Abstract
Objective
Reliability of the most commonly used duplex ultrasound (DUS) velocity thresholds for internal carotid artery (ICA) stenosis has been questioned since these thresholds were developed using less precise methods to grade stenosis severity based on angiography. In this study, maximum percent diameter carotid bulb ICA stenosis (European Carotid Surgery Trial [ECST] method) was objectively measured using high resolution B-mode DUS validated with computed tomography angiography (CTA) and used to determine optimum velocity thresholds for ≥50% and ≥80% bulb internal carotid artery stenosis (ICA).
Methods
B-mode DUS and CTA images of 74 bulb ICA stenoses were compared to validate accuracy of the DUS measurements. In 337 mild, moderate, and severe bulb ICA stenoses (n = 232 patients), the minimal residual lumen and the maximum outer bulb/proximal ICA diameter were determined on longitudinal and transverse images. This in contrast to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) method using normal distal ICA lumen diameter as the denominator. Severe calcified carotid segments and patients with contralateral occlusion were excluded. In each study, the highest peak systolic (PSV) and end-diastolic (EDV) velocities as well as ICA/common carotid artery (CCA) ratio were recorded. Using receiver operating characteristic (ROC) analysis, the optimum threshold for each hemodynamic parameter was determined to predict ≥50% (n = 281) and ≥80% (n = 62) bulb ICA stenosis.
Results
Patients mean age was 74 ± 8 years; 49% females. Clinical risk factors for atherosclerosis included coronary artery disease (40%), diabetes mellitus (32%), hypertension (70%), smoking (34%), and hypercholesterolemia (49%). Thirty-three percent of carotid lesions (n = 110) presented with ischemic cerebrovascular symptoms and 67% (n = 227) were asymptomatic. There was an excellent agreement between B-mode DUS and CTA (r = 0.9, P = .002). The inter/intraobserver agreement (κ) for B-mode imaging measurements were 0.8 and 0.9, respectively, and for CTA measurements 0.8 and 0.9, respectively. When both PSV of ≥155 cm/s and ICA/CCA ratio of ≥2 were combined for the detection of ≥50% bulb ICA stenosis, a positive predictive value (PPV) of 97% and an accuracy of 82% were obtained. For a ≥80% bulb ICA stenosis, an EDV of ≥140 cm/s, a PSV of ≥370 cm/s and an ICA/CCA ratio of ≥6 had acceptable probability values.
Conclusion
Compared with established velocity thresholds commonly applied in practice, a substantially higher PSV (155 vs 125 cm/s) was more accurate for detecting ≥50% bulb/ICA stenosis. In combination, a PSV of ≥155 cm/s and an ICA/CCA ratio of ≥2 have excellent predictive value for this stenosis category. For ≥80% bulb ICA stenosis (NASCET 60% stenosis), an EDV of 140 cm/s, a PSV of ≥370 cm/s, and an ICA/CCA ratio of ≥6 are equally reliable and do not indicate any major change from the established criteria. Current DUS ≥50% bulb ICA stenosis criteria appear to overestimate carotid bifurcation disease and may predispose patients with asymptomatic carotid disease to untoward costly diagnostic imaging and intervention.
Over the last decade, a number of randomized clinical trials have established evidence in support of carotid endarterectomy (CEA) for asymptomatic and symptomatic patients with hemodynamically significant internal carotid artery stenosis (ICA) as determined by arteriography.1,2,3 Biplane arteriography has been the gold standard for investigating and grading the degree of carotid bifurcation disease for decades. The multicenter carotid trials used angiography to grade stenosis severity in the proximal ICA using different criteria in North America vs Europe (North American Symptomatic Carotid Endarterectomy Trial [NASCET] and European Carotid Surgery Trial [ECST] methods, Fig. 1). With improved resolution of current imaging modalities, including duplex ultrasound (DUS), computed tomography angiography (CTA), and magnetic resonance angiography (MRA), it is now possible to visualize the carotid bifurcation plaque burden as well as the outer wall boundaries, and potentially obtain more accuracy than with bulb diameter estimates made from arteriography using the ECST method.
Fig. 1.
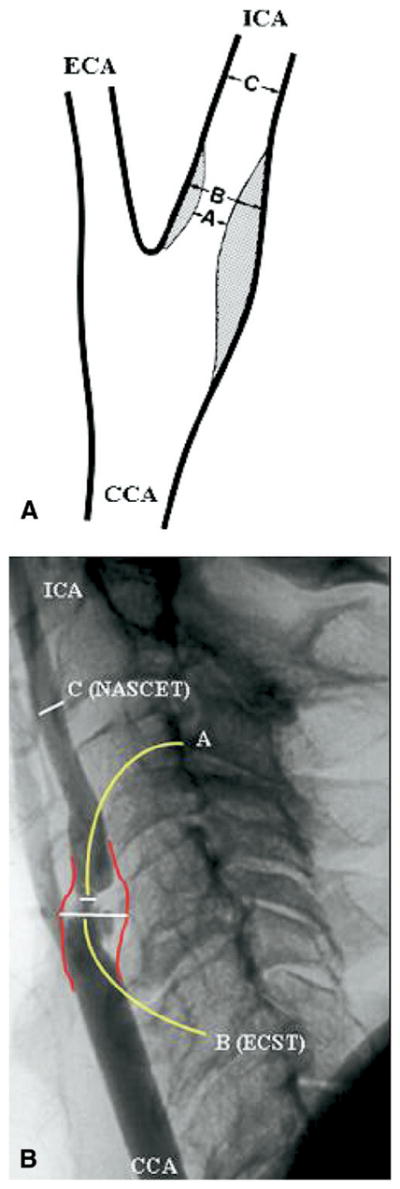
Schematic (A) and arteriographic (B) representations of NASCET (C-A/C) and ECST (B A/B) methods of bulb internal carotid artery (ICA) stenosis severity measurement. The bulb outer wall is subjectively estimated on arteriography. CCA, Common carotid artery; ECA, external carotid artery; NASCET, North American Symptomatic Carotid Endarterectomy Trial; ECST, European Carotid Surgery Trial.
