Abstract
While the salivary gland has been recognized as an important effector site of the common mucosal immune system, a useful model for studying anti-viral salivary gland immune responses in vivo and for exploring the role of the salivary gland within the common mucosal system has been lacking. Murine cytomegalovirus (MCMV) is a beta-herpesvirus that displays a strong tropism for the salivary gland and produces significant morbidity in susceptible mice when introduced by intraperitoneal (i.p.) inoculation. This study tested the hypothesis that MCMV morbidity and pathology could be reduced by injecting the virus directly the submandibular salivary gland (intraglandular (i.g.)), using either in vivo derived MCMV or the less virulent, tissue culture-derived MCMV (tcMCMV). Peak salivary gland viral titers were completely unaffected by infection route (i.p vs. i.g.) after inoculation with either MCMV or tcMCMV. However, i.g. tcMCMV inoculation reduced viremia in all systemic tissues tested compared to i.p. inoculation. Further, systemic organ pathology observed in the liver and spleen after i.p. inoculation with either MCMV or tcMCMV was completely eliminated by i.g. inoculation with tcMCMV. Cellular infiltrates in the salivary glands, after i.p. or i.g. inoculation were composed of both B and T cells, indicating the potential for a local immune response to occur in the salivary gland. These results demonstrate that a focused MCMV infection of the salivary gland without systemic organ pathology is possible using i.g. delivery of tcMCMV.
Keywords: rodent, viral, MCMV, mucosa, spleen and lymph nodes, salivary gland, antibodies, mucosal immunity
Introduction
The nature of the common mucosal immune system allows that immunization at one mucosal surface results in protective immunity at mucosal sites both proximal as well as distal to the original site of immunization. Finding a useful model to study immune responses generated by the salivary gland is an important step in understanding and optimizing mucosal and systemic immunity to viruses, which infect through mucosal surfaces. Murine cytomegalovirus (MCMV) is a species-specific, β-herpesvirus and is an ideal pathogen to stimulate immune responses in the salivary gland since it (i) infects cells in the salivary gland, (ii) replicates to high titers in the salivary gland, and (iii) elicits MCMV-specific immune responses (Britt and Alford, 1996; Ho, 1991). However, in animal models, the virus is most often administered by the intraperitoneal (i.p.) route and results in systemic pathology in the spleen and liver leading to morbidity and mortality in MCMV-susceptible Balb/c mice (Britt and Alford, 1996; Ho, 1991). In addition, this route does not mimic the natural route of infection in mice, which is transmission through saliva and respiratory secretions (Britt and Alford, 1996; Ho, 1991). Therefore, in order to develop a model of a focused salivary gland infection and clearly study the role of the salivary gland as a component of the mucosal immune system, an infection protocol that (i) limits the virus to the salivary gland without high viral titers and pathology in other organs, and (ii) stimulates a local host immune response is needed.
This study utilized a rodent model of CMV infection, MCMV, to investigate the effect of infection route on CMV disease and localization to the salivary gland. In order to accomplish this goal, we manipulated three variables of infection: (i) route of inoculation [i.e. intraperitoneal (i.p.) versus intraglandular (i.g.) routes], (ii) virus preparation [i.e. salivary gland-derived (MCMV) and tissue culture-derived (tcMCMV) virus, which is attenuated with respect to its ability to infect systemic tissues (Ho, 1991; Tonari and Minamishima, 1983; Manning et al., 1992; Lee et al., 2000; Jordan and Takagi, 1983; Osborn and Walker, 1970; MacDonald et al., 1998) and (iii) mouse strain (i.e. MCMV-susceptible BALB/cByJ mice and MCMV-resistant C57Bl6/J mice) (Allan and Shellam, 1984; Lee et al., 2001). Viral pathology was determined by observation of mice for gross signs of morbidity, viral titers in tissues over time as well as the degree of accumulation of lymphocytic infiltrates in tissues, and tissue necrosis. In this study, we demonstrate that i.g. tcMCMV inoculation of susceptible Balb/c mice limits infection to the salivary gland while also limiting systemic organ pathology normally observed after i.p. MCMV inoculation. This focused salivary gland infection also induced infiltrates of B220+ B cells and macrophages, CD4+ helper T cells, and CD8+ cytotoxic T cells in the salivary gland and resulted in production of MCMV-specific antibodies (Abs). These data suggest that i.g. tcMCMV inoculation will be a useful model for studying anti-viral salivary gland immune responses in vivo and for exploring the role of the salivary gland within the mucosal immune system.
Materials and Methods
Mice and Virus
Female CD1 mice (Charles Rivers Laboratories, Wilmington, MA) were used to propagate MCMV in vivo (Jordan and Takagi, 1983). Briefly, Smith strain MCMV (ATCC, Manassas, VA) was injected i.p. into CD1 mice, salivary glands were homogenized after 14 days, and the homogenate was used to infect naïve CD1 mice. This process was repeated twice and third passage MCMV was used for inoculation. Control salivary gland antigen was prepared from salivary gland homogenates from uninfected CD1 mice. tcMCMV was generated by infecting 3T12 mouse embryo fibroblasts (ATCC) with third passage MCMV (multiplicity of infection (MOI) = 0.1). After 6 days of culture, tcMCMV was isolated from the supernatant and infected fibroblasts as described (Osborn and Walker, 1970; Castellano et al., 1977). Control tissue culture antigen was prepared similar to tcMCMV from cells that were incubated with salivary gland homogenate from uninfected CD1 mice.
Four- to six-week old female Balb/cByJ or C57BL6J mice (Jackson Laboratories, Bar Harbor, ME) were maintained in a BL2 facility. Mice were anesthetized i.p. with 20% Ketaset (Fort Dodge Animal Health, Fort Dodge, IA) + 2% Promace (Boehringer Ingelheim Vetmedical, St. Joseph, MT) in 0.9% saline (150 μl). Mice were inoculated with virus in 60 mM sodium bicarbonate buffer by the i.g. or i.p. routes. Mice inoculated by the i.p. route were immunized with 100 μL containing 105 plaque forming units (PFUs)/mouse of MCMV or tcMCMV, as indicated. Mice immunized by the i.g. route were deeply anesthetized and a 0.5 – 1cm incision was made over the salivary glands. 10 μL (5 μL per lobe) containing 105 PFUs/mL of MCMV or tcMCMV, as indicated, was injected into the submandibular gland. Control tissue culture antigen was used for controls in mice infected with tcMCMV and control salivary gland antigen was used for controls in mice infected with MCMV. Control mice were sacrificed 7 days after inoculation, if not otherwise indicated.
Antibodies
The following Abs were used in this study. Rat mAbs to B220 (Clone RA3-6B2, Caltag, Burlingame, CA), CD4 (Clone RM4-5, BD-Pharmingen, San Diego, CA), and CD8 (Clone CT-CD8a, Caltag). The secondary Ab used in immunohistochemistry was a biotinylated rabbit anti-rat Ig (H + L) (Vector Laboratories, Burlingame, CA).
Plaque Assay
Viral titers were determined using a standard plaque assay (Rubin et al., 1985). Briefly, tissue homogenates were incubated on confluent monolayers of 3T12 mouse embryo fibroblasts (ATCC) and incubated under solid agar containing media for 7 days, with media-agar (1:1) overlays on day 1 and day 3 – 4. Cells were fixed, stained, and virus plaques were counted. Virus titers are reported as PFU per milligram tissue (wet weight) (PFU/mg tissue). Virus titers in serum and saliva are reported as PFU per milliliter of sample.
