Abstract
The kinetics of phototransduction of phytochrome A (phyA) and phytochrome B (phyB) were compared in etiolated Arabidopsis thaliana seedlings. The responses of hypocotyl growth, cotyledon unfolding, and expression of a light-harvesting chlorophyll a/b-binding protein of the photosystem II gene promoter fused to the coding region of β-glucuronidase (used as a reporter enzyme) were mediated by phyA under continuous far-red light (FR) and by phyB under continuous red light (R). The seedlings were exposed hourly either to n min of FR followed by 60 minus n min in darkness or to n min of R, 3 min of FR (to back-convert phyB to its inactive form), and 57 minus n min of darkness. For the three processes investigated here, the kinetics of phototransduction of phyB were faster than that of phyA. For instance, 15 min R h−1 (terminated with a FR pulse) were almost as effective as continuous R, whereas 15 min of FR h−1 caused less than 30% of the effect of continuous FR. This difference is interpreted in terms of divergence of signal transduction pathways downstream from phyA and phyB.
In higher plants phytochrome is a family of photoreceptors, the apoproteins of which are encoded by divergent genes (Clack et al., 1994). In Arabidopsis thaliana, phyA and phyB are the most abundant members of the family and their deficiencies are most evident under continuous FR or R, respectively (Reed et al., 1994; Quail et al., 1995). Phytochromes exhibit three modes of responses: VLFR, LFR, and HIR. The HIR operate under continuous FR and are mediated by phyA (Nagatani et al., 1993; Parks and Quail, 1993; Whitelam et al., 1993). The LFR operate under pulsed or continuous R and are mediated by phyB (Botto et al., 1995; Mazzella et al., 1997). The VLFR are mediated by phyA, do not require continuous light, and are induced by R, FR, or any wave band between 300 and 800 nm (Botto et al., 1996; Shinomura et al., 1996; Mazzella et al., 1997).
Although phyA and phyB activities occur under different light conditions, the end-point responses (e.g. hypocotyl growth, cotyledon unfolding, flowering, etc.) controlled by phyA and phyB are largely the same. The phototransduction pathways of phyA and phyB obviously converge at some point. The relative position of the point of convergence is not known, and this is a matter of current debate. Ahmad and Cashmore (1996) have proposed that phyA and phyB share the same reaction partner. Their view is based on the observations that the carboxy-terminal domain of phyA and phyB bear a small, common region important for signal transduction (Quail et al., 1995; Wagner and Quail, 1995; Xu et al., 1995) and that mutants such as pef1 affect both phyA- and phyB-mediated responses (Ahmad and Cashmore, 1996). Wagner et al. (1997) recently proposed the hypothesis of specific pathways of signal transduction downstream from phyA and phyB. This possibility is supported by the observations that loci such as fhy1, fhy3 (Whitelam et al., 1993), vlf1, and vlf2 (Yanovsky et al., 1997) affect phyA- but not phyB-mediated responses, whereas pef2, pef3 (Ahmad and Cashmore, 1996), and red1 (Wagner et al., 1997) affect phyB- but not phyA-mediated responses. Since regulatory elements present in the amino-terminal domain differ between phyA and phyB (Wagner et al., 1996), these domains could be involved in recognition of specific cognate partners (Quail, 1997; Wagner et al., 1997). Confirmation of the latter view would require the identification of the function of the products of FHY1, FHY3, PEF2, PEF3, and RED1 in the transduction chains of phyA or phyB.
In vivo dark reversion can be observed in both dark- and light-grown tissue (Mancinelli, 1994). In vitro phyA and phyB Pfr dark revert to Pr (Kunkel et al., 1996; Braslavsky et al., 1997; Ruddat et al., 1997). In a strict sense, with available information it cannot be fully excluded that fhy1, fhy3, pef2, pef3, and red1 enhance dark reversion of Pfr to Pr to rates not fully compensated by light fields of standard irradiance. If this were the case, fhy1, fhy3, pef2, pef3, and red1 would reduce the levels of active phyA or phyB rather than affecting their transduction chains. Although it is unlikely that all of these mutations affect dark reversion, complementary approaches should help to test independently the extended view that the transduction chains of phyA and phyB are different at some point.