In contemporary clinical practice, DUS is the primary noninvasive screening modality used for the assessment of carotid stenosis in patients with possible carotid bifurcation disease. Studies have shown accuracies in the range of 92% to 96% in predicting severe carotid stenosis.4 Advances in image resolution have improved DUS to an extent that it is arguably the most commonly used primary noninvasive tool prior to CEA and endovascular interventions or for pursuing additional diagnostic testing with CTA or MRA.
Numerous duplex velocity criteria have been developed and widely adopted for grading carotid bifurcation disease severity.5–10 The reliability of the most commonly used DUS velocity thresholds for carotid stenosis has been questioned as these thresholds were developed using less precise estimates, ie, wall calcification of the bulb stenosis outer wall diameter on arteriography.2,11 B-mode imaging (BMI) holds potential as an accurate and reliable predictor of carotid bifurcation disease. The ability of the gray-scale or BMI to accurately measure the degree of carotid stenosis independent of velocity criteria has been demonstrated.12–14 We have shown previously that BMI measurements are accurate among experienced technologists and are useful adjunct to duplex-derived velocity parameters and that BMI improves the accuracy and predictive values of DUS evaluation for different thresholds of bulb/ICA stenosis.15
Current state of the art CTA, performed with multidetector helical high-speed CT hardware, allows for three-dimensional (3D)-postprocessing of the extra- and intracranial carotid systems.16–18 Both noncontrast and contrast-enhanced CT axial source images can be used to visualize arterial lumen and surrounding tissues and to grade carotid stenosis with spatial resolution and anatomic detail superior to other imaging modalities.19 CTA has the potential to replace or complement arteriography for the assessment of carotid bifurcation disease.
There is still an ongoing debate over the most accurate image modalities and grading criteria for carotid lesions. The purpose of the present study was therefore to objectively measure the maximum percent diameter carotid bulb/proximal ICA stenosis using high resolution B-mode DUS validated with CTA and to use such measurements to determine optimal peak systolic (PSV) and end-diastolic (EDV) velocities and PSV ICA/common carotid artery (CCA) ratio thresholds for ≥50% and ≥80% carotid bulb ICA stenosis categories.
METHODS
Study population
In this retrospective case-controlled study, we reviewed the results of 1093 patients who underwent duplex ultrasound imaging of 2186 carotid arteries in an Intersocietal Commission for the Accreditation of Vascular Laboratories (ICAVL)-accredited noninvasive vascular laboratory between January 2004 and December 2006. The indications for duplex ultrasound carotid imaging included suspected symptomatic or asymptomatic extracranial cerebrovascular disease. Patients with aneurysm, pseudoaneurysm, dissection, carotid body tumor, or trauma were all excluded. Inclusion criteria for the present study were patients who had abnormal carotid DUS findings (22% of total carotid studies; n = 481) with mild (<50%), moderate (50%–79%), or severe (80%–99%) bulb ICA stenosis. Exclusion criteria from this cohort included: ipsilateral carotid occlusions (n = 22; 4.5%), carotid stenoses with contralateral occlusion or contralateral carotid stenosis greater than 80% (n = 29; 6%), hemodynamically significant common carotid disease (n = 7; 1.5%), prior carotid intervention including stent placement (n = 10; 2%), suboptimal B-mode images (n = 10; 2%), and acoustic shadowing from plaque calcification which prohibited accurate B-mode or Doppler velocity assessment at the tightest stenosis (n = 66; 14%). Finally, a total number of 232 patients (105 bilateral and 127 unilateral lesions) with 337 carotid stenoses were included in the study. During the same time period, 37 of these patients (74 carotids) underwent imaging of the extracranial carotid arteries with both DUS and CTA within 6 months of each other, followed by comparison of B-mode DUS and CTA images for validation of BMI measurements.
Carotid duplex ultrasound
All carotid duplex scans were performed with ATL HDI 3000 (Advanced Technology Laboratories, Bothel, Wash), ATL HDI 5000, or Acuson Sequoia 512 (Acuson Corp, Mountain View, Calif) ultrasound scanner with linear array 4–7 MHz or 5–10 MHz transducers. In our institution, all DUS carotid studies were performed by registered vascular technologists and were subsequently reviewed by board-certified vascular surgeons. The carotid arteries were examined in supine position with the head slightly elevated and turned towards the contralateral side. The standard protocol for carotid duplex examination included examination of the CCA and the extracranial segments of ICA, external carotid artery (ECA), and vertebral (VA) artery using spectral analysis and BMI complemented with color flow mapping. All velocity and B-mode data were recorded in the patient’s vascular laboratory chart with representative images of velocity measurements and B-mode images that were produced on photographic film paper using a Sony UP 5600 color printer (Sony Electronics Inc, Park Ridge, NJ).
Pulsed Doppler spectral analysis
An initial Doppler sweep of the CCA, ICA, and the proximal ECA was performed to identify areas of increased velocity. While the Doppler beam angle was maintained as close as possible to 60 degrees at all times, representative values of peak systolic velocity (PSV) and end-diastolic velocity (EDV) were recorded at specified locations of the CCA, ICA, ECA, and vertebral arteries. The ratio of PSV between ICA and CCA was calculated and recorded for each patient. The criteria used for diagnosing a hemodynamically significant stenosis (≥50%) and for grading of carotid stenosis severity were based on previously published criteria from the University of Washington (Strandness criteria)5,6 (Table I). In each study, the highest PSV, EDV, and ICA/CCA ratio were recorded.
Table I.
University of Washington (Strandness) velocity criteria for the diagnosis of bulb internal carotid artery stenosis
| Grade | Degree of stenosis | PSV cm/s | EDV cm/s | Flow characteristics |
|---|---|---|---|---|
| A | 0% | <125 | <140 | No spectral broadening |
| B | 1%–15% | <125 | <140 | Minimal spectral broadening |
| C | 16%–49% | <125 | <140 | Marked spectral broadening |
| D | 50%–79% | ≥125 | <140 | Marked spectral broadening |
| D+ | 80%–99% | ≥125 | ≥140 | Marked spectral broadening |
| E | Total occlusion | N/A | N/A | No flow |
PSV, Peak systolic velocity; EDV, end diastolic velocity.
