Summary
Pseudomonas aeruginosa is an important opportunistic pathogen causing nosocomial infections, especially in immunocompromised patients such as burn patients. Pseudomonas aeruginosa is potentially resistant to different broad-spectrum antibiotics due to its ability to produce extended-spectrum β-lactamase (ESBL) and metallo-β-lactamase (MBL). In the present 6 month study, 220 strains of multidrug-resistant (MDR) Pseudomonas aeruginosa were isolated from male and female burn patients who had been hospitalized for at least one week in Motahari Hospital in Tehran. These strains were screened by the disc diffusion and double disc methods to determine the capacity of producing ESBL and MBL. Of all strains, 18% were ESBL-positive, resulting in a significant inhibition zone (≥5 mm) with cefotaxime and ceftazidime plus clavulanic acid discs when compared to the plain cefotaxime or ceftazidime discs. 38% of the strains were MBL-positive, showing at least 7 mm difference between the inhibition zone around the imipenem discs alone in comparison with imipenem plus EDTA discs, and at least 5 mm difference between the inhibition zone around imipenem plus EDTA discs and EDTA discs alone. In the light of our results, the rapidly spreading resistance among bacterial populations due to the extensive use of antibiotics is a matter of concern for the optimal treatment of patients, particularly in burn wards, and the determination of ESBL and MBL production of MDR Pseudomonas aeruginosa strains is essential.
Keywords: Pseudomonas aeruginosa, extended-spectrum β-lactamase (ESBL), metallo-β-lactamase (MBL), burn patients
Abstract
Pseudomonas aeruginosa est un pathogène opportuniste important qui cause des infections nosocomiales, en particulier chez les patients immunodéprimés, par exemple les patients atteints de brûlures. Pseudomonas aeruginosa est potentiellement résistant aux divers antibiotiques à large spectre en raison de sa capacité à produire β-lactamase à spectre étendu (BLSE) et métallo-β-lactamase (MBL). Les Auteurs de cette étude conduite pour une période de 6 mois ont isolé 220 souches de Pseudomonas aeruginosa multirésistante (MR) dans patients brûlés masculins et féminins hospitalisés pendant au moins une semaine à l’hôpital Motahari à Téhéran. Ces souches ont été criblées moyennant la méthode du disque et du double disque pour déterminer la capacité de production de BLSE et MBL. De toutes les souches, 18% étaient positives à BLSE, ce qui produisait une zone d’inhibition significative (≥ 5 mm) avec céfotaxime et ceftazidime avec les disques d’acide clavulanique par rapport aux simples disques de céfotaxime ou de ceftazidime. 38% des souches étaient positives à MBL: elles ont montré une différence de 7 mm au moins entre la zone d’inhibition autour des disques d’imipénème seuls par rapport à l’imipénème avec les disques de EDTA, et une différence de 5 mm entre la zone d’inhibition autour des disques imipénème avec EDTA et les disques de EDTA seuls. Sur la base de nos résultats, la résistance à l’utilisation étendue des antibiotiques qui se propage rapidement parmi les populations bactériennes soulève des préoccupations pour ce qui concerne le traitement optimal des patients, en particulier dans les services des grands brûlés. Pour ces raisons l’individuation des souches de Pseudomonas aeruginosa multirésistantes aux BLSE et de MBL se démontre essentielle.
Introduction
Pseudomonas aeruginosa is a common gram-negative opportunistic pathogen associated with nosocomial infections, especially in immunocompromised patients admitted to an intensive care unit, such as burn patients.1,2 These bacteria are the major cause of infections in burn wounds, and threaten the life of many patients all over the world.3 Secondary systemic infections caused by Pseudomonas aeruginosa occur in a wide variety of conditions and are associated with high rates of morbidity and mortality.2 Pseudomonas aeruginosa is a highly adaptable micro-organism that can rapidly develop resistance to different types of broad-spectrum antibiotics. It can grow in hospital environments characterized by heavy antimicrobial use, and consequently it can be transmitted rapidly among hospitalized burn patients.4 Nosocomial infections caused by Pseudomonas aeruginosa are often difficult to treat.5
Multidrug-resistant (MDR) Pseudomonas aeruginosa is responsible for most nosocomial infections in burn patients. 3,6 Also, biofilm formation can enhance ability of resistance in Pseudomonas aeruginosa.7 Carbapenems are typically used as the last-line of antibiotic defence for the treatment of infections caused by MDR Pseudomonas aeruginosa. However, these MDR pathogens are capable of producing enzymes that can inactivate beta-lactams, such as Extended spectrum beta lactamase (ESBL) and metallo-β-lactamase (MBL).8,9 ESBL and MBL are the main resistance mechanism against beta-lactams and carbapenem resistance in MDR Pseudomonas aeruginosa.10 ESBL-producing Pseudomonas aeruginosa was first detected in Western Europe in the mid-1980s, and MBL-producing Pseudomonas aeruginosa was first reported from Japan in 1991,11 and they have been described from different countries all over the world since then.12 As mentioned above, the rapid spread of bacterial resistance due to the extensive use of antibiotics remains a matter of concern for the optimal treatment of patients, and in particular burn patients.