One of the predictions of the hypothesis that the transduction chains of phyA and phyB are different is that, at least under certain conditions, the time required by active phyA or active phyB to complete its action should be different, i.e. it is unlikely that two different pathways have exactly the same kinetics. The purpose of this work was to test this prediction in etiolated Arabidopsis seedlings.
MATERIALS AND METHODS
Seeds of Arabidopsis thaliana (L.) Heynh of the ecotype Columbia, and of the phyA-211 (Reed et al., 1994) and phyB-9 (Reed et al., 1993) mutants (both in the Columbia background), were provided by the Arabidopsis Biological Research Center (Columbus, OH). Seeds of the Columbia ecotype carrying a transgenic Arabidopsis Lhcb1*2 promoter fused to the GUS gene (line pOCA 107–2, Susek et al., 1993) were kindly provided by Dr. Joanne Chory (The Salk Institute, La Jolla, CA). The phyA-211 mutant was obtained from the pOCA 107–2 line (Reed et al., 1994). The phyB-9 mutant was crossed to the pOCA 107–2 line and tall seedlings were selected in the F2 generation under continuous R from plates containing kanamycin. Homozygosity was indicated by the lack of segregation in subsequent generations.
Approximately 15 (growth and cotyledon-unfolding experiments) or 30 seeds (gene-expression experiments) of a given genotype were sown in clear, plastic boxes (40 × 33 mm2 × 15 mm in height) on 3 mL of 0.8 g/100 mL agar. The boxes were incubated in darkness at 7°C for at least 3 d and given a R pulse followed by darkness to induce germination. The light treatments started 1 d (hypocotyl-growth and cotyledon-unfolding experiments) or 2 d (Lhcb1*2 gene expression) after the R pulse and continued for 3 d or for 20 h, respectively. R (10 μmol m−2 s−1) was provided by light-emitting diodes and FR (15 μmol m−2 s−1) was provided by incandescent bulbs in combination with water filters, a red acetate filter, and six blue acrylic filters (model 2031, Paolini, La Casa del Acetato, Buenos Aires, Argentina). When R was followed by a short FR pulse (3 min, 100 μmol m−2 s−1), R was given from below the boxes and FR was given from above.
Hypocotyl length was measured to the nearest 0.5 mm with a ruler and the largest 10 seedlings of each box (i.e. one replicate) were averaged. The angle between the cotyledons was measured with a protractor using the same seedlings that were used for length measurements, and the 10 values obtained per box were also averaged before statistical analysis. For the measurements of GUS activity the plants were harvested under a dim-green light, homogenized in 50 μL of ice-cooled extraction buffer, and microcentrifuged at 4°C. The supernatant was stored at −80°C (usually less than 1 week) and GUS activity was measured according to the method of Jefferson et al. (1987), using 4-methylumbelliferyl-β-d-glucuronide (Sigma) as a substrate. The standard curves were prepared with 4-methylumbelliferone (Sigma). Protein content was measured according to the method of Lowry et al. (1951).
RESULTS
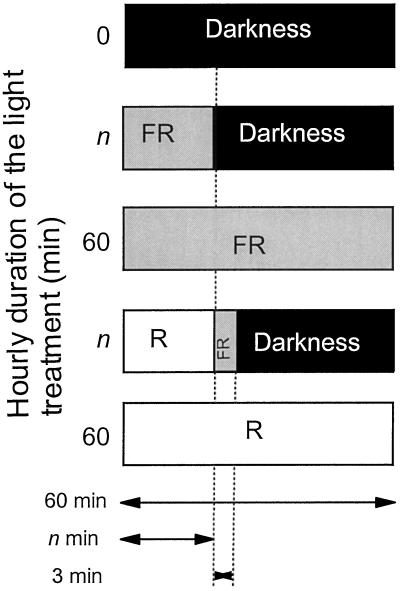
To investigate the phototransduction kinetics of phyA and phyB, WT seedlings were exposed hourly for 3 d (hypocotyl growth and cotyledon unfolding) or for 20 h (Lhcb1*2 gene expression) to either (a) n min FR followed by 60 minus n min in darkness or (b) n min of R, 3 min of FR (to photoconvert phyB Pfr to Pr), and 57 minus n min of darkness (Fig. 1). The extreme conditions were darkness, continuous FR, or continuous R (in which case the 3-min FR pulse was not given). Both phyA and phyB mutants were also grown in darkness, continuous R, and continuous FR. All of the experiments were conducted in the Columbia ecotype plants (Yanovsky et al., 1997) because in Landsberg the phyB mutant shows residual responses to R (or FR pulses) because of a VLFR mediated by phyA (Mazzella et al., 1997; Smith et al., 1997). Thus, during the dark period of each hourly cycle no phytochrome activity was predicted: the VLFR was absent because of the use of the ecotype Columbia, the induction of LFR was canceled because of Pfr removal by the FR pulse, and HIR are assumed not to operate in darkness (given the nature of the results, this assumption will have no consequences in terms of interpretation). In Sinapis alba (Heim and Schäfer, 1982) and A. thaliana (Mazzella et al., 1997), short (3–5 min) hourly pulses of R are known to substitute for continuous R. However, in previous experiments the exposures to R were not followed by a FR pulse to remove phyB Pfr that is known to be relatively stable. Thus, the kinetics of phototransduction of phyB (as well as phyA) cannot be estimated from already available data.
