Abstract
Overinduced CD4+CD25+high regulatory T cells (Treg) and downregulated NK cells contribute to tumor-relevant immune tolerance and interfere with tumor immunity. In this study, we aimed to design a novel strategy with cytokine combination to correct the dysregulated Treg and NK cells in malignant patients. Initially, a total of 58 healthy individuals and 561 malignant patients were analyzed for their cellular immunity by flow cytometry. The average percentages of CD4+CD25+high/lymphocyte were 1.30 ± 1.19 % (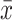 ± SD) in normal adults and 3.274 ± 4.835 % in malignant patients (p < 0.001). The ratio of CD4+CD25+high to CD4+ was 3.58 ± 3.19 % in normal adults and 6.01 ± 5.89 % to 13.50 ± 23.60 % in different kinds of malignancies (p < 0.001). Of normal adults, 15.52 % had >3 % Treg and 12.07 % had <10 % NK cells. In contrast, the Treg (>3 %) and NK (<10 %) percentages were 40.82 and 34.94 % in malignant patients, respectively. One hundred and ten patients received the immunomodulation therapy with IFN-α and/or IL-2. The overinduced Treg in 86.3 % and the reduced NK cells in 71.17 % of the patients were successfully modulated. In comparison, other lymphocyte subpopulations in most patients were much less affected by this treatment. No other treatment-relevant complications except slight pyrexia, fatigue, headache, and myalgia were observed. In conclusion, dysregulated Treg and/or NK cells were common in malignant patients. Different from any regimens ever reported, this strategy was simple and effective without severe complications and will become a basic regimen for other cancer therapies.
± SD) in normal adults and 3.274 ± 4.835 % in malignant patients (p < 0.001). The ratio of CD4+CD25+high to CD4+ was 3.58 ± 3.19 % in normal adults and 6.01 ± 5.89 % to 13.50 ± 23.60 % in different kinds of malignancies (p < 0.001). Of normal adults, 15.52 % had >3 % Treg and 12.07 % had <10 % NK cells. In contrast, the Treg (>3 %) and NK (<10 %) percentages were 40.82 and 34.94 % in malignant patients, respectively. One hundred and ten patients received the immunomodulation therapy with IFN-α and/or IL-2. The overinduced Treg in 86.3 % and the reduced NK cells in 71.17 % of the patients were successfully modulated. In comparison, other lymphocyte subpopulations in most patients were much less affected by this treatment. No other treatment-relevant complications except slight pyrexia, fatigue, headache, and myalgia were observed. In conclusion, dysregulated Treg and/or NK cells were common in malignant patients. Different from any regimens ever reported, this strategy was simple and effective without severe complications and will become a basic regimen for other cancer therapies.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-012-1297-2) contains supplementary material, which is available to authorized users.
Keywords: Cytokine, Treg and NK, Cellular immunity, Immunotherapy, Immune modulation
Introduction
Immune tolerance can be considered as another cause of cancer progression in malignant patients, and so far, clinical failures in cancer immunotherapy have largely been attributed to dysfunction of tumor immunity in most malignant patients. It has been well demonstrated that tumor-induced immune tolerance mostly arises from elevated immunosuppressive factors such as TGFβ [1], IL-10 [2], or PGE [3] and from tumor-induced modulatory cells with immunosuppressive properties such as those belonging to the subpopulation of T cells, dendritic cells (DCs), and the like [4, 5]. Particularly, the CD4+CD25+high regulatory T cells (Treg) derived from naturally existing Treg cells or mostly converted from peripheral naive CD4+ T cells are considered to play a crucial role in tumor immunotolerance [6–8]. Moreover, the effect of natural killer (NK) cell dysfunction on tumor immunity has also been well investigated [9, 10].
Treg has a wide inhibitory effect on the immune system, that is, CD4+ T cells [11], CD8+ T cells [12], NK cells [13], B cells [14], natural killer T (NKT) cells [15], and dendritic cells [16, 17], and is highly induced in various types of tumors [18–21]. Several studies on animal models have proved that the tumor immunosurveillance can be augmented when CD4+CD25+ Treg is inhibited or depleted [22–24]. Therefore, multiple strategies have been designed to inhibit or deplete them to evoke tumor immunity, that is, CD25 monoantibody-toxin such as daclizumab, chemical anticancer drugs such as cyclophosphamide, or immunosuppressors such as fludarabine [25–27]. Nevertheless, the use of cyclophosphamide and fludarabine for the depletion/inhibition of Treg is either nonspecific or less efficient with chemical drug toxicities. The immunotoxin denileukin diftitox (Ontak) has been proved to be much reliable to selectively eliminate CD25-expressing Treg from PBMCs of malignant patients without toxicity. It is specific and effective but cannot be used repeatedly [23]. Moreover, the number of Treg cells after the depletion of CD25+ cells is generally restored over time, and the capacity to mount an antitumor response progressively diminishes [23, 25, 27]. Other studies have shown that depleting Treg cells also raises the possibility of autoimmunity [28].
NK cells play a crucial role in immunosurveillance [29]. It has been proved that inhibition of NK function contributes to poor tumor immunity [30, 31]. In addition, they are key effectors of antibody-dependent cell-mediated cytotoxicity (ADCC), being validated in the study of rituximab, a chimeric mouse–human antibody that recognizes the CD20 antigen expressed on mature B cells, and several other antibodies [32, 33]. These facts above suggest that either the number or the function of NK cells is important to tumor immunity. Several strategies have been investigated to enhance NK-cell responses to tumors experimentally or clinically, including the combined use of cytokines (such as interleukin-2 (IL-2)) and transfusion of in vitro expanded or activated autologous or allogeneic NK cells [34–36]. However, the result seems promising but still far from satisfactory.