In this study, our aim was to determine the incidence of ESBL and metallo-β-lactamase MBL enzymes in the isolates collected from burn patients admitted to Motahari Hospital in Tehran.
Material and methods
In the present study, 220 strains of Pseudomonas aeruginosa were isolated and analysed over a period of 6 months (October 2010 to March 2011) from 106 adults and children hospitalized for at least one week with burn infections in the burn unit of Motahari hospital in Tehran. Prophylactic antimicrobial therapy was performed based on clinical conditions and wound infection.13 The samples were collected by swabbing from burn wound exudates, and immediately transported in transport culture media under standard conditions to the central laboratory of the Antimicrobial Resistance Research Center. Pseudomonas aeruginosa ATTCC27853 was used as the negative control. Determination of Pseudomonas aeruginosa strains was confirmed by standard biochemical tests.
Antimicrobial susceptibility testing was performed on Mueller-Hinton agar plates with: cefepime (30μg), cefotaxime (30μg), ceftazidime (30μg), aztreonam (30μg), imipenem (10μg), amikacin (30μg), ticarcillin (75μg), ticarcillin- clavulanic acid (75/10μg), piperacillin (100μg), piperacillin-tazobactam (100/10μg), ciprofloxacin (5μg), gentamicin (10μg), tobramycin (10μg), and colestin (10μg) discs by disc diffusion method and interpreted as per Clinical and Laboratory Standards Institute (CLSI) recommendations. All discs used in this method were purchased from Mast Company in England. Screening of ESBL- and MBL-producing strains was performed by combined double disc synergy test. Despite the different definitions of MDR micro-organisms,14 MDR was used in this study for isolates that were resistant to at least three classes of antibiotics.
Phenotypic detection of ESBL:
one disc of ceftazidim (30μg) alone, and one in combination with clavulanic acid (30μg/10μg) were placed at a distance of 20mm, centre to centre, on a Muller Hinton agar plate inoculated with a bacterial suspension of 0.5 McFarland turbidity standards, and incubated overnight at 37° C. The ESBL-producing strains showed at least 5mm differentiation between the inhibition zone around cefotaxime or ceftazidime discs alone in comparison with the inhibition zone around cefotaxime+clavulanic acid or ceftazidime+ clavulanic acid discs.
Phenotypic detection of MBL:
One disc of imipenem (10μg) alone and one with imipenem (10μg) in combination with EDTA were placed at a distance of 20mm, centre to centre, on a Muller Hinton agar plate inoculated with a bacbacterial suspension of 0.5 McFarland turbidity standards and incubated overnight at 37° C. The MBLproducing strains showed a greater than 7mm variation between the inhibition zone around imipenem discs alone and the inhibition zone around imipenem+ EDTA discs, and they showed a greater than 5mm variation between the inhibition zone around imipenem+EDTA discs and EDTA discs alone.