B-mode ultrasound imaging (BMI)
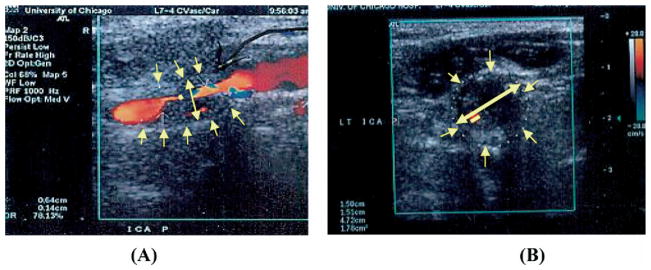
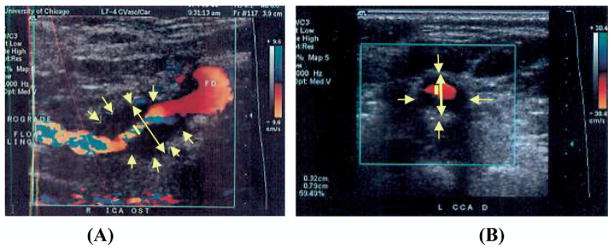
The carotid bifurcation was imaged with B-mode and color flow in the transverse and longitudinal planes. B-mode US images were digitized using a scanner. Measurements were done on the digitized images using commercially available software (Adobe Photoshop, San Jose, Calif). The absolute measurements were calibrated to the B-mode depth scale present on each image. The minimal residual lumen (measured at the point of tightest stenosis) and the corresponding outer ICA or bulb diameters were determined on longitudinal and transverse images. (Figs. 2 and 3) Measurements were taken perpendicular to the axis of the vessel. The percent stenosis was calculated using the minimal residual lumen (A) and the diameter of the corresponding ICA or bulb true lumen (B), unlike the NASCET method measuring normal ICA distal to the stenosis, in the following formula:
Fig. 2.
B-mode imaging (BMI) diameter measurement of bulb internal carotid artery (ICA) stenosis in longitudinal (A) and transverse (B) views of a heterogeneous carotid plaque.
Fig. 3.
B-mode imaging (BMI) diameter measurement of bulb internal carotid artery (ICA) stenosis in longitudinal (A) and transverse (B) views of a homogeneous echolucent carotid plaque.
Measurements were performed by two independent examiners (W. S. and C. W.), who were blinded to the velocity parameters, and were reviewed by the principal investigator (H. B.).
Quantitative morphometry of ICA stenosis
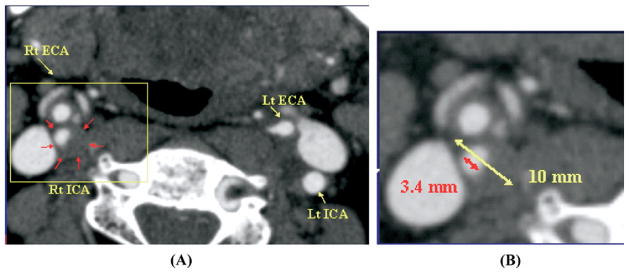
B-mode carotid DUS measurements were validated against CTA studies. CTA examinations were retrospectively collected from database using Stentor Picture Archiving and Communications System (PACS) from January 2004 through December 2006. All CTA examinations were performed using 16- or 64-slice Brilliance CT scanner (Philips, Cleveland, Ohio). CTA axial source images at 1.25-mm interval, both contrast and noncontrast, were utilized for measurements and were examined from C6 to vertex. Coronal and sagittal MPRs (multiplanar reformats) were used to identify the carotid orientation to ensure true cross-sectional measurements in all the evaluated arteries. These measurements were verified with measures from reformats to ensure accuracy in obtaining the narrowest diameter in a true cross-sectional plane. Center-line imaging was not used. Maximum carotid stenosis was identified on the axial source images. Diameter and carotid stenosis measurements were obtained by placing measurement calipers at the edges of the residual carotid lumen (A) at the narrowest portion of the carotid bulb or the ICA and at the corresponding outer wall (B) using submillimeter scale and magnification tools on the PACS workstation. (Fig. 4) Special attention to calcified high-density regions of the plaque was reinforced with extra-windowing and contrast modification. Carotid stenosis was calculated using the same formula for BMI measurements.
Fig. 4.
Computed tomography angiography (CTA) axial source image of right internal carotid artery (RT ICA) stenosis (A). Diameter and carotid stenosis measurements were obtained by placing measurement calipers at the edges of the residual carotid lumen at the narrowest portion of the carotid bulb and at the outer wall of the carotid bulb using submillimeter scale and magnification tools (B) on the Picture Archiving and Communication System (PACS) workstation.
All images were viewed using Stentor PACS system and were evaluated by two independent examiners (W. S. and C. W.), who were blinded to the velocity parameters and BMI measurements.
Statistical analysis
All duplex ultrasound velocity profiles, BMI and CTA diameter and stenosis measurements were recorded on Excel spreadsheets. Specific parameters recorded included PSV, EDV, PSV ICA/CCA ratio, BMI diameter and stenosis measurements, CTA diameter and stenosis measurement. The inter/intraobserver agreement for BMI and CTA diameter measurements were determined with the Kappa (κ) statistics. B-mode DUS and CTA validation and agreement were tested using Pearson regression analysis and correlation coefficient (r2). Using receiver operating characteristic (ROC) analysis, with BMI measurements as the gold standard, the optimum threshold for each hemodynamic parameter (PSV, EDV, and PSV ICA/CCA ratio) was determined to predict ≥50% and ≥80% bulb ICA stenosis. BMI has previously been validated to pathologic carotid specimen at our institution (unpublished results). The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of the different velocity criteria for diagnosis of bulb ICA stenosis ≥50% and ≥80% were calculated. Statistical significance was accepted if P <.05. Medcalc statistical software (MedCalc Software, Mariakerke, Belgium) was used for statistical calculations.