Since the middle of 1990s, IFN-α and IL-2 have long been used separately or in combination in the treatment for malignancies, to evoke stronger antitumor immune response as immune modulators or to stimulate immune cells as growth factors, but the clinical antitumor outcomes were limited [37, 38]. Recently, in the application of IFN-α for the treatment of melanoma, we occasionally found that the upregulated CD4+CD25+high cells in some patients simultaneously declined following the injections of IFN-α. Though the exact mechanism is still unknown, it is believed that it may come from the tendency of the modulatory activities of IFN-α and IL-2 toward different lymphocyte subsets. This new finding led us to design a new regimen to overcome tumor-induced upregulation of Treg or inhibition of NK for therapeutic purpose.
In the present study, we found that the dysregulated Treg (>3 %) and/or NK cells (<10 %) were common in malignant patients. By combining IFN-α and IL-2, the Treg and NK cells could be selectively and effectively modulated without severe complications, achieving the balance of the cellular immunity. The strategy is promising to become a basic treatment for other cancer therapies and adds new dimension to cancer immunotherapy.
Patients and methods
Patients and eligibility
A total of 58 healthy individuals and 561 various malignant patients (aged from 25 to 76, 360 males and 201 females) from the Cancer Therapy Center in our hospital were enrolled in this study. All patients were tumor bearing on physical examination or radiographic imaging during their visits to the hospital regardless of the cancer types and the treatments received in the clinic. The cellular immunity of the patients was continuously monitored for 2–23 months, with the minimum interval over 1 month.
Analysis of cellular immunity by flow cytometry
Peripheral blood was analyzed by a 4-color flow cytometry (EPICS XL, Beckman Coulter Inc., USA). Fluorescein isothiocyanate (FITC)-, PE-Cy5-, PerCP-, allophycocyanin (APC)-, or PE-Texas Red (ECD)-conjugated antibodies against CD3, CD4, CD8, CD56, CD19, CD25, CD28, HLA-DR, CD45RA, and CD45RO were purchased from Beckman Coulter Inc. Cells were labeled according to the manufacturer’s protocols. The data were initially collected on day −3 to day 0 before treatment. The post-treatment data were collected immediately after treatment, mostly on the last day of a course. Only a few outpatients had the analysis on their convenience with days or weeks delay. The follow-up interval was generally recommended as 4–6 months.
Regimens for modulation of the cellular immunity
Upon approval by the Ethics Committee of the Affiliated Hospital of the Academy of Military Medical Sciences, a total of 110 patients received the immune modulation therapy after giving informed consent. IFN-α-1b (Beijing Tri-Prime Gene Inc.) and IL-2 (Beijing Shuanglu Pharmaceutical Inc. China) were used. The doses were 300 MU of IFN-α and 200 MU of IL-2 for each injection. The protocols of the therapy were designed according to the status of CD4+CD25+high and NK cells: (1) type 1 treatment: if CD4+CD25+high cells exceeded 3 % of the lymphocyte population or accounted for 30 % of the CD4+ population, IFN-α-1b was used subcutaneously at every other day for 3 weeks for each course of treatment. In early stage of this study, it was given daily in some patients; (2) type 2 treatment: if NK cells were <10 % with CD4+CD25+high <3 %, 1 day of IFN-α-1b was followed by 2 days of IL-2 for 3 weeks for each course of treatment; (3) type 3 treatment: if the patients had >3 % CD4+CD25+high and <10 % NK cells, IFN-α-1b was used daily until CD4+CD25+high cells were dropped to near 3 %, then 1 day of IFN-α-1b followed by 2 days of IL-2 for 3 weeks. In addition, the following prerequisites were needed for the patients to receive the therapy: the absolute neutrophil count >0.3 × 109/L and platelet count >70 × 109/L, normal liver and renal function, at least 2 weeks after chemotherapy or operation, after wound healing, without gastrointestinal tract bleeding, and without a history of allergy to cytokines. If overinduced Treg and downregulated NK were not reversed (Treg/lymphocyte <3 %, NK/lymphocyte >10 %), another course was given.
Statistical analysis
SASS 9.0 software was used for all statistical analyses. The significance of the results was determined using the rank sum test. Statistical significance was defined as p < 0.001.
Results
CD4+CD25+high T cells in normal adults and various malignant patients
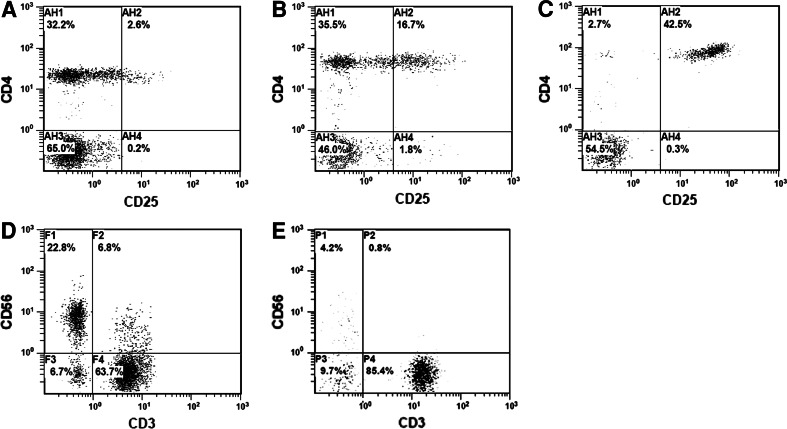
CD4+CD25+high T cells were observed in both the lymphocyte and the CD4+ populations. Among 58 normal adults, the average proportion of CD4+CD25+high cells was 1.30 ± 1.19 % (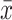 ± SD) (range 0.1–4.3 %, a representative one shown in Fig. 1a) in the lymphocyte population and 0.23–10.73 % in the CD4+ population. Nine of them (15.52 %, 9/58) had their Treg/lymphocyte over 3 %. Four (6.9 %, 4/58) had their Treg/CD4+ over 10 %, and one (1.72 %, 1/58) over 15 %. The average proportion of NK was 20.44 ± 10.09 % (range 5.8–67.6 %, a representative one shown in Fig. 1d). Seven (12.07 %, 7/58) had their NK/lymphocyte reduced to less than 10 %. Only one (1.72 %, 1/58) had both elevated Treg (Treg/lymphocyte >3 %) and downregulated NK (<10 %) (Table 1).