Figure 1.
Schematic representation of the hourly experimental protocol. In hypocotyl-growth and cotyledon-unfolding experiments, 1-d-old seedlings were exposed to the indicated protocol for 3 d. In gene-expression experiments, 2-d-old seedlings were exposed to the indicated protocol for 20 h and harvested 24 h later.
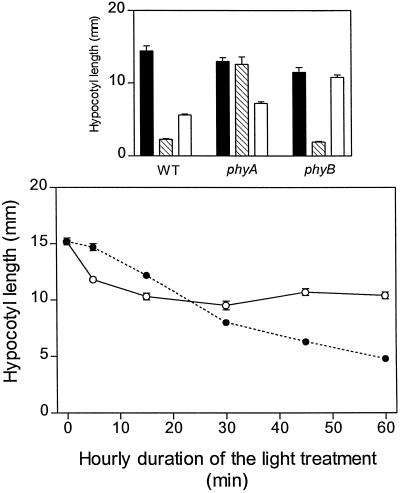
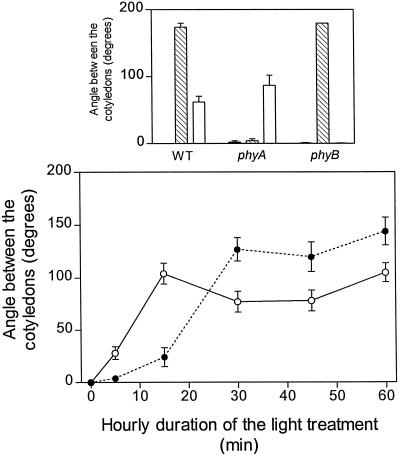
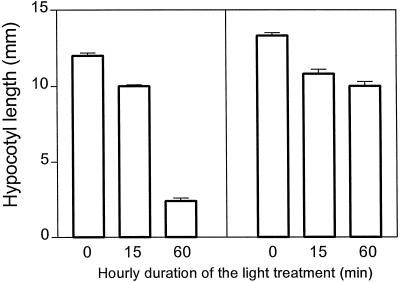
In the WT both R and FR inhibited hypocotyl growth (Fig. 2) and enhanced cotyledon unfolding (Fig. 3). As expected, the phyA mutant did not respond to FR and the phyB mutant showed no residual response to R (Figs. 2 and 3). Under FR (i.e. under conditions where phyA mediates HIR), the extent of hypocotyl growth inhibition increased with increasing durations of the hourly light exposure (Fig. 2), and cotyledon unfolding reached saturation with 30 min h−1 (Fig. 3). Under R (i.e. under conditions in which phyB mediates the response), maximum hypocotyl growth inhibition (Fig. 2) and cotyledon unfolding (Fig. 3) were reached with only 15 min of exposure to R h−1 (terminated with a FR pulse). It is interesting to note that, although continuous FR caused stronger effects than continuous R, short exposures (5 or 15 min) to R were more efficient than short exposures to FR.
Figure 2.