Histology
Paraffin-embedded tissues sections from sham-inoculated controls, from mice inoculated i.p. or i.g. with MCMV or tcMCMV were obtained from salivary gland, spleen, lung, and liver tissues (lung and liver are not shown). H&E stained sections were used to evaluate pathology. Images from low (10X) and high (40X) magnification on an Olympus CKX41 scope were captured with a Olympus DP12 digital microscope camera (Olympus, Melville, NY) and edited using Adobe Photoshop 5.0 software. Histological evaluation of salivary gland sections was performed by two blinded observers. Histological scoring of salivary gland sections was based relative to a known negative (normal, no infiltrate visible=0) and a known positive (day 21 after i.p. MCMV inoculation, severe infiltrate=3 (>50 foci)). Slides ranked as mild (score=1; <10 foci) showed some infiltrate but were close to normal. Slides ranked as moderate (score=2; 10–50 organized foci, diffuse infiltrates) showed significant infiltrate and were closer to the severe end of the scale.
Immunohistochemistry
Standard protocols for immunohistochemistry in frozen sections were followed according to the manufacturer’s instructions (Vectastain Elite ABC staining kit, Vector Laboratories). Background avidin-biotin interactions in salivary gland were blocked using Vector Avidin/Biotin blocking kit (Vector Laboratories). Tissues were incubated with primary Abs for at least 3 hours. Tissues were incubated with a biotinylated rabbit anti-rat secondary Ab for 1 hour and all other steps were followed according to manufacturer instructions. Positive cells were visualized using DAB substrate (Sigma-Aldrich) and counterstained with hematoxylin. Negative controls included control and infected tissues incubated with secondary Ab only (data not shown). Images from low (10X) and high (40X) magnification on an Olympus CKX41 scope were captured as described above.
ELISA for MCMV Specific Antibodies
Whole blood was collected by cardiac puncture and allowed to clot at room temperature. Serum was separated by spinning tubes for 5 minutes at 14K RPMs in microcentrifuge and removed to new microcentrifuge tubes. A standard ELISA protocol was followed with slight modifications (Castellano et al., 1977; Shanley et al., 1981). Briefly, 96-well plates were coated overnight with 5 μg/ml tcMCMV then blocked with 4% BSA for 2 hours. Plates were washed with PBS/Tween-20 after each incubation. Serum were added at 1:64 dilutions in PBS + 0.1% BSA and incubated at room temperature for 2 hours. Bound, specific Abs in samples were detected using horseradish peroxidase-conjugated, isotype-specific rat anti-mouse Abs against IgM, IgG, and IgA (Southern Biotech, Birmingham, AL). Pierce Immuno Pure PNPP tablets dissolved in 0.2M TRIS Base buffer (pH=9.8) was added at 50 μL/well to plate for 15 minutes at RT with shaking. Absorbance of product formation proportional to bound antibody was measured at 405 nm in a standard plate reader.
Statistical Analysis
Student’s T test with two-tailed distribution and two-sample, unequal variance was performed to determine statistical significance in pair-wise comparisons. Data points were excluded from analysis by using Grubb’s test for detecting outliers.
Results
Infection route has a significant effect on timing and extent of viral replication in several tissues
In order to investigate the potential of the salivary gland to generate a specific immune response using MCMV, it was necessary to develop a model of MCMV infection where viral titers were focused to the salivary gland with minimal systemic organ pathology. Susceptible and resistant strains of mice, various routes of inoculation, viral dosage, and preparations of the virus were variables investigated in order to obtain a model of focused salivary gland infection. Peak viral titers in the salivary gland, spleen, lungs, liver, serum, and saliva and pathology in salivary gland, spleen, and liver were used as criteria to evaluate infection. In resistant C57BL6 mice, irrespective of the route of inoculation or virus preparation (MCMV or tcMCMV), viral titers in all tissues, including salivary glands, were reduced as compared to susceptible (Balb/c) mice (data not shown). However, while reduced viral titers and reduced pathology in systemic tissues was evident, there were not sufficient numbers of infiltrates in the salivary glands of C57BL6 mice to make this a useful model for the study of salivary gland immunity. Preliminary experiments determined that a dose of 105 PFU of either MCMV or tcMCMV produced a detectable infection after i.p. inoculation without causing mortality in Balb/c susceptible mice. Therefore, this dose was used in all of the following experiments to compare i.p. and i.g. routes of viral inoculation for both virus preparations, resulting in four infection protocols: i.p. MCMV, i.p. tcMCMV, i.g. MCMV, and i.g. tcMCMV. Sham inoculations were also carried out for both routes with control antigen. Mice were harvested 0, 2, 4, 7, 14, and 21 days after infection. Viral titers in the salivary gland, spleen, lungs, and liver are plotted for the four infection protocols over a three-week time course (Figure 1).
