Abstract
Introduction
The aim of this study was the long-term prospective evaluation of the effects of estroprogestagen (EP) therapy on the bone mineral density (BMD) of girls with functional hypothalamic amenorrhea (FHA) carrying various PvuII and XbaI polymorphisms of ER-α.
Material and methods
Prospective observation included 84 FHA girls and 50 controls. The FHA patients were subjected to 4-year sequential therapy with 17β estradiol (2 mg from the 2nd to 25th day of the menstrual cycle) and dydrogesterone (10 mg from the 16th to the 25th day). Hormonal parameters, serum concentration of the bone fraction of alkaline phosphatase (BALP), urine concentration of cross-linked n-telopeptide of type I collagen (Ntx) and BMD were determined before and after the treatment.
Results
Six-month treatment resulted in a marked increase in estradiol (p = 0.001), testosterone and prolactin levels (p = 0.01 both) and a significant decrease in BALP and Ntx (p = 0.001 both). Patients with the PP polymorphism had significantly lower baseline BMD compared to carriers of other polymorphic variants of PvuII (p = 0.003). A significant increase in BMD was observed throughout the entire therapy period, with no significant differences in the yearly dynamics of BMD changes observed amongst various polymorphic variants and haplotypes of ER-α.
Conclusions
The EP therapy is effective in the treatment of BMD disorders associated with FHA, and treatment results do not depend on PvuII and XbaI polymorphisms of ER-α.
Keywords: bone mineral density, estrogen receptor, functional hypothalamic amenorrhea, hormone replacement therapy, osteoporosis
Introduction
Irregular menstrual cycles related to functional hypothalamic amenorrhea (FHA) may be a symptom of juvenile crisis in girls [1–3]. According to the literature, for the first few years after menarche, menstrual disorders are observed in 11.3% to 26.7% of patients, whereas functional amenorrhea and oligomenorrhea occur in 2.6% to 8.5% of girls [4].
The mechanism by which psychogenic factors affect the function of the hypothalamus during puberty is not fully understood. It is plausible that stress results in an increase in the hypothalamic concentration of some neuropeptides, such as corticotropin-releasing factor (CRF) and β-endorphins. These impair the pulsatile release of gonadotropin-releasing hormone (GnRH), inhibit dopamine secretion, and elevate prolactin concentrations [5]. The resulting functional hyperprolactinemia affects the pulsatile secretion of luteinizing hormone (LH), causes ovarian granulosa cell proliferation, decreased ovarian synthesis and secretion of estradiol, and as well as the inhibition of ovarian follicular growth and subsequent ovulation [6, 7]. These hormonal changes are manifested by infrequent menstrual bleeding, oligomenorrhea, and finally by functional amenorrhea [1, 8]. In some girls, prolonged exposure to stress may lead to fully symptomatic FHA which is characterized by low prolactin concentrations.
Since estrogens affect bone turnover by exerting anti-resorptive and anabolic effects, decreased blood concentrations of these hormones in pubescent girls interfere with bone mineralization and affect future peak bone mass [9, 10]. Consequently, girls with FHA have an elevated risk of bone mass depletion.
Our previous studies revealed that estroprogestagen (EP) therapy administered during puberty was reflected by improved bone mineral density in girls with FHA [11]. However, treatment response in our group was inhomogeneous – there were girls in whom the increase in BMD following EP therapy was delayed and/or diminished. We hypothesized that this variability has a molecular background, and results from diverse interactions between estrogens and the respective hormonal receptors on the host's cells. Consequently, we assumed that estrogen receptor-α (ER-α) is a potential candidate for the molecular regulator of EP therapy efficiency, since other authors have shown an association between ER-α PvuII and XbaI polymorphisms and BMD amongst prepubertal girls and postmenopausal women [12–15]. Besides its various cognitive aspects, confirmation of this hypothesis would have significant clinical implications since identification of the “unfavorable” genotype/haplotype would allow for the preliminary identification of patients with poor prognosis and enable the support of their EP treatment with other therapeutic modalities.