Kinetics of the effects of phyA and phyB on hypocotyl growth. Top, One-day-old WT and mutant seedlings were exposed for 3 d to continuous FR (hatched bars), continuous R (white bars), and darkness (black bars). Bottom, WT seedlings were exposed to hourly FR (•) or R treatments (○; terminated with an FR pulse to remove active phyB) of different durations. Note that 60 min h−1 is equivalent to continuous light. Data are means ± se of at least 5 (top) or 11 (bottom) replicate boxes.
Figure 3.
Kinetics of the effects of phyA and phyB on cotyledon unfolding. Top, One-day-old WT and mutant seedlings were exposed for 3 d to continuous FR (hatched bars), continuous R (white bars), and darkness (black bars). Bottom, WT seedlings were exposed to hourly FR (•) or R treatments (○; terminated with an FR pulse to remove active phyB) of different durations. Data are means ± se of at least 5 (top) or 11 (bottom) replicate boxes.
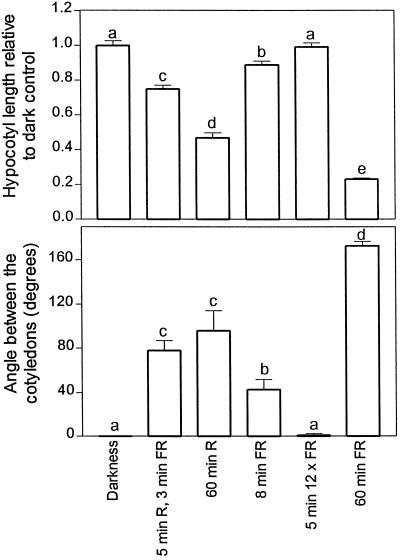
In the above experiments, 5 min R h−1 implied an actual 8 min of light exposure: 5 min of R plus 3 min of FR (FR is necessary to remove active phyB Pfr at the end of R). Furthermore, increasing durations of the hourly FR treatment implied increasing number of photons per hour. Thus, additional experiments were conducted in which some seedlings were exposed to 8 min of FR or to 5 min of FR but at a fluence rate 12-fold higher than that used for continuous FR (i.e. at the same total fluence). For both hypocotyl growth and cotyledon unfolding, the effects of 5 min of R followed by 3 min of FR (i.e. 5 min of phyB activity) were larger than the effects of 8 min of FR or 5 min of FR at a 12-fold higher fluence rate, whereas the effects of continuous FR were larger than those of continuous R (Fig. 4).
Figure 4.
Effects of different hourly light treatments on hypocotyl growth and cotyledon unfolding. One-day-old seedlings were exposed for 3 d to darkness; to hourly cycles of 5 min of R, 3 min of FR, and 52 min of darkness; to continuous R; to hourly cycles of 8 min of FR and 52 min of darkness; to hourly cycles of 5 min of FR at a fluence rate 12-fold higher than that of continuous FR (to equal hourly fluence) and 55 min of darkness; or to continuous FR. Data are means ± se of at least four replicate boxes. Different letters indicate significant differences (P < 0.05).
To rule out the possibility that the relatively faster action of phyB compared with phyA was the result of some sort of interaction between phyB and phyA, the effects of R were investigated in the phyA mutant and the effects of FR were investigated in the phyB mutant. Compared with continuous light, 15 min of R caused a larger proportion of the hypocotyl-growth inhibition response than 15 min of FR (Fig. 5).
Figure 5.
Effects of different durations of the hourly FR treatment in the phyB mutant (left) compared with the effects of R in the phyA mutant (right). One-day-old seedlings were exposed for 3 d to the indicated light or dark conditions. Data are means ± se of five replicate boxes.