Figure 1.
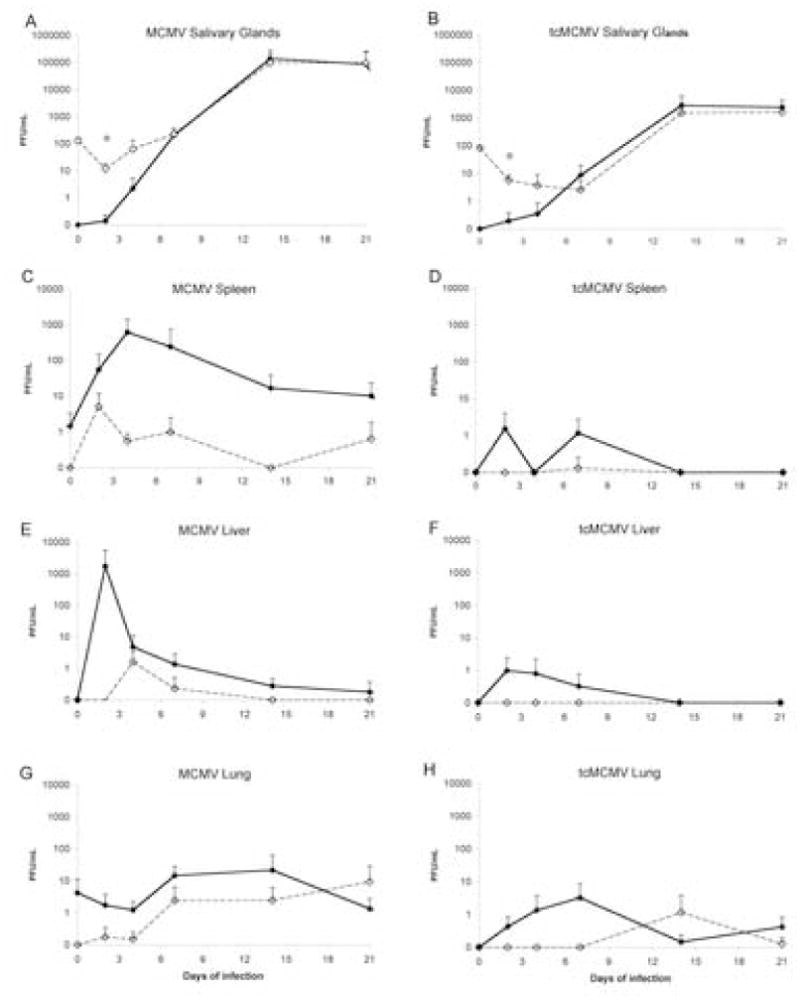
I.g. inoculation of tcMCMV limits viral titers to the salivary glands. Viral titers were determined in (A, B) salivary gland, (C, D) spleen, (E, F) liver, and (G, H) lung from Balb/c mice inoculated i.p (closed symbols, solid lines) or i.g. (open symbols, dashed lines) with MCMV (A, C, E. G) or tcMCMV (B, D, F, H) using a standard plaque assay. PFU/mg tissue sample are shown. Results are pooled averages ± standard deviation from 5–6 mice per time point from 2 – 3 independent experiments. Detection limits were 0.1 PFU/mg for salivary gland, spleen and lung, and 0.2 PFU/mg for liver. *p<0.05, i.p. MCMV vs. i.g. MCMV
Regardless of the route of inoculation (i.p. or i.g.) or virus preparation (tcMCMV or MCMV), the salivary gland was the predominant source of MCMV viral titers after the first week of infection, where viral titers peaked on day 14, and remained high 21 days after inoculation (Figure 1A and 1B). However, after tcMCMV inoculation whether by the i.p or i.g route, viral load in the salivary gland was reduced 100 fold as compared to inoculation with MCMV (Figure 1A and 1B). Peak titers were highly consistent between mice and between inoculation routes on days 14 through 21. While both i.p. MCMV and i.g. tcMCMV inoculation resulted in viral titers in the saliva (Figure 2A), virus was only detected in the serum after i.p. MCMV inoculation (Figure 2B). Following i.p. MCMV inoculation, as expected, virus was detected in all tissues collected (Figure 1). Viral titers in the spleen peaked on day 4 and remained detectable throughout the 3-week time course (Figure 1C). Viral titers in the liver peaked two days after i.p. MCMV inoculation, but quickly diminished and virus was not detected in the liver after the first week of infection (Figure 1E). Virus was also detected in the lung after i.p. MCMV inoculation throughout the time course (Figure 1G). I.g. inoculation with MCMV resulted in similar timing of viral replication in the visceral tissues (Figure 1A, 1C, 1E and 1G), but peak titers were 1–3 logs lower than after i.p. inoculation, with liver titers on day 2 and spleen titers on day 4 most different between the two routes. In tcMCMV treated mice, the effect of inoculation route on tissue titers was similar to that observed with MCMV, except that tcMCMV attenuation resulted in peak titers 1 – 3 logs lower than those produced by the same inoculation route with MCMV (Figure 1B, 1D, 1F, and 1H). The combined reduction in viral replication in the visceral tissues as a result of i.g. inoculation route and virus attenuation meant that virus was below detectable levels in spleen (Figure 1D) and liver (Figure 1F) after i.g. tcMCMV infection, and peak lung titers were very low and close to the plaque assay detection limit (Figure 1H).
Figure 2.
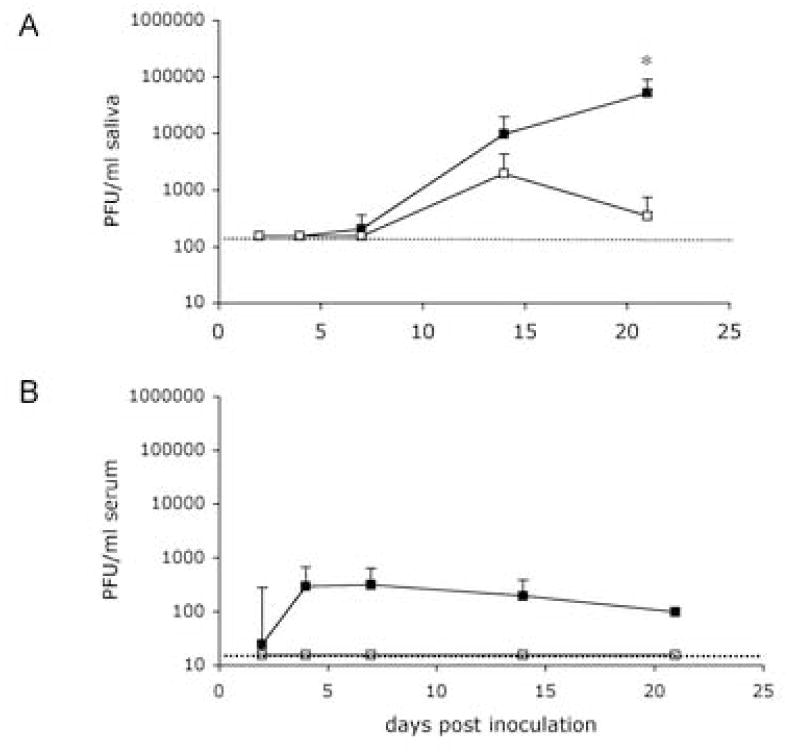
I.g. inoculation of tcMCMV results in viral titers in the saliva but not the serum. Viral titers were determined in (A) saliva or (B) serum in Balb/c mice inoculated i.p with MCMV (closed symbols) or i.g. with tcMCMV (open symbols). PFU/ml fluid are shown. Results are pooled averages ± standard deviation from 5–6 mice per time point from 2 – 3 independent experiments. Detection limits were 100 PFU/ml of saliva, and 50 PFU/ml of serum. *p<0.05, i.p. MCMV vs. i.g. tcMCMV
Infection route determines the pattern of tissue pathology after infection
The pathology of infected mice was assessed by gross observation of spleen morphology, histological examination of salivary gland, spleen, and liver (Table I and Figure 3) and qualitative evaluation of salivary gland infiltrates in H&E stained sections (Table II). Following i.p. MCMV inoculation, on gross observation, mice exhibited splenomegaly and white nodules in the spleen by day 7, and splenic necrosis was evident by day 14 in at least half the animals (not shown). In contrast to i.p. MCMV inoculation, infection following i.g. inoculation with MCMV or tcMCMV or i.p. inoculation with tcMCMV while enlarged spleens were often observed, splenic necrosis was never observed (not shown).
Table I.
Viral and Histological Analysis following MCMV Inoculation
| I.p. | I.g. | |||
|---|---|---|---|---|
| VIRUS PREPARATION | MCMV | tcMCMV | MCMV | tcMCMV |
| SG a | ||||
| 4 | +/− | − | + | + |
| 7 | +/− | + | + | ++ |
| 14 | +++ | + | + | + |
| 21 | +++ | + | ++ | ++ |
| SPLEEN b | ||||
| 4 | − | − | − | − |
| 7 | +++ | − | + | − |
| 14 | +++ | + | ++ | + |
| 21 | ++ | + | ++ | + |
| LIVER c | ||||
| 4 | +++ | + | + | − |
| 7 | ++ | ++ | ++ | − |
| 14 | ++ | + | + | + |
| 21 | ++ | + | + | + |
salivary gland pathology: + + + (severe, >50 foci), + + (moderate, 10–0 organized foci), + (mild, <10 foci), − (normal).
spleen pathology: + + + (severe, necrosis), + + (moderate, some pathology, hyperplasia of white pulp), + (mild, stimulated spleen with secondary follicles), − (normal, unstimulated spleen with primary follicles).
liver pathology: + + + (severe hepatitis, <100 foci), + + (moderate hepatitis, 10–00 foci), + (mild hepatitis, <10 foci), − (normal).