± SD) (range 0.1–4.3 %, a representative one shown in Fig. 1a) in the lymphocyte population and 0.23–10.73 % in the CD4+ population. Nine of them (15.52 %, 9/58) had their Treg/lymphocyte over 3 %. Four (6.9 %, 4/58) had their Treg/CD4+ over 10 %, and one (1.72 %, 1/58) over 15 %. The average proportion of NK was 20.44 ± 10.09 % (range 5.8–67.6 %, a representative one shown in Fig. 1d). Seven (12.07 %, 7/58) had their NK/lymphocyte reduced to less than 10 %. Only one (1.72 %, 1/58) had both elevated Treg (Treg/lymphocyte >3 %) and downregulated NK (<10 %) (Table 1).
Fig. 1.
Treg and NK cells in normal adults and malignant patients. Flow cytometry analysis shows the Treg (CD4+CD25+high) in representatives of normal adults (a) and malignant patients (b, c), and the NK cells (CD3−CD56+) in representatives of normal adults (d) and malignant patients (e)
Table 1.
Treg and NK cells in different cancers
| Individuals | CD4/CD8 | Treg/Lym (%) | Treg/CD4+ (%) | NK/Lym (%) |
|---|---|---|---|---|
| Normal adults (n = 58) | 1.30 ± 0.58 | 1.30 ± 1.19 | 3.58 ± 3.19 | 20.40 ± 10.09 |
| Lung cancer (n = 126) | 1.63 ± 1.21 | 2.94 ± 2.00 | 9.50 ± 7.42 | 16.24 ± 9.70 |
| Breast cancer (n = 52) | 1.45 ± 0.65 | 3.42 ± 7.78 | 10.35 ± 23.37 | 15.55 ± 7.66 |
| Ovarian cancer (n = 23) | 1.65 ± 1.19 | 4.35 ± 4.46 | 10.99 ± 9.36 | 17.50 ± 12.34 |
| Gastrointestinal cancer (n = 88) | 1.52 ± 0.86 | 3.00 ± 1.97 | 9.81 ± 13.11 | 17.49 ± 8.90 |
| Renal carcinoma (n = 45) | 1.68 ± 0.88 | 5.70 ± 10.74 | 13.50 ± 23.60 | 12.11 ± 8.68 |
| Melanoma (n = 14) | 1.34 ± 1.18 | 2.56 ± 2.96 | 8.62 ± 10.58 | 18.67 ± 10.05 |
| Sarcoma (n = 41) | 1.97 ± 1.26 | 2.21 ± 2.10 | 6.01 ± 5.89 | 9.98 ± 5.80 |
| Intracranial cancer (n = 10) | 2.10 ± 0.84 | 2.77 ± 1.57 | 6.77 ± 5.05 | 10.46 ± 5.49 |
| Pancreatic cancer (n = 19) | 1.58 ± 0.81 | 3.25 ± 2.89 | 8.62 ± 7.93 | 13.09 ± 6.70 |
| Liver cancer (n = 31) | 1.29 ± 1.01 | 3.71 ± 6.29 | 12.48 ± 17.89 | 15.72 ± 9.54 |
| Other cancers (n = 112) | 1.45 ± 0.86 | 3.10 ± 4.14 | 9.56 ± 13.11 | 14.22 ± 9.77 |
The value is expressed as mean ± SD. Lym lymphocytes
Among the malignant patients, the average proportion of CD4+CD25+high cells was 3.27 ± 4.86 % (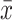 ± SD), ranging from 0.1 to 54.1 % of their lymphocyte population (two representatives shown in Fig. 1b, c) and 0.22–177.18 % of their CD4+ population, significantly higher than that in normal adults (p < 0.001); 229 patients (40.82 %, 229/561) had their Treg/lymphocyte over 3 %, and 183 cases (32.62 %, 183/561) had their Treg/CD4+ over 10 % and 96 cases (17.11 %, 96/561) over 15 %. Particularly, the percentages of patients with Treg/CD4+ > 10 % and >15 % were 2.3–5.3 and 5.8–14.5 times over those of the normal adults, respectively (see Table 2). The average proportion of NK cells was 14.93 ± 9.31 % (
± SD), ranging from 0.1 to 54.1 % of their lymphocyte population (two representatives shown in Fig. 1b, c) and 0.22–177.18 % of their CD4+ population, significantly higher than that in normal adults (p < 0.001); 229 patients (40.82 %, 229/561) had their Treg/lymphocyte over 3 %, and 183 cases (32.62 %, 183/561) had their Treg/CD4+ over 10 % and 96 cases (17.11 %, 96/561) over 15 %. Particularly, the percentages of patients with Treg/CD4+ > 10 % and >15 % were 2.3–5.3 and 5.8–14.5 times over those of the normal adults, respectively (see Table 2). The average proportion of NK cells was 14.93 ± 9.31 % (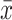 ± SD, ranging from 0.1 to 54.5 %, a representative one shown in Fig. 1e) in malignant patients, significantly lower than that of normal adults (p < 0.001). One hundred and ninety-six cases (34.94 %, 196/561) had reduced NK cells to less than 10 % of their lymphocyte population. Notably, 75 cases (13.37 %, 75/561) had both overinduced Treg (Treg/lymphocyte > 3 %) and reduced NK (<10 %). Regarding the Treg ratio, there was a significant difference between the normal adults and the patients with lung cancer (p < 0.001), gastrointestinal cancer (p < 0.001), intracranial cancer (p < 0.001), ovarian cancer (p < 0.001), pancreatic cancer (p < 0.001), renal cancer (p < 0.001), and other malignancies (p < 0.001), respectively. With respect to the NK ratio, there was a significant difference between the normal adults and the patients with intracranial cancer (p < 0.001), ovarian cancer (p < 0.001), renal cancer (p < 0.001), breast cancer (p < 0.001), sarcoma (p < 0.001), and the group of other malignancies (p < 0.001), respectively.