Therefore, the aim of this study was the long-term prospective evaluation of the effects of EP therapy on the mineral bone density of FHA girls with various PvuII and XbaI polymorphisms of ER-α.
Material and methods
The study included 134 girls between the ages of 16 and 17 years who were divided into two groups: 1) 84 patients with FHA, treated at the Department of Gynecology, Poznan University of Medical Sciences (Poland) between 2004 and 2009 (group A), and 2) 50 girls whose menstrual cycles were normal (controls, group C).
All procedures were approved by the Local Ethics Committee of the Poznan University of Medical Sciences. Both subjects and their parents gave their informed consent before the start of any procedure.
Qualification criteria for group A included: 1) at least 6 months of amenorrhea preceded by at least 3 years of oligomenorrhea, 2) psychological problems (learning and/or family problems) confirmed by a clinical psychologist.
Group A exclusion criteria, based on medical history and standardized questionnaire survey [12], included the following: 1) polycystic ovary syndrome, congenital adrenal hyperplasia or premature ovarian failure, 2) low birth weight or preterm birth, 3) at least one confirmed episode of eating disorders, 4) poor eating during childhood or puberty, 5) episodes of impaired growth and body mass gain, 6) extensive participation in sports that may have influenced bone mineralization, 7) metabolic disorders that may be associated with decreased bone mineralization, 8) prolonged use of stimulants or drugs that may affect bone metabolism, 9) familial history of osteoporosis, 10) incomplete 4-year follow-up (return of regular menstrual cycles, cessation of treatment due to medical indications or because of other reasons).
The control group comprised healthy, normally menstruating girls who gave their consent to participate in this study due to prophylactic reasons.
Anthropometric measurements (height, body weight) were taken for all participants and their body mass indices (BMI) were calculated. The development of secondary sexual characteristics was assessed using the Tanner scale [16]. Baseline values of hormonal parameters were determined for thyrotropin (thyroid-stimulating hormone – TSH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), total serum testosterone (T), sex hormone binding globulin (SHBG), estradiol (E2), and prolactin (PRL). The following bone turnover markers were also measured in both groups: serum concentration of the bone fraction of alkaline phosphatase (BALP) and urine concentration of cross-linked n-telopeptide of type I collagen (Ntx). Bone mineral density (BMD) measurements were also performed. Subjects in group A were additionally tested for blood morphology, erythrocyte sedimentation rate, and blood concentrations of calcium, phosphorus, creatinine, total protein, alkaline phosphatase, vitamin D3, and parathormone.
Estradiol, TSH, FSH, and LH were measured in serum using a solid-phase chemiluminescence immunoassay (Immulite; Diagnostic Products Co, Los Angeles, CA, USA). Assay sensitivity was 20 pg/ml for estradiol, 0.01 mU/l for TSH, 0.1 mIU/ml for FSH, and 0.1 mIU/ml for LH. The intra-assay and inter-assay coefficients of variation (CVs) for estradiol were 9.3% and 10.5%, respectively, whereas for FSH they were 1.9% and 5.0%, respectively, and for LH 3.6% and 5.0%, respectively. Total serum testosterone was measured using radioimmunoassay (RIA) (Orion Diagnostica, Espoo, Finland) with 0.1 nmol/l limit of detection, with both-phase two-site chemiluminescent enzyme immunometric assay and with an inter-assay CV intra-assay and inter-assay CVs of 6%. Sex hormone binding globulin (SHBG) was measured using a fully automated system (Immulite: DPC, Inc., Los Angeles, CA, USA) which uses a solid of less than 8%. Prolactin concentrations were determined by microparticle enzyme immunoassay using an automated Abbott AxSYM system (Abbott Laboratories, Chicago, IL, USA). For prolactin, the within-run CV was less than 3% and the total CV was less than 6%.