Figure 3.

I.g. tcMCMV inoculation of Balb/c mice reduces spleen pathology and induces salivary gland infiltrates. Spleens (A - F) and salivary glands (G - L) were isolated from sham-inoculated mice (A, D, G, J), mice inoculated i.g. with tcMCMV (B, E, H, K), or i.p. with MCMV (C, F, I, L). H&E stained sections are shown on day 14 post-infection. 10X magnification (A-C, G-I), 40X magnification of A - C are shown in D - F and 40X magnification of G - I are shown in J - L, respectively, as indicated by the boxes. Results are representative of at least 3 independent experiments for each condition. PF=primary follicle, GC=germinal center, WP=white pulp, RP=red pulp, SD=secretory duct, V=blood vessel. Arrows indicate cellular infiltrates. Arrowheads indicate MCMV-infected cells.
Table II.
Infiltrates in the Salivary Gland after MCMV Inoculation
| I.g. tcMCMVa | I.g. MCMVa | I.p. MCMVa | |
|---|---|---|---|
| Controlb | 0.17±0.41c | 0.08±0.20c | 0±0c |
| Day 4 | 1.63±0.48d | 1.42±1.16g | 0.75±05 |
| Day 7 | 1.83±0.41d | 1.08±0.20g | 1.83±0.75 |
| Day 14 | 1.83±0.75d,e | 1.92±0.49g | 2.92±0.20 |
| Day 21 | 2.00±0.89d,e,f | 3.00±0g | 3.00±0 |
H&E stained sections of salivary gland tissues from infected BALB/c mice were compared after i.g. tcMCMV, i.g. MCMV or i.p. MCMV inoculation. Sections were scored by three blinded observers with 2 – 3 slides for each time point. Values were obtained by pooling scores from both observers from each time point.
Mice i.p. sham-inoculated with salivary gland homogenate or i.g. sham-inoculated with 3T12 homogenate were used as controls.
0=normal, 1=mild, 2=moderate, 3=severe (as described in Methods and Materials)
p <0.01. I.g. tcMCMV inoculation as compared to i.g. sham-inoculated control.
p <0.05. I.g. tcMCMV inoculation as compared to i.p. MCMV inoculation.
P<0.05. i.g. tcMCMV inoculation as compared to i.g. MCMV inoculation.
P<0.01. i.g. MCMV inoculation as compared to i.g. sham-inoculated control.
Histological observations of spleens from i.p. (Figure 3A and 3D) or i.g. (not shown) sham-inoculated mice were similar to naive mice. White pulp (WP) and red pulp (RP) are clearly observed and the WP consisted of primary B cell follicles (PF), indicating naïve, unstimulated spleen tissue (Figure 3A and 3D). However, histological evaluation of the spleens after i.p. MCMV inoculation on day 14 revealed extensive destruction of the WP and large numbers of phagocytic cells, dead cells, and debris (Figure 3C and 3F), as previously reported (Ho 1991; Leung et al., 1986). In contrast, unlike the necrotic spleen observed after i.p. MCMV inoculation (Figure 3C and 3F), spleen from i.g. tcMCMV inoculated mice on day 14 contained a high number of secondary B cell follicles with germinal centers (GC), indicating an active immune response (Figure 3B and 3E). Thus, while i.p. MCMV inoculation leads to the destruction of the spleen, the spleen remains intact and capable of participating in an immune response after i.g. tcMCMV inoculation.
Histological evaluation of the salivary gland was also examined for hallmarks of MCMV pathology. Normal salivary gland architecture was observed after either i.p. (Figure 3G and 3J) or i.g. sham-inoculation (data not shown). However, typical hallmarks of MCMV infection (“owl’s eye” type nucleus, viral inclusion bodies, cytomegaly) were present after i.g. tcMCMV inoculation (Figure 3K) as well as after i.p. MCMV inoculation (Figure 3L, arrowheads).
Cellular infiltrates in the salivary glands were also present after either i.g. tcMCMV inoculation (Figure 3H, arrows) or i.p. MCMV inoculation (Figure 3I, arrows). The infiltrates organized into cellular foci and many of these foci were proximal to secretory ducts (SD) (periductal) or blood vessels (V) (perivascular). Many of the cellular foci that were present after i.p. MCMV inoculation grew large enough to coalesce with neighboring foci, which made it difficult to quantitate the number of foci as a measure of salivary gland pathology (Figure 3I). Since quantitative evaluation of pathology was difficult, qualitative scoring of H&E sections of the submandibular gland was blindly evaluated by two independent observers and the results are presented in Table II on a scale of zero (normal) to 3 (severe inflammatory infiltrates, loss of normal tissue architecture). Significant inflammatory infiltrates were present throughout the 21 day time course after i.p. MCMV inoculation (Table 2). I.g. tcMCMV inoculation resulted in significant inflammatory infiltrates in the salivary gland as compared to controls at all time points (p<0.01). Inflammatory infiltrates in the salivary gland after i.g. tcMCMV inoculation remained throughout the 21 day time course. In addition, inflammatory infiltrates in the salivary gland after i.g. MCMV inoculation were also present and at day 21 post-infection were graded significantly higher than infiltrates observed after i.g. tcMCMV inoculation at the same time point (P<0.05).
Both i.p. MCMV and i.g. tcMCMV inoculation result in salivary gland infiltrates composed of B and T cells
Salivary gland infiltrates following sham-inoculation, and the extremes of systemic and focused salivary gland infection (i.p. MCMV and i.g. tcMCMV, respectively), were examined in more detail for differences in the make up of the lymphocytic infiltrates observed in the salivary gland in situ using immunohistochemistry (Figure 4). Frozen sections of salivary gland from sham-inoculated mice were stained with Abs against B cells and macrophages (B220, Figure 4A), helper T cells (CD4, Figure 4B), and cytotoxic T cells (CD8, Figure 4C) and showed little positive staining and no cellular infiltrates. In contrast, i.g. tcMCMV inoculation (Figure 4D - I) and i.p. MCMV inoculation (Figure 4J – O) induced cellular infiltrates containing B220+ B cells and macrophages (Figure 4D, 4G, 4J, and 4M), CD4+ helper T cells (Figure 4E, 4H, 4K, 4N), and CD8+ cytotoxic T cells (Figure 4F, 4I, 4L, and 4O). Based on these three markers, there were no significant differences in the cellular infiltrates induced by either i.p. or i.g. inoculation of virus. In general, these infiltrates localized to the perivascular and periductal regions. Although the submandibular gland is shown and remained the primary site where cellular infiltrates were found (Figure 4), similar lymphocytic infiltrates were also seen in the sublingual and less frequently in the parotid glands of mice infected i.g. with tcMCMV or i.p. with MCMV (data not shown).
Figure 4.