± SD, ranging from 0.1 to 54.5 %, a representative one shown in Fig. 1e) in malignant patients, significantly lower than that of normal adults (p < 0.001). One hundred and ninety-six cases (34.94 %, 196/561) had reduced NK cells to less than 10 % of their lymphocyte population. Notably, 75 cases (13.37 %, 75/561) had both overinduced Treg (Treg/lymphocyte > 3 %) and reduced NK (<10 %). Regarding the Treg ratio, there was a significant difference between the normal adults and the patients with lung cancer (p < 0.001), gastrointestinal cancer (p < 0.001), intracranial cancer (p < 0.001), ovarian cancer (p < 0.001), pancreatic cancer (p < 0.001), renal cancer (p < 0.001), and other malignancies (p < 0.001), respectively. With respect to the NK ratio, there was a significant difference between the normal adults and the patients with intracranial cancer (p < 0.001), ovarian cancer (p < 0.001), renal cancer (p < 0.001), breast cancer (p < 0.001), sarcoma (p < 0.001), and the group of other malignancies (p < 0.001), respectively.
Table 2.
Proportion of patients with overinduced Treg and downregulated NK cells in different cancers
| Individuals | CD4/CD8 (<1) (%) |
NK/Lym (<10) (%) |
Treg/Lym (>3) (%) |
Treg/CD4+
(>10) (%) |
Treg/CD4+
(>15) (%) |
|---|---|---|---|---|---|
| Normal adults (n = 58) | 35.59 | 12.07 | 15.52 | 6.90 | 1.72 |
| Lung cancer (n = 126) | 35.62 | 28.09 | 41.10 | 32.88 | 17.13 |
| Breast cancer (n = 52) | 24.57 | 19.30 | 26.32 | 24.57 | 10.53 |
| Ovarian cancer (n = 23) | 39.13 | 34.78 | 47.83 | 30.43 | 17.39 |
| Gastrointestinal cancer (n = 88) | 34.09 | 23.86 | 36.35 | 30.68 | 15.91 |
| Renal carcinoma (n = 45) | 14.59 | 43.75 | 41.67 | 33.34 | 24.44 |
| Melanoma (n = 14) | 44.45 | 16.67 | 22.23 | 16.67 | 11.12 |
| Sarcoma (n = 41) | 22.73 | 52.28 | 24.39 | 20.46 | 11.37 |
| Intracranial cancer (n = 10) | 0 | 40.00 | 40.00 | 20.00 | 10.00 |
| Pancreatic cancer (n = 19) | 21.05 | 26.32 | 42.11 | 36.84 | 15.79 |
| Liver cancer (n = 31) | 54.58 | 22.58 | 35.48 | 32.26 | 12.9 |
| Other cancers (n = 112) | 33.05 | 45.22 | 36.53 | 27.83 | 14.79 |
Lym lymphocytes
Selective modulation of CD4+CD25+high and NK populations in patients with different cancers
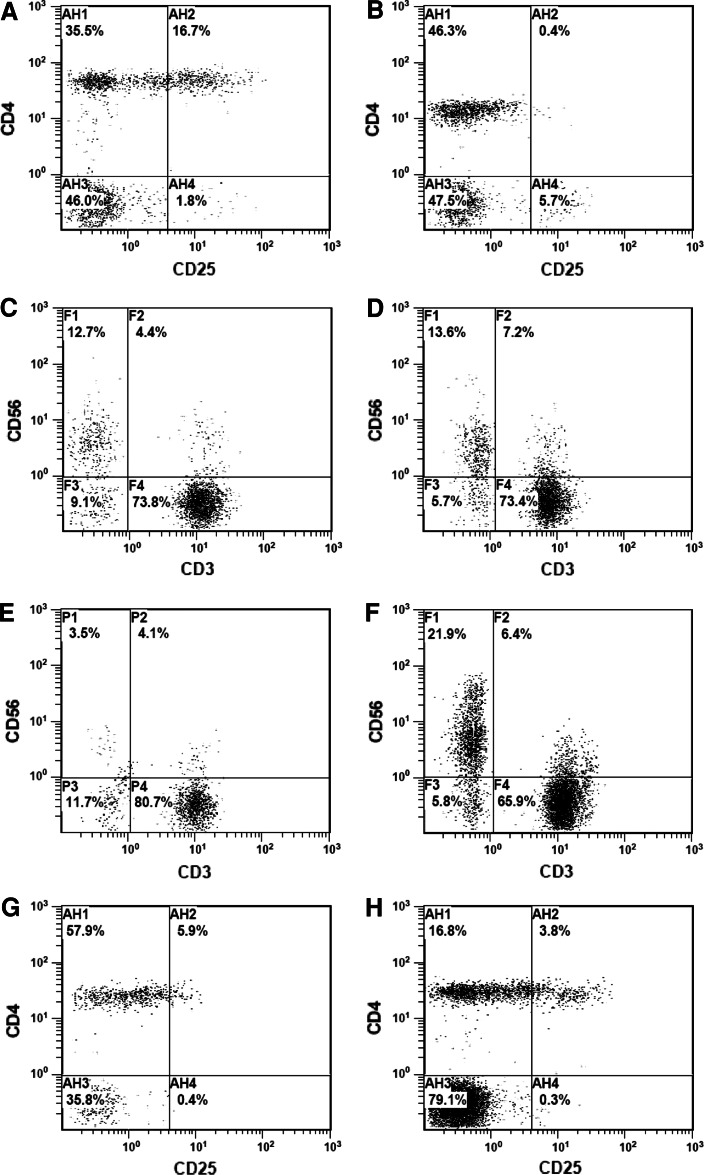
Based on the above results, 3 % of CD4+CD25+high cells and 10 % of NK cells in the lymphocyte population were determined as the reference levels. The patients who had >3 % Treg and/or <10 % NK cells were considered to have imbalance in their cellular immunity (overinduced immunosuppression) and were chosen for immunomodulatory therapy. A total of 110 patients received the immune modulation. After treatment, the Treg/lymphocyte ratio was successfully downregulated to <3 % in 86.3 % (63/73) of the patients (data from a representative patient shown in Fig. 2a–d) and the NK/lymphocyte ratio to >10 % in 71.19 % (42/59) of the patients (data from a representative patient shown in Fig. 2e–h). Most therapies