RESULTS
Patient mean (±SD) age was 74 ± 8 years and 49% of patients (n = 114) were females. Forty-four percent (n = 102) and 56% (n = 130) of patients were Caucasians and African Americans, respectively. Clinical risk factors for atherosclerosis were recorded for all patients, which included coronary artery disease (n = 93; 40%), diabetes mellitus (n = 74; 32%), hypertension (n = 162; 70%), smoking (n = 79; 34%), and hypercholesterolemia (n = 114; 49%). There were 33% (n = 110) symptomatic and 67% (n = 227) asymptomatic carotid lesions. Symptoms included: transient ischemic attacks (n = 37), stroke (n = 34), amaurosis fugax (n = 14), and nonspecific (dizziness, syncope, n = 25).
Of the 337 carotid studies included in the study for BMI measurements and analysis there were: 56 carotids <50% bulb ICA stenosis and 281 carotids ≥50% including 219 carotids with 50% to 79% and 62 carotids with 80% to 99% bulb ICA stenosis. The inter/intraobserver agreement (κ) for BMI measurements was 0.8 and 0.9, respectively.
BMI validation with CTA
For BMI validation with CTA, 74 carotid studies were available including: <50% (n = 29), 50% to 79% (n = 23), and 80% to 99% (n = 22) bulb ICA stenosis. The inter/intraobserver agreement (κ) for CTA measurements was 0.8 and 0.9, respectively. In light of the low interobserver variability, the measurements from the two examiners were averaged to obtain a mean narrowest stenosis diameter by BMI and CTA for each carotid study. Using Pearson regression analysis, there was a strong agreement between B-mode ultrasound and CTA measurements (r = 0.9, P = .002). (Fig. 5).
Fig. 5.
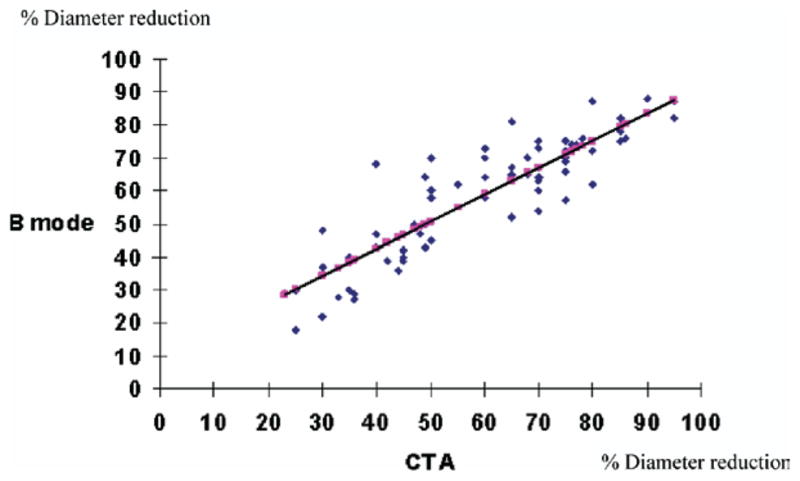
Regression analysis of B-mode and computed tomography angiography (CTA) diameter and bulb internal carotid artery (ICA) stenosis measurements. (r = 0.9, P = .002).
ROC analysis
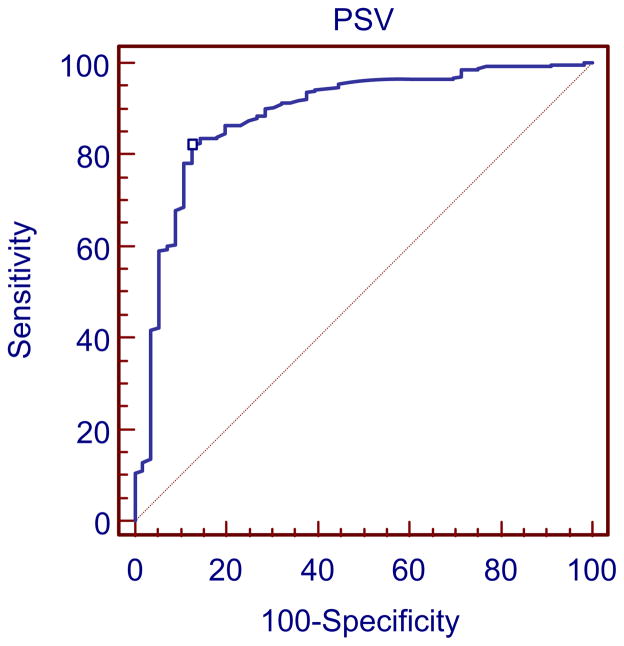
The ROC curve analysis of different PSV thresholds for detection of ≥50% bulb ICA stenosis showed that a PSV ≥155 cm/s was the most accurate (Fig. 6). The sensitivity, specificity, PPV, and NPV of different PSV thresholds for the diagnosis of ≥50% carotid stenosis are shown in Table II. Table III shows the probability values of different ICA/CCA ratio thresholds for the diagnosis of ≥50% carotid stenosis. When both PSV of ≥155 cm/s and ICA/CCA ratio of ≥2 were combined for the detection of ≥50% bulb ICA stenosis, a sensitivity of 77%, a specificity of 89%, a PPV of 97%, a NPV of 83%, and an accuracy of 82% were obtained. Using a PSV threshold of 125 cm/s and BMI as the gold standard, there was 11% chance (31/281) of false positive diagnosis of ≥50% carotid stenosis. On the other hand, there was only 3% chance (8/281) of false positive diagnosis of ≥50% bulb ICA stenosis if PSV a threshold of 155 cm/s was used (P < .0001). The sensitivity, specificity, PPV, and NPV of different hemodynamic thresholds (PSV, EDV, and ICA/CCA ratio) for the diagnosis of ≥80% carotid stenosis are shown in Tables IV, V, and VI, respectively. For a ≥80% bulb ICA stenosis, an EDV of ≥140 cm/s, a PSV of ≥370 cm/s, and an ICA/CCA ratio of ≥6 had acceptable probability values.
Fig. 6.
Receiver operating characteristic (ROC) curve analysis depicting the sensitivity and specificity of peak systolic velocity (PSV) threshold of 155 cm/s for detection of ≥50% bulb internal carotid artery (ICA) stenosis. The sensitivity and specificity were 82% and 88%, respectively.
Table II.