Results
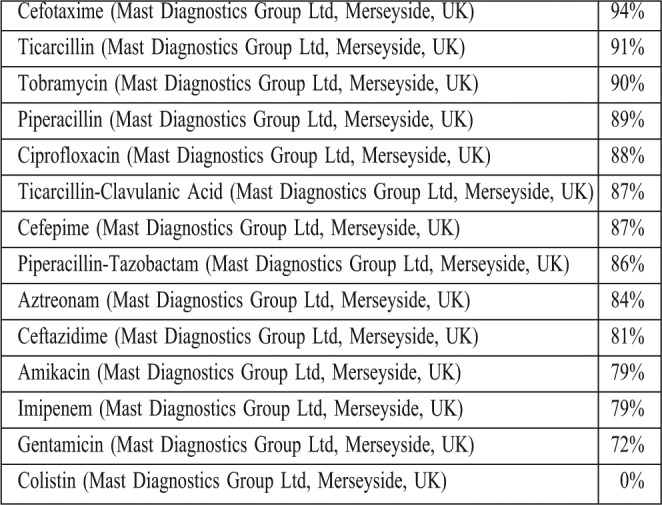
In this study, 220 clinical isolates of MDR Pseudomonas aeruginosa were collected from 106 burn patients (25% females, age range, 1-88 yr) in six months. Among all samples, 112 isolates (50.9%) were MDR. Antimicrobial susceptibilities were determined according to the interpretative criteria of the CLSI guidelines (Table I). No resistance was seen against colistin. In the present study, the overall prevalence of ESBL- positive strains was 18%, and 38% of the isolates were MBL-positive.
Table I. Antibiotic resistance among multidrug-resistant Pseudomonas aeruginosa isolated from burn patients admitted to the burn unit of Motahari hospital in Tehran.
Discussion
Carbapenems were the effective antibiotics for MDR gram-negative bacteria infections, especially in high-risk hospital settings,15 such as burn wards. Resistance is the result of different mechanisms, such as ESBL and MBL production by micro-organisms.1 The increasing use of extended spectrum antibiotics such as carbapenems would provide the selective pressure for selection of these enzymes. 16 In many studies across the world, different resistance ranges (4-60%) have been reported towards ESBI (carbapenems). For instance, the incidence of MBL production in Pseudomonas aeruginosa has been reported to be 10-30% from a variety of clinical specimens across India.15 However, in a study by Didier Hocquet et al.18 in France, 9.3% ESBL, MBL, and extended spectrum oxacillinase-production was found in Pseudomonas aeruginosa. Our prevalence of ESBL and MBL in Pseudomonas aeruginosa does not correlate with the Didier Hocquet study (18% for ESBL positive and 38% for MBL positive strains). Also, our prevalence of MBL in Pseudomonas aeruginosa does not correlate with other studies across the country. In a study by Saderi et al.,5 53.2% of MBL production was reported in Pseudomonas aeruginosa, and in another study performed in Ahwaz19 19.51% of Pseudomonas aeruginosa strains isolated from burn patients were reported as MBL producers.11 This variation seems to reflect the different diagnostic methods and the different rates of antibiotics used in different hospitals. The emergence of ESBL- and MBL-mediated resistance in our country, Iran, is a matter of concern for the treatment of patients.2 The phenotypic screening of resistance is an important step for epidemiological purposes and for developing policies for effective infection control measures in order to manage and prevent the spread of resistant strains. We suggest accurate surveillance, especially of ESBL and MBL, in MDR Pseudomonas aeruginosa isolated from burn patients20,21 as an important step for optimal antibacterial treatment in the future.
Acknowledgments
This study was supported by a grant (543M/T) from Tehran University of Medical Sciences in Tehran, Iran.