finished within 3 weeks, and 17 patients finished in 1–3 months (mostly within 2 months, until Treg/lymphocyte <3 % and NK/lymphocyte >10 %). Owing to the unavailability of the methodology in our laboratory during early years, the expression of Foxp3 in the CD4+CD25+high T-cell population was not analyzed initially in this study. Regarding the Foxp3-expressing CD4+CD25+high T cells, we further analyzed 10 samples of normal adults from the blood bank in our hospital and 6 samples of malignant patients with routine immunomodulatory treatment (Supplementary Figs. 5, 6). All of the normal adults and five patients had low levels of CD4+CD25+high as well as CD4+CD25+high Foxp3+ T cells initially. One patient (#1) had overinduced CD4+CD25+high and CD4+CD25+highFoxp3+ T cells. After treatment, the overinduced CD4+CD25+high and the CD4+CD25+highFoxp3+ T cells were effectively downmodulated to low level, while those in the five other patients were maintained at low levels. Currently, we have initiated a large cohort multi-center observation regarding the modulatory efficacy of this novel strategy on the CD4+CD25+highFoxp3+ Tregs in malignancies.
Fig. 2.
Modulation of dysregulated Treg and NK cells in malignant patients. After type 1 treatment, the overinduced Treg (a, CD4+CD25+high) in a representative malignant patient could be modulated to 0.4 % (b), and the NK cells remained normal (c, d). After type 2 treatment, the downregulated NK (e, CD3−CD56+) in a representative malignant patient could be elevated to 21.9 % (f), and the Treg remained normal (g, h)
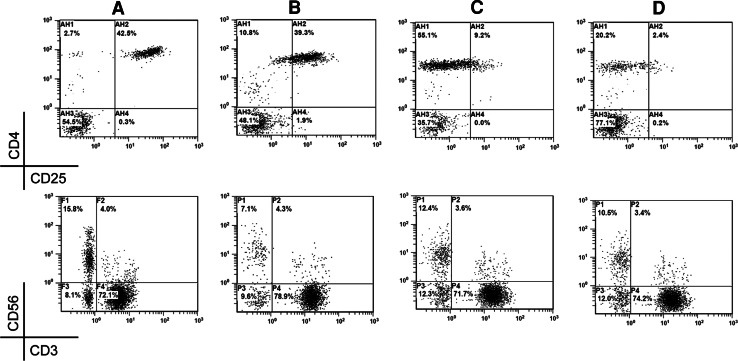
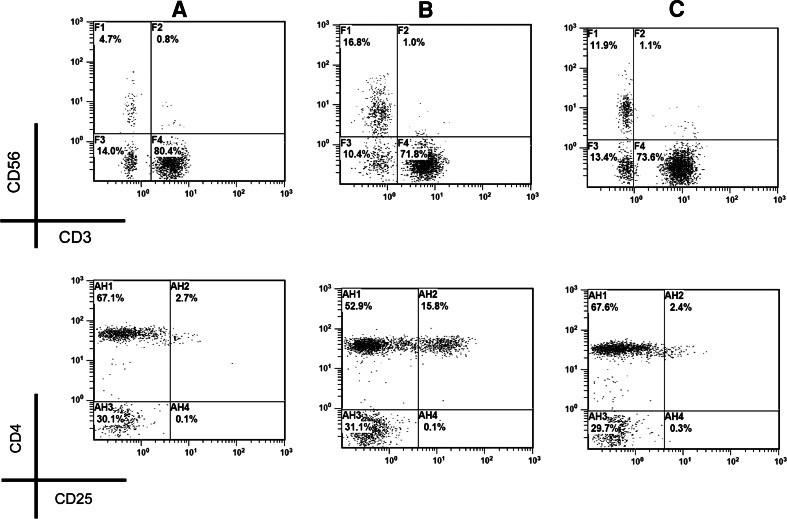
Meanwhile, after IL-2 treatment, the Treg/lymphocyte ratio was still >3 % in 47 patients, but 34 (72.34 %) of them were successfully reversed to <3 % by a following course of type 1 treatment and 13 missed the follow-up. After the use of IFN-α-1b, the NK population was still <10 % in 24 patients. Following a course of type 2 or type 3 treatments, the ratio was successfully upregulated in 12 patients (50 %), and the other 12 missed the follow-up. Notably, some patients had frequent fluctuation of Treg and NK during the treatment. Therefore, consecutive monitoring and repeated modulation were required. As shown in Fig. 3, after two courses of type 1 treatment against overinduced Treg (42.5 %), the NK population dropped to 7.1 %. Then, two courses of type 3 treatment were given, and the Treg and NK reached 9.2 and 12.4 %, respectively. Thereafter, another type 1 treatment was given, and the final Treg and NK population were 2.4 and 10.5 %, respectively. Figure 4 shows another patient with primary downregulated NK (4.7 %) and normal Treg (2.7 %). After two courses of type 2 treatment, the NK population became 16.8 % but the Treg increased to 15.8 %. After several courses of type 3 and type 1 treatments, the NK and Treg were finally modulated to 11.9 and 2.4 %, respectively.
Fig. 3.