Serum BALP was measured using a specific monoclonal antibody (Alkphase-B; Metra Biosystems, Mountain View, CA, USA), with a sensitivity of 0.7 U/l, and with intra-assay and inter-assay CVs at 28 U/l amounting to 3.3% and 7.9%, respectively. The Ntx was measured from morning samples of urine and corrected for urinary creatinine. Measurements were carried out by means of an enzyme-linked immunoassay (VitrosTM NTX reagent pack; Ortho-Clinical Diagnostics, Amersham, UK) with a detection limit of 20 nmol bone collagen equivalent and an inter-assay CV of 4.1%.
The BMD measurements of the lumbar spine (L2-L4) were performed with the DXA method (GE Lunar Prodigy Advance, Madison, WI, USA; software enCORE version 8.8), using automatic scan mode. Results were presented as absolute values (g/cm2) and relative changes between consecutive measurements (ΔBMD%).
Additionally, ER-α PvuII and XbaI polymorphisms were determined in subjects in group A. For the purpose of genomic DNA extraction from peripheral blood mononuclear cells (PBMCs), 10 ml of venous blood was collected and stored in EDTA anticoagulant. XbaI and PvuII polymorphisms were analyzed according to Carling et al. [17]. DNA amplification was performed by means of the PCR method with pairs of the following primers flanking both polymorphic sites: 5’-CAACCAAGACTACAAGTACCGCGTCAGTGA-3’ as a sense ER-se primer, and 5’-CAACCAAGACTACAAGTACCGCGTCAGTGA-3’ as an antisense ER-as primer. A 1300 b.p.-long product was obtained. The PCR product and restriction fragment analysis was performed by electrophoresis on 3% ethidium bromide stained agarose gel, and recorded after visualization in UV light.
Patients from group A underwent 4-year sequential EP therapy with a preparation which included the natural female sex hormone 17β estradiol (2 mg from the 2nd to 25th day of the menstrual cycle) and dydrogesterone (10 mg from the 16th to the 25th day of the menstrual cycle). The goal of the treatment was for the patients to resume regular menstrual bleeding. Spontaneous menstruations did not resume in any of the participants and therefore the therapy was continued for 4 years. In addition to hormonal treatment, patients were encouraged to modify their lifestyles and dietary habits. They were prescribed calcium and vitamin D3 preparations at individual doses adjusted for their dietary content and season of the year. Moreover, regular physical activity (15 min of recreational gymnastics twice a day) was suggested.
Follow-up measurements of TSH, FSH, LH, T, SHBG, E2, and PRL, BALP and Ntx were performed after 6 months and after 4 years of EP treatment in patients belonging to group A. Moreover, BMD measurements were carried out on a yearly basis, starting 12 months after the initiation of EP therapy.
Statistical analysis
Continuous variables are presented as arithmetic means and their standard deviations (SD). Normal distribution was tested using the Kolmogorov-Smirnov test. Logarithmic transformation was used for the Ntx variable. Arithmetic means between groups A and C and amongst certain genotypes or haplotypes of ER-α in group A were compared using ANOVA and the Tukey post-hoc test. Calculations were performed using Statistica 7 (StatSoft®, Poland) software, and statistical significance was defined as p ≤ 0.05.
Results
There was no difference between patients from group A and group C in terms of somatic-sexual development assessed according to the Tanner scale [16]. Other clinical characteristics of the study participants are summarized in Table I. Patients from group A had significantly lower anthropometric parameters (height, body weight and BMI) compared to their peers in group C. Menarche occurred at a markedly older age in group A subjects, while their gynecological age was significantly lower than in regularly menstruating controls. Group A subjects also had significantly lower baseline levels of E2, testosterone, and gonadotropins. Moreover, girls with amenorrhea had significantly poorer baseline BMD. Subjects in group A were also characterized by markedly higher concentrations of bone turnover markers (BALP and Ntx) when compared to their healthy peers.