Probability values of different PSV thresholds for the detection of ≥50% bulb internal carotid artery stenosis
| PSV threshold | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| 125 cm/s | 96% | 52% | 52% | 96% | 74% |
| 135 cm/s | 91% | 66% | 59% | 94% | 80% |
| 145 cm/s | 86% | 77% | 67% | 92% | 81% |
| 155 cm/s* | 82% | 88% | 78% | 90% | 84% |
| 165 cm/s | 78% | 88% | 77% | 89% | 83% |
| 175 cm/s | 77% | 89% | 79% | 88% | 82% |
PSV, Peak systolic velocity; PPV, positive predictive value; NPV, negative predictive value.
denotes optimum value.
Table III.
Probability values of different ICA/CCA ratio thresholds for the detection of ≥50% bulb internal carotid artery stenosis
| ICA/CCA ratio | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| 1.7 | 85% | 68% | 59% | 89% | 76% |
| 1.8 | 83% | 71% | 61% | 89% | 77% |
| 1.9 | 82% | 79% | 67% | 89% | 80% |
| 2.0* | 80% | 84% | 73% | 89% | 81% |
| 2.1 | 78% | 84% | 72% | 89% | 80% |
| 2.2 | 76% | 84% | 72% | 87% | 79% |
| 2.3 | 74% | 86% | 74% | 87% | 79% |
ICA, Internal carotid artery; CCA, common carotid artery; PPV, positive predictive value; NPV, negative predictive value.
denotes optimum value.
Table IV.
Probability values of different PSV thresholds for the detection of ≥80% bulb internal carotid artery stenosis
| PSV threshold | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| 340 cm/s | 89% | 84% | 75% | 93% | 86% |
| 350 cm/s | 89% | 86% | 77% | 93% | 87% |
| 360 cm/s | 87% | 87% | 79% | 93% | 88% |
| 370 cm/s* | 87% | 90% | 82% | 93% | 89% |
| 380 cm/s | 82% | 90% | 82% | 91% | 86% |
| 390 cm/s | 82% | 91% | 83% | 90% | 85% |
| 400 cm/s | 79% | 91% | 83% | 90% | 85% |
PSV, Peak systolic velocity; PPV, positive predictive value; NPV, negative predictive value.
denotes optimum value.
Table V.
Probability values of different EDV thresholds for the detection of ≥80% bulb internal carotid artery stenosis
| EDV threshold | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| 125 cm/s | 90% | 86% | 78% | 94% | 83% |
| 130 cm/s | 90% | 88% | 79% | 94% | 85% |
| 135 cm/s | 87% | 88% | 80% | 93% | 88% |
| 140 cm/s* | 84% | 91% | 83% | 91% | 90% |
| 145 cm/s | 79% | 92% | 84% | 89% | 89% |
| 150 cm/s | 76% | 94% | 86% | 88% | 88% |
| 155 cm/s | 74% | 94% | 87% | 87% | 87% |
EDV, End diastolic velocity; PPV, positive predictive value; NPV, negative predictive value.
denotes optimum value.
Table VI.
Probability values of different ICA/CCA ratio thresholds for the detection of ≥80% bulb internal carotid artery stenosis
| ICA/CCA ratio threshold | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| 4.5 | 87% | 82% | 73% | 92% | 82% |
| 5.0 | 86% | 85% | 76% | 92% | 84% |
| 5.5 | 84% | 89% | 81% | 91% | 87% |
| 6.0* | 82% | 91% | 83% | 91% | 89% |
| 6.5 | 76% | 92% | 83% | 88% | 88% |
| 7.0 | 68% | 94% | 85% | 84% | 87% |
| 7.5 | 58% | 95% | 85% | 81% | 85% |
ICA, Internal carotid artery; CCA, common carotid artery; PPV, positive predictive value; NPV, negative predictive value.
denotes optimum value.
DISCUSSION
Multicenter randomized trials have shown that carotid endarterectomy (CEA) significantly reduces the risk of ipsilateral stroke in patients with severe symptomatic or asymptomatic carotid stenosis.1,2,3 These large randomized trials demonstrated that CEA is beneficial in symptomatic patients with severe stenosis (70%–99%) and in subgroups of patients with moderate stenosis (50%–69%). In asymptomatic patients with greater than or equal to 60% stenosis, CEA reduces the risk of stroke by approximately 1% per annum overall. The benefit is greatest for men and younger patients, and there may be no benefit for women or for older patients. The indications for CEA in asymptomatic patients are less robust than in symptomatic patients, however, it is customary to intervene on patients with ≥80% stenosis who have a reasonable life expectancy and in whom a low morbidity and mortality following intervention can be achieved.20
Although the natural history of asymptomatic carotid stenosis remains controversial, studies using serial noninvasive testing have concluded that a 50% or greater carotid stenosis carries a risk of subsequent stroke on the order of 4% per year.21 In addition, progression of a lesion to more than 80% carries an even higher risk of stroke; up to 35% risk of ischemic symptoms or ICA occlusion within 6 months.22 It is therefore recommended that patients with asymptomatic stenosis ≥50% of the carotid artery should be serially imaged with DUS at 6- to 12-month intervals.23,24
The degree of internal carotid stenosis is critical in considering if a patient should undergo carotid intervention. There is still an ongoing debate over the most accurate image modalities and grading criteria for carotid lesions. Duplex ultrasonography (DUS) is the primary noninvasive imaging method for evaluation of bulb ICA stenosis and is widely used in clinical practice.25 This imaging modality is increasingly becoming the only examination performed before surgical intervention. Exclusion of 70% to 99% arteriographic stenosis can be achieved by DUS with a sensitivity of up to 98%.26 Advantages of DUS, include relatively low cost, noninvasiveness, mobility, availability of high image resolution, and color flow. The technology is limited of poor image quality with calcified vessels as well as poor visualization of high bifurcations and proximal arch vessel disease.27 To ensure accuracy and reduced operator variability, serial accreditation (ICAVL) and validation of DUS in individual laboratories is essential. Notwithstanding, there is wide variability in the criteria used to estimate ECST bulb ICA and NASCET ICA stenosis.