Correction of fluctuated Treg and NK cells by multiple types of treatment. After type 1 treatment, the NK cells (CD3−CD56+, lower row) in a malignant patient with overinduced Treg (CD4+CD25+high, upper row) (a) were downregulated to 7.1 % (b). Upon subsequent two courses of type 3 treatment, the NK population became >10 % (c). After another type 1 treatment, the Treg was <3 % with >10 % NK population (d)
Fig. 4.
Correction of fluctuated NK and Treg cells by multiple types of treatment. After type 2 treatment, the Treg (CD4+CD25+high, lower row) in a malignant patient with downregulated NK population (CD3−CD56+, upper row) (a) was overinduced to 15.8 % (b). Upon subsequent several courses of type 1 treatment, the Treg population became <3 % with >10 % NK (c)
The influence of the therapy on other lymphocytes, such as CD3+, CD3+CD4+, CD3+CD8+, CD3−CD19+ subpopulations, was separately analyzed (Supplemental Table 2). When dysregulated Treg and NK cells were successfully corrected, the CD3+, CD3+CD4+, CD3+CD8+, and CD3−CD19+ subpopulations were slightly affected in both up and down directions in most cases. In comparison, more patients had CD3+ and CD3+CD4+ cells downmodulated and CD3+CD8+ cells upmodulated. To minimize the interference of the Treg and NK modulations on other lymphocyte populations, the dose was limited to 200 MU of IL-2 and/or 300 MU of IFN-α-1b per day, the length of each course limited to less than 3 weeks, and the interval of treatment courses or follow-up limited to 4–6 months or longer.
Of all the patients received the treatment, no other treatment-relevant complications except pyrexia, fatigue, headache, and myalgia were observed. No anaphylactic or autoimmune disease was observed in any of the patients.
Discussion
In this study, to overcome the tumor-induced immunotolerance, a simple and safe strategy with IFN-α and/or IL-2 was used to selectively and efficiently downregulate Treg and upregulate NK cells in malignant patients. The result showed that the overinduced Treg in 86.3 % and the reduced NK cells in 71.17 % of the patients were successfully modulated by one course of treatment.
As all the patients enrolled had detectable tumor burden on imaging examination, the changes in Treg and NK cells may reflect in vivo perturbation of the cancer-relevant factors on cellular immunity. The results revealed that Treg cells were highly induced in tumor-bearing patients, which confirmed to the reports from the literatures [18–21]. Interestingly, the significant difference (p < 0.001) in Treg ratio was observed between the normal adults and the patients with lung cancer, gastrointestinal cancer, intracranial cancer, ovarian cancer, pancreatic cancer, renal cancer, or group of other malignancies. For each kind of cancer, a significant proportion of the patients had their Treg/lymphocyte > 3 % and Treg/CD4+ > 10 % or >15 %, respectively. These results indicated that Treg-mediated immunosuppression seemed to be a general mechanism of tumor immunotolerance, but depended on random/ongoing immunosuppressive induction of malignancies. In addition, the NK proportion in malignant patients was also frequently downregulated. As documented previously, Treg can inhibit NK in both direct and indirect ways [37, 39, 40]. In this study, 13.37 % (75/561) of the patients had both overinduced Treg and downregulated NK cells. In the 196 patients with primarily downregulated NK cells (<10 %), only 38.27 % (75/196) had overinduced Treg. The result indicated that there was no significant correlation between the highly induced Treg and downregulated NK cells. The other lymphocytes, such as CD3+, CD3+CD4+, CD3+CD8+, CD3−CD19+ subpopulations, were slightly affected by the immunomodulatory treatment in most cases. The influence and its importance were much less in comparison with the correction of dysregulated Treg and NK cells. Moreover, as each course of the treatment was limited to about 3 weeks and these lymphocyte populations could be automatically remodulated by the immune system itself in weeks, we did not observe any impact of them to the immune function.
To date, many strategies have been employed clinically to induce tumor immunity, such as cytokines, vaccines, in vitro activated immune cells, and even donor lymphocyte infusion (DLI) [38, 41–43]. Despite the documented effects, the clinical outcome is far from satisfactory. It is believed that tumor-induced immunotolerance in vivo is one of the main obstacles that block or interfere with the antitumor function of the immune system or the adoptive immune cells [39]. Therefore, it is critical for cancer treatment to effectively reverse the immunotolerance prior to or during any clinical antitumor therapy. The selective downregulation of overinduced Treg described here may contribute to cancer treatment based on at least the partial reduction of Treg-related immunotolerance and the improvement of the immunoenvironment of malignant patients. Concerning the successful modulation of NK cells in vivo, the approach may also be significant for NK-mediated cancer therapy, apart from other conventional regimens (i.e., transfusion of autologous or allogeneic NK cells and even administration of IL-2) [31, 34–36]. Moreover, IFN-α and/or IL-2 has been used in the treatment for malignancies for decades via modulating immunity. Several systematic and long-term studies have proved their effectiveness and safety, including the combined use of them for the treatment of advanced renal cell carcinoma [37–41]. In this study, the administration of cytokines is mainly for overcoming tumor-induced immunotolerance and maintaining the balance of cellular immunity. Importantly, different from other strategies for Treg inhibition/depletion or NK activation, this strategy mimics the physical immunoregulation by cytokines in vivo, and can be used timely and repeatedly, based on the status of cellular immunity and the balance between different lymphocyte subsets.
Furthermore, by monitoring the shifting of Treg and NK as well as the balance of the cellular immunity during disease progression, clinical treatment, and the immunomodulation itself, we observed frequent up- or downregulation of the lymphocyte subsets, including the overinduced Treg and downregulated NK at an interval of weeks to months. It may be caused by factors from tumors and administration of IL-2 (overinducing Treg) or IFN-α (downregulating NK) [18–21]. It is believed that various factors from diseases and agents/drugs may induce such shifting of the cellular immunity (e.g., from their natural immunoregulatory activities). The immunoregulatory activity of cytokines is the basis of our novel strategy, which may also cause new imbalance of cellular immunity. Such new imbalance may have the same impact on tumor immunity from cancers and should receive more attention when they are used.