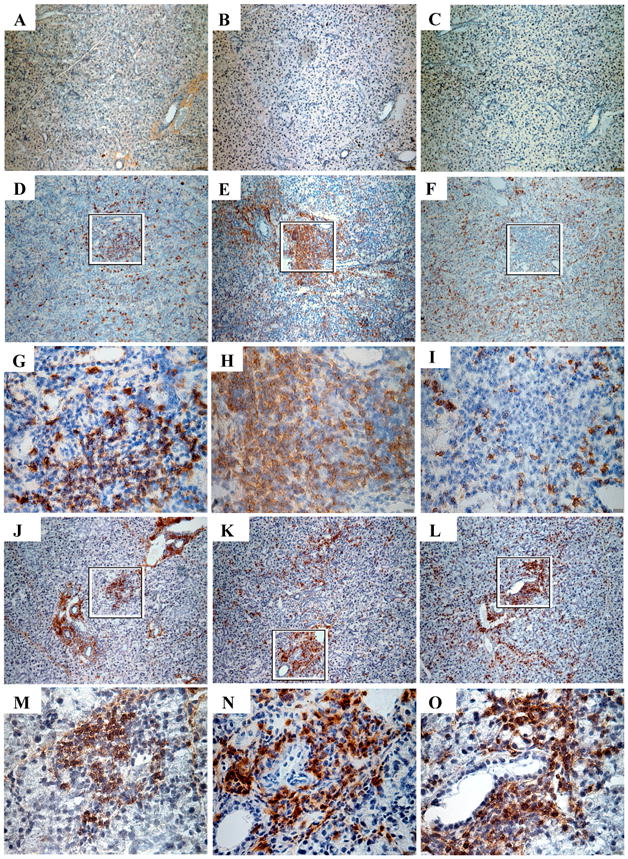
I.p. MCMV and i.g. tcMCMV inoculation result in salivary gland infiltrates composed of B and T cells. Salivary glands on day 14 from sham inoculated mice (A - C) or mice inoculated i.g. with tcMCMV (D - I) or i.p. with MCMV (J - O) were stained with Abs against B220 (A, D, G, J, M), CD4 (B, E, H, K, N), or CD8 (C, F, I, L, O). 10X magnification (A - F, J - L), 40X magnification (G - I, M - O) from the indicated boxed regions are shown. Results are representative of at least 3 independent experiments for each condition.
Both i.p. MCMV and i.g. tcMCMV inoculation result in MCMV-specific IgM and IgG in the serum during MCMV infection
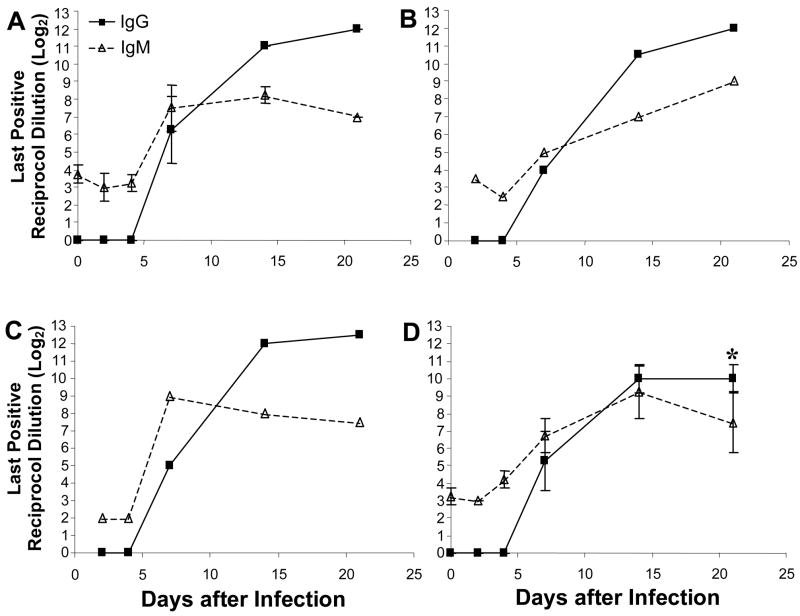
In order to determine the efficacy of i.g. inoculation with tcMCMV in stimulating systemic immunity, we measured MCMV specific IgM, IgG, and IgA Abs in the serum following both i.g. and i.p. inoculation with either MCMV or tcMCMV. No IgA in serum was detected at any time point or inoculation route. However, regardless of route or virus preparation, MCMV-specific IgM and IgG Abs were detected in serum of infected BALB/c mice (Figure 5). Serum IgM peaked on day 7 or 14 after most infections, with the exception of i.p. inoculation with tcMCMV during which serum IgM rose steadily until day 21 (Figure 5B). Interestingly, sera antibody kinetics were nearly identical for i.p. inoculation with MCMV and i.g. inoculation with MCMV (Figure 5A and 5C). Analysis of serum antibody following i.g. inoculation with tcMCMV revealed a robust systemic immune response with immunoglobulin levels and kinetics that were not significantly different from those detected after i.p. routes of infection, except for IgG on day 28 (Figure 5D).
Figure 5.
I.p. and i.g. inoculation of either MCMV or tcMCVM results in MCMV specific IgM and IgG serum Abs. BALB/c mice were infected either i.p (A, B) or i.g. (C, D) with either MCMV (A, C) or tcMCMV (B, D) and MCMV-specific Abs in serum was isotyped for IgM and IgG. Samples with OD>0.200 above background were considered positive and the last positive reciprocal dilution (log2) is shown. Detection limit for IgM was slightly higher than IgG and values of 2 – 3 were typically obtained in control samples. Results are from 1 representative experiment each: i.p. MCMV (N=3 mice/time point), i.p. tcMCMV (N=2 mice/time point), i.g. MCMV (N=2 mice/time point), i.g. tcMCMV (N=4 mice/time point). *p<0.05, compared to same time point after i.p. inoculation with MCMV; all other time points after i.g. inoculation with tcMCMV were not significantly different from i.p. inoculation with MCMV.
Discussion
The nature of the common mucosal immune system allows that immunization at one mucosal surface results in protective immunity at mucosal sites both proximal as well as distal to the original site of immunization. While the salivary gland has been recognized as an important effector site of the common mucosal immune system, a useful model for studying anti-viral salivary gland immune responses in vivo and for exploring the role of the salivary gland within the mucosal immune system has been lacking. These studies were aimed at developing a model system by which we could investigate the role of the salivary gland as a component of the common mucosal immune system. Susceptible and resistant strains of mice (susceptible Balb/c and resistant C57BL6), various routes of inoculation (i.p., i.g., intranasal (i.n.), and periglandular (p.g.)), and preparations of the virus (MCMV and tcMCMV) were variables investigated in order to obtain a model of focused salivary gland infection. In resistant C57BL6 mice, irrespective of the route of inoculation or virus preparation (MCMV or tcMCMV), viral titers in all tissues, including salivary glands, were reduced as compared to susceptible (Balb/c) mice (data not shown). However, while reduced viral titers and reduced pathology in systemic tissues was evident, there were not sufficient numbers of infiltrates in the salivary glands of C57BL6 mice to make this a useful model for the study of salivary gland immunity. Here, using i.g. tcMCMV inoculation to obtain a focused salivary gland infection in susceptible Balb/c mice, we were able to (i) limit viral replication to the salivary gland, (ii) prevent systemic organ pathology associated with MCMV infection, and (iii) stimulate a MCMV virus-specific humoral immune response. Therefore, by focusing the infection to the salivary gland, both mucosal and systemic immune responses to MCMV can now be studied without complications due to systemic pathology commonly seen following systemic MCMV inoculation protocols.