Lumen stenosis measurements in large randomized carotid trials were based solely on digital subtraction angiography. The ECST study used the estimated normal lumen diameter at the site of the lesion and the NASCET trial used the diameter of a visible portion of normal ICA distal to the stenosis.1,2 These two different measurement methods produce different values for the same “stenosis”. A NASCET 50% to 69% stenosis is equivalent to an ECST 70% to 85%, while a NASCET 70% to 99% stenosis equates to an ECST 85% to 99%.27 The limitations of angiography includes its invasiveness and a risk of complications ranging from 1% to 4% as well as technical limitations such as the limited number of projections and the lack of precise delineation of the outer vessel wall.28 Computed tomographic angiography (CTA) is an accurate modality for detection of severe carotid artery disease. The advantages of CTA are its fast and noninvasive nature, visualization of the arterial lumen and the exact delineation of the outer vessel wall from the axial source images, and the possibility to reconstruct 3D images of the artery. The pooled sensitivity and specificity for detection of a 70% to 99% carotid stenosis are 85% and 93%, respectively.18
We found that a substantially higher PSV (155 cm/s) is more accurate for detecting ≥50% bulb ICA stenosis compared with previous reported velocity thresholds. When both PSV of ≥155 cm/s and ICA/CCA ratio of ≥2 were combined for the detection of ≥50% bulb ICA stenosis, a PPV of 97% and an accuracy of 82% were obtained. For a ≥80% bulb ICA stenosis, an EDV of 3140 cm/s, a PSV of ≥370 cm/s and an ICA/CCA ratio of ≥6 were equally reliable and do not indicate any major change from previous reported criteria. The Strandness criteria developed at the University of Washington used primarily bulb ICA velocity criteria.6 They recommended a PSV velocity threshold for an ICA stenosis 50% to 79% of ≥125 cm/s and an EDV threshold <140 cm/s. For an ICA stenosis 80% to 99% an EDV threshold of ≥140 cm/s was chosen. These criteria were based on the ECST method of angiography measurements. The disadvantage of the ECST method is the subjective estimate of the stenosis outer wall diameter as the outline of the bulb is not seen on arteriography. A further disadvantage of the ECST method arises when the plaque does not involve the bulb, where expressing the residual lumen as a percentage of the diameter of the bulb gives erroneous impression of the size of the plaque. A recent study found the optimal PSV and EDV, for diagnosing a ≥80% stenosis with the ECST grading method using angiographic data, to be ≥215 cm/s and 90 cm/s, respectively.29 A national validation study recommended a PSV cutpoint value of 260 cm/s for the diagnosis of bulb ICA stenosis ≥80% (ECST method).30 However, the optimal PSV differed substantially (210 cm/s and 320 cm/s) between the two Doppler angles used, 0° to 49° and 50° to 62°, respectively. The ability to identify high-grade bulb ICA stenosis was significantly better at small Doppler angles (0°–49°).
In general, the sensitivity decreases and the specificity increases with increasing velocity thresholds for a given degree of stenosis. For intervention, a high positive predictive value is of importance to avoid disease overestimation resulting in unnecessary procedures for patients with lesser degree of stenosis. A further improvement in accuracy can be obtained using ratios of ICA velocities to CCA velocities.31 Looking at the NASCET method, a consensus report from the Society of Radiologists in Ultrasound recommended a PSV threshold of 125 to 230 cm/s and sonographically visible plaque for a 50% to 69% ICA stenosis.23 Additional criteria included ICA/CCA PSV ratio of 2.0 to 4.0 and ICA EDV of 40 to 100 cm/s. A ≥70% ICA stenosis but less than near occlusion of the ICA was diagnosed when the ICA PSV is >230 cm/s and visible plaque and luminal narrowing were seen on grayscale and color DUS. Additional criteria included ICA/CCA PSV ratio >4 and ICA EDV >100 cm/s. A recent meta-analysis of the relation between the degree of ICA stenosis by DUS criteria and degree of stenosis by arteriography, using the NASCET method, showed optimal thresholds of PSV ≥130 cm/s and ≥200 cm/s in identification of arteriographic stenosis of ≥50% and ’70%, respectively. 32 These criteria23,32 seem to overdiagnose the NASCET stenosis corresponding to the findings for ≥80% bulb ICA stenosis in the present study. The disadvantage of the NASCET method is that it underestimates the size of the plaque in the carotid bulb. For example, a plaque that uniformly fills the bulb will produce a lumen measurement equal to the distal ICA. In the present study, we measured the minimal residual lumen (at the point of tightest stenosis in the bulb or the proximal ICA) and the corresponding outer ICA or bulb diameters on longitudinal and transverse images both for duplex and CTA.
Previous investigations utilizing angiography as a gold standard have concluded that CTA may underestimate while MRA tends to overestimate the degree of carotid stenosis, and DUS provides an intermediate estimate.33,34 A combination strategy of DUS and MRA has previously yielded a high accuracy compared with digital subtraction angiography in diagnosis of carotid artery stenosis.35 Conventional arteriography compared with MRA and CTA seems to underestimate carotid stenoses.36,37 We found a strong agreement between B-mode ultrasound and CTA measurements with a low inter- and intraobserver variability for the CTA measurements. Our group previously showed that B-mode imaging stenosis measurements are accurate among experienced technologists and are a useful adjunct to duplex-derived velocity parameters.15 A recent report evaluated CTA and published velocity DUS criteria in the grading of ICA stenosis.38 Carotid lesions >70% resulted in clinically relevant discrepancies, with higher grades of stenoses assessed by CTA according to the NASCET criteria. The choice of applied DUS grading criteria was also of clinical importance because all applied criteria revealed comparable results for the detection of stenoses >50%, but significant differences in the detection of lesions >70%. It is our contention that CTA provides a realistic representation of the carotid atherosclerotic burden and allows for more precise quantitative morphometry of vessel lumen and outer wall boundaries. CTA is invaluable in validating BMI and should be used more frequently as a gold standard in developing DUS velocity thresholds for various degrees of carotid stenosis as demonstrated in this study.