To maintain tumor immunity and prevent the propagation of tumor immunotolerance in malignant patients, continuously monitoring and timely correcting the imbalance of the cellular immunity (overinduced Treg, downregulated NK, and the like) is needed during cancer treatment. This new treatment will provide optimal option for this purpose. Interestingly, as IFN-α can downregulate Treg, it can also prevent the induction of Treg by IL-2, calling for a much delicate design of the immunomodulation therapy and more modulators (cytokines or others). The outcome of the modulation can be further improved if more cytokines become clinically available.
With respect to the side effects of IFN-α and IL-2, it has been reported that pyrexia, fatigue, headache, and myalgia were generally observed, while severe complications such as hematopoietic inhibition or hepatic decompensation were occasionally seen in long-term use of them [42, 43]. In this study, as short-term administration and limited dose of IFN-α and IL-2 were employed, we observed no more than slight pyrexia, fatigue, headache, myalgia in some patients. Importantly, the signs directly correlated with autoimmune reaction or aggravation of pre-existed pathological phenomenon were not observed.
In conclusion, we reported a novel strategy using cytokines to selectively and effectively modulate Treg and NK cells and as a result, maintained the balance of the cellular immunity in malignant patients. The strategy itself is a kind of immunotherapy and can become a supplementary treatment for other kinds of cancer therapies. Further investigation will focus on optimization of this so called in vivo immunoediting of the cellular immunity strategy by employing more cytokines and also on its antitumor effectiveness, especially when it is employed in combination with other kinds of therapies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was supported by the National Natural Science Foundation (No. 30672387) and the National Basic Research Program of China (973 Program, No. 2010CB529404).
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Contributor Information
Guangxian Liu, Phone: +86-10-66947128, FAX: +86-10-68692034, Email: liugx270@hotmail.com.
Bing Liu, Phone: +86-10-66947281, FAX: +86-10-66947120, Email: bingliu17@yahoo.com.
References
- 1.Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, Zhang Q, Lonning S, Teicher BA, Lee C. Tumor evasion of the immune system by converting CD4+CD25–T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-β. J Immunol. 2007;178:2883–2892. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 2.Maria GR, Silvia G, Manuela B, Rosa B, Katharina F, Megan KL. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 3.Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, Haudenschild C, Lane TF, Hla T. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276:18563–18569. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 4.Von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 5.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 7.Valzasina B, Piconese S, Guiducci C, Colombo MP. Tumor-induced expansion of regulatory T cells by conversion of CD4+CD25− lymphocytes is thymus and proliferation independent. Cancer Res. 2006;66:4488–4495. doi: 10.1158/0008-5472.CAN-05-4217. [DOI] [PubMed] [Google Scholar]
- 8.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Bauernhofer T, Kuss I, Henderson B, Baum AS, Whiteside TL. Preferential apoptosis of CD56dim natural killer cell subset in patients with cancer. Eur J Immunol. 2003;33:119–124. doi: 10.1002/immu.200390014. [DOI] [PubMed] [Google Scholar]
- 10.Liljefors M, Nilsson B, Skog ALH, Ragnhammar P, Mellstedt H, Frodin JE. Natural killer (NK) cell function is a strong prognostic factor in colorectal carcinoma patients treated with the monoclonal antibody 17–1A. Int J Cancer. 2004;105:717–723. doi: 10.1002/ijc.11139. [DOI] [PubMed] [Google Scholar]
- 11.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation–mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 12.Trzonkowski P, Szmit E, Mysliwska J, Dobyszuk A, Mysliwski A. CD4+CD25+ T regulatory cells inhibit cytotoxic activity of T CD8+ and NK lymphocytes in the direct cell-to-cell interaction. Clin Immunol. 2004;112:258–267. doi: 10.1016/j.clim.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Smyth MJ, Teng MWL, Swann J, Kyparissoudis K, Hayakawa YGD. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol. 2006;176:1582–1587. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- 14.Azuma T, Takahashi T, Kunisato A, Kitamura T, Hirai H. Human CD4+ CD25+ regulatory T cells suppress NKT cell functions. Cancer Res. 2003;63:4516–4520. [PubMed] [Google Scholar]
- 15.Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005;175:4180–4183. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- 16.Chen W. Dendritic cells and CD4+CD25+ T regulatory cells: crosstalk between two professionals in immunity versus tolerance. Front Biosci. 2006;11:1360–1370. doi: 10.2741/1889. [DOI] [PubMed] [Google Scholar]
- 17.Scheffold A, Murphy KM, Höfer T. Competition for cytokines: Treg cells take all. Nat Immunol. 2007;8:1285–1287. doi: 10.1038/ni1207-1285. [DOI] [PubMed] [Google Scholar]
- 18.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4+CD25+ T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 19.Siddiqui SA, Frigola X, Bonne-Annee S, Mercader M, Kuntz SM, Krambeck AE, Sengupta S, Dong H, Cheville JC, Lohse CM, Krco CJ, Webster WS, Leibovich BC, Blute ML, Knutson KL, Kwon ED. Tumor-infiltrating Foxp3−CD4+CD25+ T cells predict poor survival in renal cell carcinoma. Clin Cancer Res. 2007;13(7):2075–2081. doi: 10.1158/1078-0432.CCR-06-2139. [DOI] [PubMed] [Google Scholar]