I.g. tcMCMV inoculation selectively lowers viral titers in systemic tissues and serum while maintaining virus replication in the salivary gland and shedding in saliva. In addition, following i.g. tcMCMV inoculation, perivascular and periductal cellular foci within the salivary gland were comprised of B220+ cells (B cells and macrophages) capable of antigen presentation, CD4+ TH lymphocytes, and CD8+ cytotoxic T cells (Fernandez et al., 1999; Weekes et al., 1999; Ye et al., 2002). These results suggest that the initial MCMV immune response could potentially occur within the salivary gland. It is also well known that CD4+ T cells are essential for clearance of MCMV in salivary glands (Lucin et al., 1992; Koszinowski, 1991; He et al., 1995) and this critical subset of T cells is present in the salivary glands after i.g. tcMCMV inoculation. In addition, it has been recently reported that MCMV-specific CD8 cells are present in the salivary gland and draining PGLN following i.p. MCMV inoculation using MHC class I tetramers loaded with MCMV peptide (Cavanaugh et al., 2003). However, further in situ studies after i.g. tcMCMV inoculation to detect viral antigen and MCMV-specific CD8+ T cells using MHC tetramers are required to address this issue directly. It is interesting to note that CD8 staining of salivary gland infiltrates was diffuse and not necessarily associated with cellular foci following systemic i.p. MCMV inoculation. It is likely, evidenced by high salivary gland viral titers that widespread infection of the salivary gland resulted in presentation to and engagement of CD8+ cytotoxic T cells throughout the gland. In contrast, i.g. tcMCMV inoculation resulted in CD8 staining associated primarily with periductal and perivascular cellular foci. Focal infiltrates were also seen with all other routes (i.n., p.g.), virus preparations, and mouse strains (data not shown). It is not clear what factors may be at work to organize salivary gland infiltrates into discrete cellular foci. It is possible that infection through i.n., p.g., and i.g. routes may be able to elicit help from cells in the mucosal immune system. It is well known that a major role of immunity at mucosal sites (i.e. lungs and salivary gland in the case of i.n., p.g., and i,g, inoculation) is tolerance to environmental antigens. Antigen encountered at mucosal surfaces does not necessarily invoke an inflammatory immune response. This dampened ability to cause inflammation may play a role in organization of salivary gland infiltrates. These observations further indicate that i.g. tcMCMV inoculation results in a more restricted salivary gland infection associated primarily with the infiltrating lymphocyte foci.
We also demonstrated that infecting mice with tcMCMV reduces viral titers and pathology in all tissues compared to MCMV infection, regardless of the route of infection. Hepatitis was lessened in mice after i.p. infection with tcMCMV in contrast to i.p. infection with MCMV. This illustrates the reduced ability of tcMCMV to cause pathology in systemic tissues (Fernandez et al., 1999, Weekes et al., 1999, Olver et al., 1994; Olver et al., 1998). It is thought that the mechanism of attenuation is a difference in proteins and other moieties that decorate the surface of the virus when the virus is grown in tissue culture versus the salivary gland that may affect the ability of the virus to infect target cells (Ravindranath and Graves, 1990). It has also been shown that preparations of tissue-culture derived MCMV contain more incomplete virions (noninfectious “naked” capsids, multicapsid virions, and fragments of viral genome) compared to MCMV (Chong et al., 1981). Although the number of PFUs was the same for MCMV versus tcMCMV infections in our experiments, additional viral byproducts are not equalized between these two treatments. Non-infectious material may act as an “adjuvant” to boost anti-MCMV responses, which, in turn, reduces viral titers and pathology. Salivary gland infiltrates appear to be less extensive after using tcMCMV than those seen after MCMV infection. Attenuation of virus by tissue culture passage seems to alter the pathogenicity of the virus in the spleen but not its ability to productively infect the salivary gland when administered by the same route (Tonari and Minamishima, 1983; Manning et al., 1992; Lee et a, 200; Jordan and Takagi, 1983; Osborn and Walker, 1970). It is also likely that tcMCMV is “converted” to salivary gland-derived MCMV once the virus infects the salivary gland, begins to replicate, and is disseminated to other organs. It has been shown that MCMV derived from other tissues, such as the spleen, is attenuated in its ability to cause morbidity and mortality in mice (Jordan and Takagi, 1983).
Alternate routes of infection have been utilized with MCMV in an attempt to lessen systemic organ pathology and to study various aspects of the disease process caused by MCMV infection. MCMV infection in mice following i.n. administration of MCMV and immunosuppression with cyclophosphamide has been found to mimic pneumonitis often found in humans following organ transplants (Shanley et al., 1982; Brody and Craighead, 1974). In contrast, when immunosuppression is not used, pathology in systemic organs is lessened following i.n. MCMV inoculation compared to pathology after i.p. MCMV inoculation (Shanley et al., 1982, Jordan, 1978; Shanley et al., 1997). Therefore, using routes of infection in contrast to i.p. MCMV infection without the complications of systemic organ pathology would allow the study of the immune responses of the salivary gland and other tissues. We have evaluated two other routes of inoculation with MCMV, the i.n. and periglandular (p.g.) routes (data not shown), in addition to the i.p. and i.g. routes of inoculation. While p.g. inoculation was effective at limiting viral titers to the salivary gland, this route did not prevent mild hepatitis in susceptible Balb/c susceptible mice. In contrast, i.n. and i.g. routes of inoculation were effective at limiting viral titers to the salivary gland and lungs. However, only i.g. inoculation with tcMCMV was effective at preventing splenic necrosis and hepatitis while limiting viral titers to the salivary gland and maintaining sufficient salivary gland infiltrates. While it was obvious why virus was found in the lungs after i.n. infection with MCMV, it was not immediately apparent why the lungs would contain virus after p.g. and i.g. infections. It is possible that virus was able to enter the lungs through the network of lymph nodes that drain the head, neck, and lungs. The superficial cervical lymph nodes, or periglandular lymph nodes, drain the salivary gland and parts of the head and neck, while the deep cervical lymph nodes drain the neck and parts of the chest (Tilney, 1971). It has also been shown by a number of trafficking experiments that lymphocytes that are stimulated in the head and neck (termed cranial, oral, and nasal associated lymphoid tissue or CONALT) can circulate into the lungs (Csencsits et al., 2002, Csencsits and Pascual, 2002; Csencsits et al., 2001; Csencsits et al., 1999). Therefore, it is possible that infected lymphocytes from the salivary gland or in the cervical lymph nodes could have spread the virus to the lungs after i.g. and p.g. infection. In support of this idea, most of the initial viral inoculum can be recovered from the salivary gland on the same day of i.g. injection of virus, but on day 2 after i.g. infection with MCMV, salivary gland viral titers drop before rising again on day 4 (data not shown). This delay is slightly more prolonged after i.g. inoculation with tcMCMV. Viral titers drop until 7 days after infection when titers then begin to rise (data not shown). This delay may be due to a number of factors including the draining of virus or virally infected cells to the lymph nodes, innate mechanisms of viral clearance, or the lag phase between uncoating and production of progeny virions. In addition, the prolonged delay after i.g. inoculation with tcMCMV may be a result of an infection that is less efficient due to the “conversion” of tcMCMV to salivary gland-derived MCMV. This conversion may be necessary for the virus to spread to other tissues or infect cells that may traffic to other tissues. By evaluating these additional routes of MCMV infection, we have initially characterized a spectrum of animal models that may be utilized to address questions concerning MCMV infection. For example, systemic infection can be accomplished with reduced systemic organ pathology by i.p. inoculation with tcMCMV rather than salivary gland-derived MCMV. I.g. and p.g. inoculation can be used to answer questions regarding the trafficking of MCMV-specific lymphocytes to other mucosal and systemic sites following salivary gland inoculation. In addition, i.n. inoculation may also be used to understand the nature of lymphocyte trafficking between the lungs and salivary glands during MCMV infection. Further molecular studies of virus isolated from different tissues versus tissue-culture derived virus and trafficking studies of infected cells from salivary glands would have to be done to fully understand these phenomena.