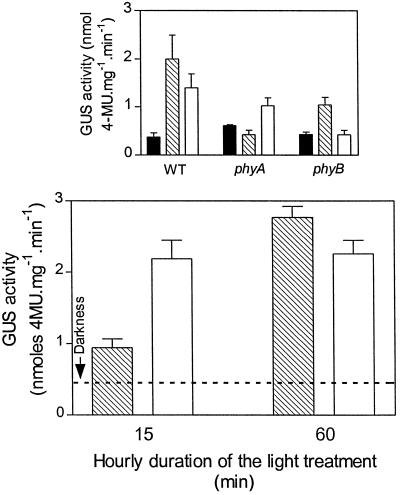
To investigate the kinetics of phyA and phyB activity in a response at the molecular level, 2-d-old A. thaliana seedlings transformed with the homologous Lhcb1*2 promoter fused to the coding region of GUS were exposed to R or FR for 20 h and immediately harvested. The activity of GUS was enhanced in the WT by both continuous R and continuous FR (Fig. 6). The phyA mutant failed to respond to FR and the phyB mutant failed to respond to R. WT seedlings were also exposed every hour to 15 min of FR followed by 45 min of darkness, to 15 min of R terminated with 3 min of FR (to remove active phyB Pfr) followed by 42 min of darkness, to 60 min of FR (i.e. continuous FR), or to 60 min of R (i.e. continuous R). The effect of 15 min R h−1 was significantly higher than the effect of 15 min FR h−1, whereas continuous R or FR had similar effects (Fig. 6).
Figure 6.
Kinetics of the effects of phyA and phyB on the activity of the Arabidopsis Lhcb1*2 promoter fused to the gene of GUS. Top, Two-day-old WT and mutant seedlings were exposed for 20 h to FR (hatched bars), R (white bars), and darkness (black bars). Bottom, WT seedlings were exposed to FR (hatched bars) or R treatments (white bars; terminated with a FR pulse to remove active phyB) of different durations. Data are means ± se of at least 5 (top) or 11 (bottom) replicate boxes. 4-MU, 4-Methylumbelliferone.
DISCUSSION
For the three processes investigated here the kinetics of phototransduction of phyB were faster than those of phyA (acting in the HIR mode). For instance, 15 min R h−1 (terminated with a FR pulse to remove active phyB during the rest of the hour) was almost as effective as continuous R, whereas 15 min FR h−1 (acting via phyA) caused less than 30% of the effect of continuous FR. The differences between the molecules of phyA and phyB are reflected in (a) differential stability in the Pfr form (Somers et al., 1991), (b) differential effectiveness under continuous R and FR (Reed et al., 1994; Quail et al., 1995), (c) apparently different cellular location (Sakamoto and Nagatani, 1996; Pratt et al., 1997), and (d) different kinetics of phototransduction (this work). The N terminus domain of the phytochrome molecule contains determinants for the differential turnover and spectral activity of phyA and phyB (Clough and Viestra, 1997; Quail, 1997).
The analysis used here yielded similar kinetics for processes taking place in different organs (cf Figs. 2 and 3). The latter observation suggests that the differences in kinetics between phyA and phyB are likely to occur early in the processes of phototransduction, before branching toward the specific end-point processes under phytochrome control. The process of phototransduction involves two basic steps: photoperception, i.e. the processes leading to the formation of the active photoreceptor, and signal transduction downstream from the photoreceptor.
In principle, the difference in phototransduction kinetics between phyA and phyB could be the result of divergence at one or both of these steps. However, the following considerations point strongly toward differences in the kinetics of signal transduction. First, particularly for hypocotyl growth and cotyledon unfolding, the effects of short hourly periods of phyA activity were smaller than the effects of similar periods of phyB activity, whereas the opposite is true when prolonged periods of activity are compared (Figs. 2 and 3). Based on this observation phytochrome synthesis or destruction cannot be the processes giving origin to the differential kinetics of phototransduction. Synthesis and destruction are predicted to occur during both the dark and light periods of each hourly cycle (note that the fluence rates are low to cause significant phytochrome photoprotection, Smith et al., 1988) and should therefore affect the response to both short or prolonged hourly periods of illumination. Second, differences in photochemical reactions cannot account for the different kinetics of phyA and phyB because short periods of phyA activity were not effective even when provided at high-fluence rates to equal the hourly fluence of continuous FR (Fig. 4). Third, dark reversion can also be ruled out as the origin of the different kinetics of phototransduction because we are dealing with the action of phyA or phyB during the light period at fluence rates that virtually saturate the response (fluence rate-response curves are not shown). With the available knowledge, the most likely interpretation of the observations presented here is that phyA and phyB had different transduction chains. This conclusion is consistent with the hypothesis derived from the analysis of loci selectively affecting phyA- or phyB-mediated responses (Wagner et al., 1997).