- 20.Mittal S, Marshall NA, Duncan L, Culligan DJ, Barker RN, Vickers MA. Local and systemic induction of CD4+CD25+ regulatory T-cell population by non-Hodgkin lymphoma. Blood. 2008;111(11):5359–5370. doi: 10.1182/blood-2007-08-105395. [DOI] [PubMed] [Google Scholar]
- 21.Beyer M, Kochanek M, Giese T, Endl E, Weihrauch MR, Knolle PA, Classen S, Schultze JL. In vivo peripheral expansion of naive CD4+CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood. 2006;107(10):3940–3949. doi: 10.1182/blood-2005-09-3671. [DOI] [PubMed] [Google Scholar]
- 22.Ko K, Yamazaki S, Nakamura K, Nishioka T, Hirota K, Yamaguchi T, Shimizu J, Nomura T, Chiba T, Sakaguchi S. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med. 2005;202:885–891. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Attia P, Maker AV, Haworth LR, Freezer RL, Rosenberg SA. Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J Immunother. 2005;28:582–592. doi: 10.1097/01.cji.0000175468.19742.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colombo MP, Piconese S. Regulatory T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer. 2007;7:880–887. doi: 10.1038/nrc2250. [DOI] [PubMed] [Google Scholar]
- 25.Lutsiak C, Semnani RT, Pascalis RD, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4+CD25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 26.Beyer M, Kochanek M, Darabi K, Popov A, Jensen M, Endl E, Knolle PA, Thomas RK, von Bergwelt-Baildon M, Debey S, Hallek M, Schultze JL. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106(6):2018–2025. doi: 10.1182/blood-2005-02-0642. [DOI] [PubMed] [Google Scholar]
- 27.Kohm AP, McMahon JS, Podojil JR, Begolka WS, DeGutes M, Kasprowicz DJ, Ziegler SF, Miller SD. Cutting Edge: anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J Immunol. 2006;176:3301–3305. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- 28.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grundy MA, Zhang T, Sentman CL. NK cells rapidly remove B16F10 tumor cells in a perforin and interferon-gamma independent manner in vivo. Cancer Immunol Immunother. 2007;56(8):1153–1161. doi: 10.1007/s00262-006-0264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu J, Lanier LL. Natural killer cells and cancer. Adv Cancer Res. 2003;90:127–156. doi: 10.1016/S0065-230X(03)90004-2. [DOI] [PubMed] [Google Scholar]
- 31.Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7:329–339. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 32.Dall’Ozzo S, Tartas S, Paintaud G, Cartron G, Colombat P, Bardos P, Watier H, Thibault G. Rituximab-dependent cytotoxicity by natural killer cells: influence of FCGR3A polymorphism on the concentration-effect relationship. Cancer Res. 2004;64:4664–4669. doi: 10.1158/0008-5472.CAN-03-2862. [DOI] [PubMed] [Google Scholar]
- 33.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nature Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 34.Gluck WL, Hurst D, Yuen A, Levine AM, Dayton MA, Gockerman JP, Lucas J, Mize KD, Tong B, Navis D, Difrancesco A, Milan S, Wilson SE, Wolin M. Phase I studies of interleukin (IL)-2 and rituximab in B-cell non-Hodgkin’s lymphoma: IL-2 mediated natural killer cell expansion correlations with clinical response. Clin Cancer Res. 2004;10:2253–2264. doi: 10.1158/1078-0432.CCR-1087-3. [DOI] [PubMed] [Google Scholar]
- 35.Ruggeri L, Cappani M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A. Effectiveness of donor natural killer cell all reactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 36.Miller JS, Soignier Y, Mortari AP, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 37.Soori G, Dillman RO, Wiemann MC, Stark JJ, Tai F, DePriest CB, Church CK, Schulof R. Phase II trial of subcutaneous interleukin-2, subcutaneous interferon-alpha, 5-fluorouracil and cis-retinoic acid in the treatment of renal cell carcinoma: final results of cancer biotherapy research group 94–10. Cancer Biother Radiopharm. 2002;17(2):165–173. doi: 10.1089/108497802753773784. [DOI] [PubMed] [Google Scholar]
- 38.Atzpodien J, Kirchner H, Jonas U, Bergmann L, Schott H, Heynemann H, Fornara P, Loening SA, Roigas J, Muller SC, Bodenstein H, Pomer S, Metzner B, Rebmann U, Oberneder R, Siebels M, Wandert T, Puchberger T, Reitz M. Interleukin-2- and interferon Alfa-2a-based immunochemotherapy in advanced renal cell carcinoma: a prospectively randomized trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN) J Clin Oncol. 2004;22(7):1188–1194. doi: 10.1200/JCO.2004.06.155. [DOI] [PubMed] [Google Scholar]
- 39.Kaufman HL, Disis ML. Immune system versus tumor: shifting the balance in favor of DCs and effective immunity. J Clin Invest. 2004;113:664–667. doi: 10.1172/JCI21148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaffen SL, Liu KD. Overview of interleukin-2 function, production and clinical applications. Cytokine. 2004;28:109–123. doi: 10.1016/j.cyto.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Klatte T, Ittenson A, Rohl FW, Ecke M, Allhoff EP, Bohm FM. Perioperative immunomodulation with interleukin-2 in patients with renal cell carcinoma: results of a controlled phase II trial. Br J Cancer. 2006;95:1167–1173. doi: 10.1038/sj.bjc.6603391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niederau C, Heintges T, Lange S, Goldmann G, Niederau CM, Mohr L, Häussinger D. Long-term follow-up of HBeAg-positive patients treated with interferon alfa for chronic hepatitis B. NEJM. 1996;334:1422–1427. doi: 10.1056/NEJM199605303342202. [DOI] [PubMed] [Google Scholar]
- 43.Janssen HLA, Gerken G, Carreno V, Marcellin P, Naoumov NV, Craxi A, Ring-Larsen H, Kttis G, van Hattum J, de Vries RA, Michielsen PP, Kate FJWT, Hop WCJ, Heijtink RA, Honkoop P, Schalm SW. Interferon Alfa for chronic hepatitis B infection: increased efficacy of prolonged treatment. The European concerted action on viral hepatitis (EUROHEP) Hepatology. 1999;30(1):238–243. doi: 10.1002/hep.510300113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.