In conclusion, i.g. inoculation of BALB/c mice with tcMCMV is the best method for producing a focused salivary gland infection model in mice. This mode of experimental infection resulted in the lowest viral titers in spleen, lungs, and liver while still maintaining a significant number of infiltrating cells in the salivary gland for future immunological studies. In addition, our initial characterization of other additional routes of MCMV infection have created a spectrum of animal models of infection that can be used to answer a variety of questions about immunity to MCMV. Since mucosal surfaces of the body are a significant portal of entry for many pathogens, including viruses, understanding the mechanisms of defense in these tissues is an important endeavor in vaccine development (Jackson et al., 1981; Wu and Russell, 1993; Bergmeier et al., 1995; Kawabata et al., 1999; Tucker et al., 2003, Tucker et al., 2004). Mucosally administered vaccines are of interest since induction of an immune response at one mucosal site may lead to protection at multiple mucosal sites within the context of a common mucosal immune system. Therefore, methods for induction of immune responses in the common mucosal immune system are an important field of investigation and characterizing inductive sites in this system will lead to further advances in the development of vaccines for pathogens that impinge on mucosal surfaces. In addition, since herpesviruses are thought to be transmitted via respiratory and/or oral secretions (i.e. saliva), developing an animal model to study anti-viral immunity in the salivary glands could be an important step in understanding and optimizing mucosal and systemic immunity to herpesviruses involved in their natural route of transmission.
Acknowledgments
The authors would like to thank Drs. Edward Balish, and Amy Hodson-Thompson for critical review of the manuscript, Drs. Brad Neville and William Tyor for histological scoring presented in Table I, Margaret Romano for preparation of H & E slides, and Dr. Damir Hamamdzic for helpful advice. The authors would also like to dedicate this paper in memory of Dr. Carwile LeRoy (1933 – 2002), Professor Emeritus (2001 – 2002) and Chairman of the Department of Microbiology and Immunology, MUSC (1995 – 2001), who provided his insightful observations in these studies.
Abbreviations
- Ab
antibody
- GC
germinal centers
- i.g
intraglandular
- i.n
intranasal
- i.p
intraperitoneal
- tcMCMV
tissue culture-derived MCMV
- MCMV
murine cytomegalovirus
- MLN
mesenteric lymph nodes
- MOI
multiplicity of infection
- PF
primary B cell follicles
- PFU
plaque forming unit
- p.g
periglandular
- PGLN
periglandular lymph nodes
- PLN
peripheral lymph nodes
- RP
red pulp
- SD
secretory ducts (periductal)
- V
blood vessels (perivascular)
- WP
White pulp
Footnotes
This work was supported in part by the Medical University of South Carolina Institutional Research Funds by NIH/NIDCR grant number 1R01 DE016652-01 (SDL) and NIH grant No. CO6 RR015455 (MUSC).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allan JE, Shellam GR. Genetic control of murine cytomegalovirus infection: virus titers in resistant and susceptible strains of mice. Arch Virol. 1984;81(1–2):139–150. doi: 10.1007/BF01309303. [DOI] [PubMed] [Google Scholar]
- Bergmeier LA, Tao L, Gearing AJM, Adams S, Lehner T. Induction of IgA and IgG antibodies in vaginal fluid, serum and saliva following immunization of genital and gut associated lymphoid tissue. In: Mestecky J, editor. Advances in Mucosal Immunology. Plenum Press; New York: 1995. pp. 1567–1573. [PubMed] [Google Scholar]
- Britt WJ, Alford CA. Cytomegalovirus. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. Lippincott-Raven; Philadelphia: 1996. pp. 2493–2523. [Google Scholar]
- Brody AR, Craighead JE. Pathogenesis of pulmonary cytomegalovirus infection in immunosuppressed mice. J Infect Dis. 1974;129(6):677–89. doi: 10.1093/infdis/129.6.677. [DOI] [PubMed] [Google Scholar]
- Castellano G, Hazzard, Madden DL, Sever JL. Comparison of the enzyme-linked immunosorbent assay and the indirect hemagglutination test for detection of antibody to cytomegalovirus. J, Infect, Dis. 1977;136:S337–S340. doi: 10.1093/infdis/136.supplement_2.s337. [DOI] [PubMed] [Google Scholar]
- Cavanaugh VJ, Deng Y, Birkenbach MP, Slater JS, Campbell AE. Vigorous innate and virus-specific cytotoxic T-lymphocyte responses to murine cytomegalovirus in the submaxillary salivary gland. J Virol. 2003;77:1703–1717. doi: 10.1128/JVI.77.3.1703-1717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong KT, Gould JJ, Mims CA. Neutralization of different strains of murine cytomegalovirus (MCMV)-effect of in vitro passage. Arch Virol. 1981;69(2):95–104. doi: 10.1007/BF01315153. [DOI] [PubMed] [Google Scholar]
- Csencsits KL, Jutila MA, Pascual DW. Nasal-associated lymphoid tissue: phenotypic and functional evidence for the primary role of peripheral node addressin in naive lymphocyte adhesion to high endothelial venules in a mucosal site. J Immunol. 1999;163(3):1382–9. [PubMed] [Google Scholar]
- Csencsits KL, Jutila MA, Pascual DW. Mucosal addressin expression and binding-interactions with naive lymphocytes vary among the cranial, oral, and nasal-associated lymphoid tissues. Eur J Immunol. 2002;32(11):3029–39. doi: 10.1002/1521-4141(200211)32:11<3029::AID-IMMU3029>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Csencsits KL, Pascual DW. Absence of L-selectin delays mucosal B cell responses in nonintestinal effector tissues. J Immunol. 2002;169(10):5649–59. doi: 10.4049/jimmunol.169.10.5649. [DOI] [PubMed] [Google Scholar]
- Csencsits KL, Walters N, Pascual DW. Cutting edge: dichotomy of homing receptor dependence by mucosal effector B cells: alpha(E) versus L-selectin. J Immunol. 2001;167(5):2441–5. doi: 10.4049/jimmunol.167.5.2441. [DOI] [PubMed] [Google Scholar]
- Fernandez JA, Zavala F, Tsuji M. Phenotypic and functional characterization of CD8+ T cell clones specific for a mouse cytomegalovirus epitope. Virol. 1999;255:40–49. doi: 10.1006/viro.1998.9575. [DOI] [PubMed] [Google Scholar]
- He X, Yoshida H, Minamishima Y, Nomoto K. Analysis of the role of CD4+ T-cells during murine cytomegalovirus infection in different strains of mice. Virus Res. 1995;36(2–3):233–45. doi: 10.1016/0168-1702(95)00010-n. [DOI] [PubMed] [Google Scholar]
- Ho M. Murine Cytomegalovirus. In: Ho M, editor. Cytomegalovirus: Biology and Infection. Plenum Medical Book Co; New York: 1991. pp. 327–353. [Google Scholar]