A novel protocol was used here to maintain phyA or phyB in their active forms for different fractions of each hourly cycle. This protocol differs from classical analyses of the kinetics of loss of reversibility or “escape” in two respects. First, continuous light is used instead of light pulses because HIR of phyA require continuous light (see above) and to rule out a potential contribution of dark reversion to the loss of reversibility in the case of phyB. Second, short light/dark cycles are repeated hourly to amplify the effects of small differences in time (in the range of minutes). The kinetics of phyA in the VLFR mode was not investigated here because once phyA is phototransformed to Pfr (the active form of phyA in VLFR) by R or FR there is no technical way available to back-convert all Pfr to Pr. To avoid the induction of VLFR by the R or FR treatments used to activate phyB and phyA, all of the experiments were conducted in the Columbia background. The Columbia alleles of the recently described VLF1 and VLF2 loci cause severely deficient VLFR compared with the Landsberg alleles (Yanovsky et al., 1997). Kinetic studies provide a useful basis to test the role of putative elements of the transduction chain identified in molecular terms. For instance, no element can be considered the first step of the phyB transduction chain (at least for hypocotyl growth, cotyledon unfolding, and Lhcb1*2 gene expression) if its status is not significantly altered within 15 min of the beginning of phyB activity.
ACKNOWLEDGMENTS
We thank the Arabidopsis Biological Research Center (Ohio State University, Columbus) and Dr. Joanne Chory for their kind provision of original seed batches.
Abbreviations:
- FR
far-red light
- HIR
high-irradiance response(s)
- LFR
low-fluence response(s)
- phyA
phytochrome A
- phyB
phytochrome B
- R
red light
- VLFR
very-low-fluence response(s)
- WT
wild type
Footnotes
This work was supported by grants from the University of Buenos Aires (no. AG041 to J.J.C. and no. 01/X304 to R.J.S.), Fundación Antorchas (no. A-13434/1 to J.J.C.), and Consejo Nacional de Investigaciones Cientificas y Tecnicas (no. PIA 6524 to J.J.C. and no. PICT 0295 to R.J.S.).
LITERATURE CITED
- Ahmad M, Cashmore AR. The pef mutants of Arabidopsis thaliana define lesions early in the phytochrome signaling pathway. Plant J. 1996;10:1103–1110. doi: 10.1046/j.1365-313x.1996.10061103.x. [DOI] [PubMed] [Google Scholar]
- Botto JF, Sánchez RA, Casal JJ. Role of phytochrome B in the induction of seed germination by light in Arabidopsis thaliana. J Plant Physiol. 1995;l46:307–312. [Google Scholar]
- Botto JF, Sánchez RA, Whitelam GC, Casal JJ. Phytochrome A mediates the promotion of seed germination by very low fluences of light and canopy shade light in Arabidopsis. Plant Physiol. 1996;110:439–444. doi: 10.1104/pp.110.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braslavsky SE, Gärtner W, Schaffner K. Phytochrome photoconversion. Plant Cell Environ. 1997;20:700–706. [Google Scholar]
- Clack T, Mathews S, Sharrock RA. The phytochrome apoprotein family in Arabidopsis is encoded by five genes: the sequences and expression of PHYD and PHYE. Plant Mol Biol. 1994;25:413–427. doi: 10.1007/BF00043870. [DOI] [PubMed] [Google Scholar]
- Clough RC, Viestra RD. Phytochrome degradation. Plant Cell Environ. 1997;20:713–721. [Google Scholar]
- Heim B, Schäfer E. Light-controlled inhibition of hypocotyl growth in Sinapis alba L. seedlings. Fluence rate dependency of hourly light pulses and continuous irradiation. Planta. 1982;154:150–155. doi: 10.1007/BF00387909. [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan M. GUS fusions: glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T, Neuhaus G, Batschauer A, Chua N-H, Schäfer E. Functional analysis of yeast-derived phytochrome A and B phycocyanobilin adducts. Plant J. 1996;10:625–636. doi: 10.1046/j.1365-313x.1996.10040625.x. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mancinelli AL. The physiology of phytochrome action. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants, Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 211–269. [Google Scholar]