In conclusion, a substantially higher PSV (155 cm/s) was more accurate for detecting ≥50% bulb ICA stenosis compared to established velocity thresholds commonly applied in practice. In combination, a PSV of ≥155 cm/s and an ICA/CCA ratio of 2 have excellent predictive value for this stenosis category. For ≥80% bulb ICA stenosis (roughly NASCET 60% stenosis), an EDV of 140 cm/s, a PSV of ≥370 cm/s, and an ICA/CCA ratio of ≥6 are equally reliable. For measurement of the degree of carotid stenosis, the corresponding outer wall of the carotid bulb or the proximal ICA were used as reference rather than a disease free point in the ICA above the stenosis. Clinicians involved in the diagnosis and management of carotid artery disease should view with caution hemodynamic criteria in current use as patients with asymptomatic disease may be overdiagnosed and subjected to unnecessary and costly diagnostics, intervention and its related morbidity.
Footnotes
Competition of interest: none.
Presented at the Society for Vascular Surgery Annual Meeting, Baltimore, Md, Jun 7–10, 2007.
AUTHOR CONTRIBUTIONS
Conception and design: HB
Analysis and interpretation: WS, CW, HB
Data collection: WS
Writing the article: CW, WS, HB
Critical revision of the article: CW, HB, WS, TD, GP, CS
Final approval of the article: HB, CW, WS, TD, GP, CS
Statistical analysis: WS
Obtained funding: HB
Overall responsibility: HB, CW
References
- 1.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–53. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 2.European Carotid Surgery Trialists’ Collaborative Group. Randomized trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST) Lancet. 1998;351:1379–87. [PubMed] [Google Scholar]
- 3.Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273:1421–8. [PubMed] [Google Scholar]
- 4.Blakeley DD, Oddone EZ, Hasselblad V, Simel DL, Matchar DB. Noninvasive carotid artery testing: a meta-analytic review. Ann Intern Med. 1995;122:360–7. doi: 10.7326/0003-4819-122-5-199503010-00007. [DOI] [PubMed] [Google Scholar]
- 5.Zierler RE, Strandness DE., Jr . Noninvasive dynamic and real-time assessment of extracranial cerebrovasculature. In: Wood JH, editor. Cerebral blood flow: physiologic and clinical aspects. New York: McGraw Hill; 1987. pp. 311–23. [Google Scholar]
- 6.Strandness DE., Jr . Duplex scanning in vascular disorders. New York: Raven Press; 1993. [Google Scholar]
- 7.Moneta GE, Edwards EM, Chitwood RW. Correlation of North American Symptomatic Carotid Endarterectomy Trial (NASCET) angiographic definition of 70% to 99% internal carotid artery stenosis with duplex scanning. J Vasc Surg. 1993;17:152–9. doi: 10.1067/mva.1993.42888. [DOI] [PubMed] [Google Scholar]
- 8.AbuRahma AF, Pollack JA, Robinson PA. New duplex criteria for threshold stenosis used in the asymptomatic carotid atherosclerosis study. Vasc Surg. 1999;33:23–32. [Google Scholar]
- 9.Faught WE, Mattos MA, van Bemmelen PS, Hodgson KJ, Karkmeier LD, Ramsey DE, et al. Color flow duplex scanning of carotid arteries: new velocity criteria based on receiver operator characteristic analysis for threshold stenoses used in the symptomatic and asymptomatic carotid trials. J Vasc Surg. 1994;19:818–28. doi: 10.1016/s0741-5214(94)70006-0. [DOI] [PubMed] [Google Scholar]
- 10.Kreske ED, Wolk SW, Shanley CJ. Duplex ultrasonography to predict internal carotid artery stenosis exceeding 50% and 70% as defined by NASCET: the need for multiple criteria. Vasc Surg. 1999;33:497–506. [Google Scholar]
- 11.De Bray JW, Glatt B. Quantification of atheromatous stenosis in the extracranial internal carotid artery. Cerebrovasc Dis. 1995;5:414–26. [Google Scholar]
- 12.Beebe HG, Salles-Cunha SX, Scissons RP. Carotid arterial ultrasound scan imaging: A direct approach to stenosis measurement. J Vasc Surg. 1999;29:838–44. doi: 10.1016/s0741-5214(99)70211-9. [DOI] [PubMed] [Google Scholar]
- 13.Schulte-Altedorneburg G, Droste DW, Felszegby S. Detection of carotid artery stenosis by in vivo duplex ultrasound: correlation with planimetric measurements of the corresponding postmortem specimens. Stroke. 2002;33:2402–7. doi: 10.1161/01.str.0000030111.34093.02. [DOI] [PubMed] [Google Scholar]
- 14.Jmor S, El-Atrozy T, Griffin M. Grading internal carotid artery stenosis using B-mode ultrasound (in vivo study) Eur J Vasc Endovasc Surg. 1999;18:315–22. doi: 10.1053/ejvs.1999.0884. [DOI] [PubMed] [Google Scholar]
- 15.MacKenzie KS, French-Sherry E, Burns K, Pooley T, Bassiouny HS. B-mode ultrasound measurement of carotid bifurcation stenoses: is it reliable? Vasc Endovasc Surg. 2002;36:123–35. doi: 10.1177/153857440203600207. [DOI] [PubMed] [Google Scholar]
- 16.Randoux B, Marro B, Koskas F. Carotid artery stenosis: prospective comparison of CT, three-dimensional gadolinium-enhanced MR, and conventional angiography. Radiology. 2001;220:179–85. doi: 10.1148/radiology.220.1.r01jl35179. [DOI] [PubMed] [Google Scholar]
- 17.Porsche C, Walker L, Mendelow D, Birchall D. Evaluation of cross-sectional luminal morphology in carotid atherosclerotic disease by use of spiral CT angiography. Stroke. 2001;32:2511–5. doi: 10.1161/hs1101.098153. [DOI] [PubMed] [Google Scholar]
- 18.Koelemay MJW, Nederkoorn PJ, Reitsma JB, Majoie CB. Systematic review of computed tomographic angiography for assessment of carotid artery disease. Stroke. 2004;35:2306–12. doi: 10.1161/01.STR.0000141426.63959.cc. [DOI] [PubMed] [Google Scholar]