- Jackson DE, Lally ET, Nakamura MC, Montgomery PC. Migration of IgA-bearing lymphocytes into salivary glands. Cell Immunol. 1981;63:203–209. doi: 10.1016/0008-8749(81)90042-3. [DOI] [PubMed] [Google Scholar]
- Jordan MC. Interstitial pneumonia and subclinical infection after intranasal inoculation of murine cytomegalovirus. Infection and Immunity. 1978;21(1):275–280. doi: 10.1128/iai.21.1.275-280.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan MC, Takagi JL. Virulence characteristics of murine cytomegalovirus in cell and organ cultures. Infection and Immunity. 1983;41:841–843. doi: 10.1128/iai.41.2.841-843.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata S, Terao Y, Fujiwara T, Nakagawa I, Hamada S. Targeted salivary gland immunization with plasmid DNA elicits specific salivary immunoglobulin A and G antibodies and serum immunoglobulin G antibodies in mice. Infection and Immunity. 1999;67:5863–5868. doi: 10.1128/iai.67.11.5863-5868.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszinowski UH. Molecular aspects of immune recognition of cytomegalovirus. Transplantation Proceedings. 1991;23(3 Suppl 3):70–74. [PubMed] [Google Scholar]
- Lee SH, Girard S, Macina D, Busa M, Zafer A, Belouchi A, Gros P, Vidal SM. Susceptibility to mouse cytomegalovirus is associated with deletion of an activating natural killer cell receptor of the C-type lectin superfamily. Nature Genetics. 2001;28(1):42–5. doi: 10.1038/ng0501-42. [DOI] [PubMed] [Google Scholar]
- Lee M, Xiao J, Haghjoo E, Zhan X, Abenes G, Tuong T, Dunn W, Liu F. Murine cytomegalovirus containing a mutation at open reading frame M37 is severely attenuated in growth and virulence in vivo. J Virol. 2000;74:11099–11107. doi: 10.1128/jvi.74.23.11099-11107.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung WCT, Hashimoto K, Hata J. Murine cytomegalovirus infection model in Balb/c mice: 1. Virological and pathological profiles in mice inoculated with various doses. Tokai J Exper Clin Med. 1986;11:293–301. [PubMed] [Google Scholar]
- Lucin PI, Pavic B, Polic S, Jonjic U, Koszinowski H. Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J Virol. 1992;66:1977–1984. doi: 10.1128/jvi.66.4.1977-1984.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald MR, Li X-Y, Stenberg RM, Campbell AE, Virgin HW., IV Mucosal and parenteral vaccination against acute and latent murine cytomegalovirus (MCMV) infection by using an attenuated MCMV mutant. J Virol. 1998;72(1):442–451. doi: 10.1128/jvi.72.1.442-451.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning WC, Stoddart CA, Lagenaur LA, Abenes GB, Mocarski ES. Cytomegalovirus determinant of replication in salivary glands. J Virol. 1992;66:3794–3802. doi: 10.1128/jvi.66.6.3794-3802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olver SD, Price P, Shellam GR. Cytomegalovirus hepatitis: characterization of the inflammatory infiltrate in resistant and susceptible mice. Clin Exper Immunol. 1994;98:375–381. doi: 10.1111/j.1365-2249.1994.tb05500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olver SD, Price P. Contrasting phenotypes of liver-infiltrating leucocytes isolated from MCMV-infected BALB/c and C57BL/6 mice. Intern J Exper Path. 1998;79(1):33–46. [PMC free article] [PubMed] [Google Scholar]
- Osborn JE, Walker DL. Virulence and attenuation of murine cytomegalovirus. Infection and Immunity. 1970;3:228–236. doi: 10.1128/iai.3.2.228-236.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindranath RMH, Graves MC. Attenuated murine cytomegalovirus binds to N-acetylglucosamine, and shift to virulence may involve recognition of sialic acids. J Virol. 1990;64:5430–5440. doi: 10.1128/jvi.64.11.5430-5440.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DH, Kornstein MJ, Anderson AO. Reovirus serotype 1 intestinal infection: a novel replicative cycle with ileal disease. J Virol. 1985;53:391–398. doi: 10.1128/jvi.53.2.391-398.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley JD, Pesanti EL, Nugent KM. The pathogenesis of pneumonitis due to murine cytomegalovirus. J Infect Dis. 1982;146(3):388–396. doi: 10.1093/infdis/146.3.388. [DOI] [PubMed] [Google Scholar]
- Shanley JD, Thrall RS, Forman SJ. Murine cytomegalovirus replication in the lungs of athymic BALB/c nude mice. J Infect Dis. 1997;175:309–315. doi: 10.1093/infdis/175.2.309. [DOI] [PubMed] [Google Scholar]
- Shanley JD, Jordan MC, Stevens JG. Modification by adoptive humoral immunity of murine cytomegalovirus infection. J Infect Dis. 1981;143:231–237. doi: 10.1093/infdis/143.2.231. [DOI] [PubMed] [Google Scholar]
- Tilney NL. Patterns of lymphatic drainage in the adult laboratory rat. J Anat. 1971;109(3):369–383. [PMC free article] [PubMed] [Google Scholar]
- Tonari Y, Minamishima Y. Pathogenicity and immunogenicity of temperature-sensitive mutants of murine cytomegalovirus. J Gen Virol. 1983;64:1983–1990. doi: 10.1099/0022-1317-64-9-1983. [DOI] [PubMed] [Google Scholar]
- Tucker SN, Lin K, Stevens S, Scollay R, Bennett MJ, Olson DC. Systemic and mucosal antibody responses folowing retroductal gene transfer to the salivary gland. Mol Ther. 2003;8:392–399. doi: 10.1016/s1525-0016(03)00180-1. [DOI] [PubMed] [Google Scholar]
- Tucker SN, Lin K, Stevens S, Scollay R, Bennett MJ, Olson DC. Salivary gland genetic vaccination: a scalable technology for promoting distal mucosal immunity and heightened systemic immune responses. Vaccine. 2004;22:2500–2504. doi: 10.1016/j.vaccine.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Weekes MP, Wills MR, Mynard K, Carmichael AJ, Sissons JG. The memory cytotoxic T-lymphocyte (CTL) response to human cytomegalovirus infection contains individual peptide-specific CTL clones that have undergone extensive expansion in vivo. J Virol. 1999;73(3):2099–108. doi: 10.1128/jvi.73.3.2099-2108.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HY, Russell MW. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with the cholera toxin B subunit. Infection and Immunity. 1993;61:314–322. doi: 10.1128/iai.61.1.314-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M, Morello CS, Spector DH. Strong CD8 T-cell responses following co-immunization with plasmids expressing the dominant pp89 and subdominant M84 antigens of murine cytomegalovirus correlate with long-term protection against subsequent viral challenge. J Virol. 2002;76(5):2100–2112. doi: 10.1128/jvi.76.5.2100-2112.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]