Biochem. J. 1997;327(Pt 2):487–492. doi: 10.1042/bj3270487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss SJ, Test ST, Eckmann CM, Roos D, Regiani S. Brominating oxidants generated by human eosinophils. Science. 1986;234:200–203. doi: 10.1126/science.3018933. [DOI] [PubMed] [Google Scholar]
- 5.Kouichiro Tsuge MK, Yasuo Seto. Cyanide and thiocyanate levels in blood and saliva of healthy adult volunteers. J. Health Sci. 2000;46:343–350. [Google Scholar]
- 6.Fonteh FA, Grandison AS, Lewis MJ. Variations of lactoperoxidase activity and thiocyanate content in cows’ and goats’ milk throughout lactation. J. Dairy Res. 2002;69:401–409. doi: 10.1017/s0022029902005538. [DOI] [PubMed] [Google Scholar]
- 7.Taurog A, Dorris ML. Peroxidase-catalyzed bromination of tyrosine, thyroglobulin, and bovine serum albumin: comparison of thyroid peroxidase and lactoperoxidase. Arch. Biochem. Biophys. 1991;287:288–296. doi: 10.1016/0003-9861(91)90481-w. [DOI] [PubMed] [Google Scholar]
- 8.Taurog A, Dorris ML, Doerge DR. Mechanism of simultaneous iodination and coupling catalyzed by thyroid peroxidase. Arch. Biochem. Biophys. 1996;330:24–32. doi: 10.1006/abbi.1996.0222. [DOI] [PubMed] [Google Scholar]
- 9.Cheng G, Salerno JC, Cao Z, Pagano PJ, Lambeth JD. Identification and characterization of VPO1, a new animal heme-containing peroxidase. Free Radic. Biol. Med. 2008;45:1682–1694. doi: 10.1016/j.freeradbiomed.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng G, Li H, Cao Z, Qiu X, McCormick S, Thannickal VJ, Nauseef WM. Vascular peroxidase-1 is rapidly secreted, circulates in plasma, and supports dityrosine cross-linking reactions. Free Radic. Biol. Med. 2011;51:1445–1453. doi: 10.1016/j.freeradbiomed.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi R, Hu C, Yuan Q, Yang T, Peng J, Li Y, Bai Y, Cao Z, Cheng G, Zhang G. Involvement of vascular peroxidase 1 in angiotensin II-induced vascular smooth muscle cell proliferation. Cardiovasc. Res. 2011;91:27–36. doi: 10.1093/cvr/cvr042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Cao Z, Moore DR, Jackson PL, Barnes S, Lambeth JD, Thannickal VJ, Cheng G. Microbicidal activity of vascular peroxidase 1 in human plasma via generation of hypochlorous acid. Infect. Immun. 2012;80:2528–2537. doi: 10.1128/IAI.06337-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deskur E, Przywarska I, Dylewicz P, Szczesniak L, Rychlewski T, Wilk M, Wysocki H. Exercise-induced increase in hydrogen peroxide plasma levels is diminished by endurance training after myocardial infarction. Int. J. Cardiol. 1998;67:219–224. doi: 10.1016/s0167-5273(98)00231-9. [DOI] [PubMed] [Google Scholar]
- 14.Lacy F, Kailasam MT, O’Connor DT, Schmid-Schonbein GW, Parmer RJ. Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender, and ethnicity. Hypertension. 2000;36:878–884. doi: 10.1161/01.hyp.36.5.878. [DOI] [PubMed] [Google Scholar]
- 15.Tenovuo J, Pruitt KM, Mansson-Rahemtulla B, Harrington P, Baldone DC. Products of thiocyanate peroxidation: properties and reaction mechanisms. Biochim. Biophys. Acta. 1986;870:377–384. doi: 10.1016/0167-4838(86)90244-x. [DOI] [PubMed] [Google Scholar]
- 16.Dypbukt JM, Bishop C, Brooks WM, Thong B, Eriksson H, Kettle AJ. A sensitive and selective assay for chloramine production by myeloperoxidase. Free Radic. Biol. Med. 2005;39:1468–1477. doi: 10.1016/j.freeradbiomed.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Hawkins CL, Brown BE, Davies MJ. Hypochlorite- and hypobromite-mediated radical formation and its role in cell lysis. Arch. Biochem. Biophys. 2001;395:137–145. doi: 10.1006/abbi.2001.2581. [DOI] [PubMed] [Google Scholar]
- 18.Thomas EL, Grisham MB, Jefferson MM. Preparation and characterization of chloramines. Methods Enzymol. 1986;132:569–585. doi: 10.1016/s0076-6879(86)32042-1. [DOI] [PubMed] [Google Scholar]
- 19.Senthilmohan R, Kettle AJ. Bromination and chlorination reactions of myeloperoxidase at physiological concentrations of bromide and chloride. Arch. Biochem. Biophys. 2006;445:235–244. doi: 10.1016/j.abb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Slungaard A, Mahoney JR., Jr Thiocyanate is the major substrate for eosinophil peroxidase in physiologic fluids. Implications for cytotoxicity. J. Biol. Chem. 1991;266:4903–4910. [PubMed] [Google Scholar]
- 21.van Dalen CJ, Kettle AJ. Substrates and products of eosinophil peroxidase. Biochem. J. 2001;358:233–239. doi: 10.1042/0264-6021:3580233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang JG, Mahmud SA, Thompson JA, Geng JG, Key NS, Slungaard A. The principal eosinophil peroxidase product, HOSCN, is a uniquely potent phagocyte oxidant inducer of endothelial cell tissue factor activity: a potential mechanism for thrombosis in eosinophilic inflammatory states. Blood. 2006;107:558–565. doi: 10.1182/blood-2005-05-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lloyd MM, van Reyk DM, Davies MJ, Hawkins CL. Hypothiocyanous acid is a more potent inducer of apoptosis and protein thiol depletion in murine macrophage cells than hypochlorous acid or hypobromous acid. Biochem. J. 2008;414:271–280. doi: 10.1042/BJ20080468. [DOI] [PubMed] [Google Scholar]
- 24.Wang JG, Mahmud SA, Nguyen J, Slungaard A. Thiocyanate-dependent induction of endothelial cell adhesion molecule expression by phagocyte peroxidases: a novel HOSCN-specific oxidant mechanism to amplify inflammation. J. Immunol. 2006;177:8714–8722. doi: 10.4049/jimmunol.177.12.8714. [DOI] [PubMed] [Google Scholar]
- 25.Lane AE, Tan JT, Hawkins CL, Heather AK, Davies MJ. The myeloperoxidase-derived oxidant HOSCN inhibits protein tyrosine phosphatases and modulates cell signalling via the mitogen-activated protein kinase (MAPK) pathway in macrophages. Biochem. J. 2010;430:161–169. doi: 10.1042/BJ20100082. [DOI] [PMC free article] [PubMed] [Google Scholar]