- 19.Bartlett ES, Symons SP, Fox AJ. Correlation of carotid stenosis diameter and cross-sectional areas with CT angiography. AJNR Am J Neuroradiol. 2006;27:638–42. [PMC free article] [PubMed] [Google Scholar]
- 20.Faries PL, Chaer RA, Patel S, Lin SC, DeRubertis B, Kent KC. Current management of extracranial carotid artery disease. Vasc Endovasc Surg. 2006;40:165–75. doi: 10.1177/153857440604000301. [DOI] [PubMed] [Google Scholar]
- 21.Moore WS. Vascular surgery: a comprehensive review. 7. Philadelphia, (PA): Saunders; 2005. [Google Scholar]
- 22.Roederer GO, Langlois YE, Jager KA, Primozich JF, Beach KW, Phillips DJ, et al. The natural history of carotid arterial disease in asymptomatic patients with cervical bruits. Stroke. 1984;15:605–13. doi: 10.1161/01.str.15.4.605. [DOI] [PubMed] [Google Scholar]
- 23.Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis–Society of Radiologists in Ultrasound Consensus Conference. Radiology. 2003;229:340–6. doi: 10.1148/radiol.2292030516. [DOI] [PubMed] [Google Scholar]
- 24.AbuRahma AF, Cook CC, Metz MJ, Wulu JT, Jr, Bartolucci A. Natural history of carotid artery stenosis contralateral to endarterectomy: results from two randomized prospective trials. J Vasc Surg. 2003;38:1154–61. doi: 10.1016/j.jvs.2003.07.028. [DOI] [PubMed] [Google Scholar]
- 25.Derdeyn CP, Powers WJ. Cost-effectiveness of screening for asymptomatic carotid atherosclerotic disease. Stroke. 1996;27:1944–50. doi: 10.1161/01.str.27.11.1944. [DOI] [PubMed] [Google Scholar]
- 26.Sabeti S, Schillinger M, Mlekusch W, Willfort A, Haumer M, Nachtmann T, et al. Quantification of internal carotid artery stenosis with duplex US: comparative analysis of different flow velocity criteria. Radiology. 2004;232:431–9. doi: 10.1148/radiol.2321030791. [DOI] [PubMed] [Google Scholar]
- 27.Nicolaides AN, Shifrin EG, Bradbury A, Dhanjil S, Griffin M, Belcaro G, et al. Angiographic and duplex grading of internal carotid stenosis: can we overcome the confusion? J Endovasc Surg. 1996;3:158–65. doi: 10.1177/152660289600300207. [DOI] [PubMed] [Google Scholar]
- 28.Willinsky RA, Taylor SM, TerBrugge K, Farb RI, Tomlinson G, Montanera W. Neurologic complications of cerebral angiography: prospective analysis of 2899 procedures and review of the literature. Radiology. 2003;227:522–8. doi: 10.1148/radiol.2272012071. [DOI] [PubMed] [Google Scholar]
- 29.Staikov IN, Nedeltchev K, Arnold M, Remonda L, Schroth G, Sturzenegger M, et al. Duplex sonographic criteria for measuring carotid stenoses. J Clin Ultrasound. 2002;30:275–81. doi: 10.1002/jcu.10078. [DOI] [PubMed] [Google Scholar]
- 30.Jogestrand T, Lindqvist M, Nowak J. Swedish Quality Board for Carotid Surgery. Diagnostic performance of duplex ultrasonography in the detection of high grade internal carotid artery stenosis. Eur J Vasc Endovasc Surg. 2002;23:510–8. doi: 10.1053/ejvs.2002.1621. [DOI] [PubMed] [Google Scholar]
- 31.Bluth EI, Stavros AT, Marich KW, Wetzner SM, Aufrichtig D, Baker JD. Carotid duplex sonography: a multicenter recommendation for standardized imaging and Doppler criteria. Radiographics. 1988;8:487–506. doi: 10.1148/radiographics.8.3.3289100. [DOI] [PubMed] [Google Scholar]
- 32.Jahromi AS, Cina CS, Liu Y, Clase CM. Sensitivity and specificity of color duplex ultrasound measurement in the estimation of internal carotid artery stenosis: a systematic review and meta-analysis. J Vasc Surg. 2005;41:962–72. doi: 10.1016/j.jvs.2005.02.044. [DOI] [PubMed] [Google Scholar]
- 33.Patel SG, Collie DA, Wardlaw JM, Lewis SC, Wright AR, Gibson RJ, et al. Outcome, observer reliability, and patient preferences if CTA, MRA, or Doppler ultrasound were used, individually or together, instead of digital subtraction angiography before carotid endarterectomy. J Neurol Neurosurg Psychiatry. 2002;73:21–8. doi: 10.1136/jnnp.73.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silvennoinen HM, Ikonen S, Soinne L, Railo M, Valanne L. CT angiographic analysis of carotid artery stenosis: comparison of manual assessment, semiautomatic vessel analysis, and digital subtraction angiography. AJNR Am J Neuroradiol. 2007;28:97–103. [PMC free article] [PubMed] [Google Scholar]
- 35.Nederkoorn PJ, Mali WP, Eikelboom BC, Elgersma OE, Buskens E, Hunink MG, et al. Preoperative diagnosis of carotid artery stenosis: accuracy of noninvasive testing. Stroke. 2002;33:2003–8. doi: 10.1161/01.str.0000021900.58396.44. [DOI] [PubMed] [Google Scholar]
- 36.Anzalone N, Scomazzoni F, Castellano R, Strada L, Righi C, Politi LS, et al. Carotid artery stenosis: intraindividual correlations of 3D time-of-flight MR angiography, contrast-enhanced MR angiography, conventional DSA, and rotational angiography for detection and grading. Radiology. 2005;236:204–13. doi: 10.1148/radiol.2361032048. [DOI] [PubMed] [Google Scholar]
- 37.Bucek RA, Puchner S, Haumer M, Rand T, Minar E, Lammer J. Grading of internal carotid artery stenosis: can CTA overcome the confusion? J Endovasc Ther. 2006;13:443–50. doi: 10.1583/06-1824MR.1. [DOI] [PubMed] [Google Scholar]
- 38.Bucek RA, Puchner S, Haumer M, Rand T, Sabeti S, Minar E, et al. Grading of internal carotid artery stenosis: comparative analysis of different flow velocity criteria and multidetector computed tomographic angiography. J Endovasc Ther. 2006;13:182–9. doi: 10.1583/05-1768R.1. [DOI] [PubMed] [Google Scholar]