- Mazzella MA, Alconada Magliano TM, Casal JJ. Dual effect of phytochrome A on hypocotyl growth under continuous red light. Plant Cell Environ. 1997;20:261–267. [Google Scholar]
- Nagatani A, Reed JW, Chory J. Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol. 1993;102:269–277. doi: 10.1104/pp.102.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks BM, Quail PH. hy8, a new class of Arabidopsis long hypocotyl mutants deficient in functional phytochrome A. Plant Cell. 1993;5:39–48. doi: 10.1105/tpc.5.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt LH, Cordonier-Pratt M-M, Kelmenson PM, Lazarova GI, Kubota T, Alba RM. The phytochrome gene family in tomato (Solanum lycopersicum L.) Plant Cell Environ. 1997;20:672–677. [Google Scholar]
- Quail PH. An emerging molecular map of the phytochromes. Plant Cell Environ. 1997;20:657–665. [Google Scholar]
- Quail PH, Boylan MT, Parks BM, Short TW, Xu Y, Wagner D. Phytochromes: photosensory perception and signal transduction. Science. 1995;268:675–680. doi: 10.1126/science.7732376. [DOI] [PubMed] [Google Scholar]
- Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddat A, Schmidt P, Gatz C, Braslavsky SE, Gärtner W, Schaffner K. Recombinant type A and B phytochromes from potato. Transient absorption spectroscopy. Biochemistry. 1997;36:103–111. doi: 10.1021/bi962012w. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Nagatani A. Nuclear localization activity of phytochrome B. Plant J. 1996;10:859–868. doi: 10.1046/j.1365-313x.1996.10050859.x. [DOI] [PubMed] [Google Scholar]
- Shinomura T, Nagatani A, Hanzawa H, Kubota M, Watanabe M, Furuya M. Action spectra for phytochrome A- and phytochrome B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1996;93:8129–8133. doi: 10.1073/pnas.93.15.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H, Jackson M, Whitelam GC. Photoprotection of phytochrome. Planta. 1988;175:417–477. doi: 10.1007/BF00393067. [DOI] [PubMed] [Google Scholar]
- Smith H, Xu Y, Quail PH. Antagonistic but complementary actions of phytochromes A and B allow optimum seedling de-etiolation. Plant Physiol. 1997;114:637–641. doi: 10.1104/pp.114.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers DE, Sharrock RA, Tepperman JM, Quail PH. The hy3 long hypocotyl mutant of Arabidopsis is deficient in phytochrome B. Plant Cell. 1991;3:1263–1274. doi: 10.1105/tpc.3.12.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susek RE, Ausubel FM, Chory J. Signal transduction mutants of Arabidopsis uncouple CAB and RBCS gene expression from chloroplast development. Cell. 1993;74:787–799. doi: 10.1016/0092-8674(93)90459-4. [DOI] [PubMed] [Google Scholar]
- Wagner D, Fairchild CD, Kuhn RT, Quail PH. Chromophore-bearing NH2-terminal domains of phytochromes A and B determines their photosensory specificity and differential light lability. Proc Natl Acad Sci USA. 1996;93:4011–4015. doi: 10.1073/pnas.93.9.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D, Hoecker E, Quail PH. RED1 is necessary for phytochrome B-mediated red light-specific signal transduction in Arabidopsis. Plant Cell. 1997;9:731–743. doi: 10.1105/tpc.9.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D, Quail PH. Mutational analysis of phytochrome B identifies a small COOH-terminal-domain region critical for regulatory activity. Proc Natl Acad Sci USA. 1995;92:8596–8600. doi: 10.1073/pnas.92.19.8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelam GC, Johnson E, Peng J, Carol P, Anderson ML, Cowl JS, Harberd NP. Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell. 1993;5:757–768. doi: 10.1105/tpc.5.7.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Parks BM, Short TW, Quail PH. Missense mutations define a restricted segment in the C-terminal domain of phytochrome A critical to its regulatory activity. Plant Cell. 1995;7:1433–1443. doi: 10.1105/tpc.7.9.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky MJ, Casal JJ, Luppi JP. The VLF loci, polymorphic between ecotypes Landsberg erecta and Columbia dissect two branches of phytochrome A signalling pathways that correspond to the very-low fluence and high-irradiance responses of phytochrome. Plant J. 1997;12:659–667. doi: 10.1046/j.1365-313x.1997.00659.x. [DOI] [PubMed] [Google Scholar]