References
- 1.Foolad AAI, Rostami Z, Shapouri R. Antimicrobial resistance and ESBL prevalence in Pseudomonas aeruginosa strains isolated from clinical specimen by phenotypic and genotypic methods. J Ardebil University of Medical Sciences. 2010;10:189–98. [Google Scholar]
- 2.Japoni A, Farshad S, Alborzi S. Pseudomonas aeruginosa: Burn infection, treatment and antibacterial resistance. Iran Red Crescent Me. 2009;11:244–53. [Google Scholar]
- 3.Azimi L, Motevallian A, Namvar AME, et al. Nosocomial infections in burned patients in Motahari Hospital, Tehran, Iran. Article ID 436952, 4 pages.Dermatol Res Pr. 2011 doi: 10.1155/2011/436952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambert PA. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa. J R Soc Med. 2002;41:22–26. [PMC free article] [PubMed] [Google Scholar]
- 5.Saderi H, Karimi Z, Owlia P, et al. Phenotypic detection of Metallo-beta-Lactamase producing Pseudomonas aeruginosa strains isolated from burned patients. Iran J Pathol. 2008;3:20–4. [Google Scholar]
- 6.Hamer D.H. Treatment of nosocomial pneumonia and tracheobronchitis caused by multidrug resistant Pseudomonas aeruginosa with aerosolized colistin. Am J Respir Crit Care Med. 2000;162:328–30. doi: 10.1164/ajrccm.162.1.9910071. [DOI] [PubMed] [Google Scholar]
- 7.Drenkard E, Ausubel FM. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature. 2002;18:416, 740–3. doi: 10.1038/416740a. [DOI] [PubMed] [Google Scholar]
- 8.Moya B, Dötsch A, Juan C, et al. β-lactam resistance response triggered by inactivation of a non-essential penicillin-binding protein. PLoS Pathog. 2009;5(3) doi: 10.1371/journal.ppat.1000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borgianni L, Prandi S, Salden L, et al. Genetic context and biochemical characterization of the IMP-18 metallo-beta-lactamase identified in a Pseudomonas aeruginosa isolate from the United States. Antimicrob Agents Chemother. 2010;55:140–5. doi: 10.1128/AAC.00858-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundsfjord A, Simonsen GS, Haldorsen B, et al. Broad-spectrum beta-lactamases in Gram-negative bacteria. Tidsskr Nor Laegeforen. 2008;128:2741–5. [PubMed] [Google Scholar]
- 11.Scaife W, Young HK, Paton RH, et al. Transferable imipenemresistance in Acinetobacter species from a clinical source. J Antimicrob Chemother. 1995;36:585–6. doi: 10.1093/jac/36.3.585. [DOI] [PubMed] [Google Scholar]
- 12.De AS AS, Kumar SH, Baveja SM. Prevalence of metallo β-lactamase producing Pseudomonas aeruginosa and Acinetobacter species in intensive care areas in a tertiary care hospital. Indian J Crit Care Med. 2010;14:217–9. doi: 10.4103/0972-5229.76089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alaghebandan R, Azimi L, Rastegar Lari A. Nosocomial infections among burn patients in Tehran, Iran: A decade later. Ann Burns Fire Disasters. 2012;25:3–7. [PMC free article] [PubMed] [Google Scholar]
- 14.Falagas ME, Koletsi PK, Bliziotis LA. The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J Med Microbiol. 2006;55:1619–29. doi: 10.1099/jmm.0.46747-0. [DOI] [PubMed] [Google Scholar]
- 15.Deshpande P, Rodrigues C, Shetty A, et al. New Delhi metallob-lactamase (NDM-1) in Enterobacteriaceae: Treatment options with carbapenems compromised. J Assoc Physicians India. 2010;58:147–9. [PubMed] [Google Scholar]
- 16.Butt T, Usman M, Ahmad RN, et al. Emergence of metallo betalactamase producing Pseudomonas aeruginosa in Pakistan. J Pak Med Assoc. 2005;55:302–4. [PubMed] [Google Scholar]
- 17.Varaiya A, Kulkarni N, Kulkarni M, et al. Incidence of metallo β-lactamase producing Pseudomonas aeruginosa in ICU patients. Indian J Med Res. 2008;127:398–402. [PubMed] [Google Scholar]
- 18.Hocquet D, Plésiat P, Dehecq B, et al. ONERBA. Nationwide investigation of extended spectrum β-lactamases, metallo-β-lactamases and extended spectrum oxacillinases produced by ceftazidime resistant Pseudomonas aeruginosa strains in France. Antimicrob Agents Chemother. 2010;54:3512–5. doi: 10.1128/AAC.01646-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khosravi AD, Mihani F. Detection of metallo-beta-lactamase-producing Pseudomonas aeruginosa strains isolated from burn patients in Ahwaz, Iran. Diagn Microbiol Infect Dis. 2008;60:125–8. doi: 10.1016/j.diagmicrobio.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Birgy A, Bidet P, Genel N, et al. Phenotypic screening of carbapenemases and associated beta-lactamases in carbapenem-resistant Enterobacteriaceae. J Clin Microbiol. 2012;50:1295–302. doi: 10.1128/JCM.06131-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prashant DP, Peerapur BV. ESBL and MBL mediated resistance in Pseudomonas aeruginosa: An emerging threat to clinical therapeutics. J Clin Diag Res. 2011;5:1552–4. [Google Scholar]