Table I.
Baseline clinical characteristics (mean ± SD) of FHA patients (A) and controls (C)
| Parameter | A (n = 84) | C (n = 50) | Value of p |
|---|---|---|---|
| Age [years] | 16.7 ±1.2 | 16.7 ±1.3 | NS |
| Age at menarche [years] | 13.5 ±1.4 | 12.7 ±0.8 | 0.001 |
| Gynecological age [years] | 3.4 ±1.3 | 4.1 ±1.3 | 0.003 |
| Birth weight [g] | 3263 ±405 | 3327 ±402 | NS |
| Weight [kg] | 49.4 ±6.8 | 59.7 ±9.3 | 0.001 |
| Height [cm] | 162.3 ±6.6 | 166.8 ±6.0 | 0.001 |
| BMI [kg/m2] | 18.7 ±2.2 | 21.5 ±3.2 | 0.001 |
| E2 [pg/ml] | 27.8 ±10.3 | 64.8 ±12.3* | 0.001 |
| T [ng/ml] | 0.1 ±0.2 | 0.7 ±0.1 | 0.001 |
| SHBG [nmol/l] | 41.45 ±16.32 | 49.17 ±25.71 | NS |
| TSH [mIU/ml] | 2.1 ±1.8 | 1.9 ±2.3 | NS |
| FSH [mIU/ml] | 1.6 ±1.4 | 10.7 ±1.0 | 0.001 |
| LH [mIU/ml] | 3.2 ±1.5 | 5.8 ±3.4 | 0.001 |
| PRL0 [ng/ml] | 2.1 ±2.9 | 10.2 ±3.2 | 0.001 |
| BALP [U/ml] | 63.2 ±25.2 | 40.3 ±13.5 | 0.001 |
| Ntx [mEBCE/mg/ml CR] | 419.1 ±276.5 | 49.4 ±23.6 | 0.001 |
| BMD [g/cm2] | 0.822 ±0.09 | 1.141 ±0.09 | 0.001 |
NS – non-significant (p < 0.05, ANOVA)
5th-7th day of menstrual cycle
The laboratory parameters of Group A subjects were determined after 6 months in order to verify the efficiency of EP therapy (Table II). Treatment resulted in a marked increase in E2 levels which were similar to levels noted in group C subjects in the follicular phase of their menstrual cycles (64.8 ±12.3 pg/ml). However, E2 concentrations in group A subjects were still significantly lower compared to luteal phase values noted in group C subjects (131.3 ±46.6 pg/ml, p = 0.001). Moreover, a significant increase in testosterone and prolactin concentrations was observed in FHA patients. Six months of EP therapy were reflected by a marked decrease in BALP and Ntx values. Average post-treatment BALP concentrations in group A subjects did not significantly differ from baseline values in group C subjects, whereas average Ntx levels in group A subjects – although markedly lower compared to baseline values – still remained significantly higher than in the control group (p < 0.001).
Table II.
Hormonal parameters and bone turnover marker values (mean ± SD) in FHA patients; measurements at baseline and following 6 months of EP treatment
| Parameter | Baseline | Follow-up | Value of p |
|---|---|---|---|
| E2 [pg/ml] | 27.3 ±10.6 | 68.1 ±10.5 | 0.001 |
| T [ng/ml] | 0.1 ±0.2 | 0.5 ±0.6 | 0.01 |
| SHBG [nmol/l] | 41.45 ±16.32 | 65.55 ±17.33 | NS |
| TSH [mIU/ml] | 2.1 ±1.8 | 3.1 ±2.8 | NS |
| FSH [mIU/ml] | 1.6 ±1.4 | 5.4 ±1.9 | NS |
| LH [mIU/ml] | 3.2 ±1.5 | 6.2 ±2.8 | NS |
| PRL0 [ng/ml] | 2.1 ±2.9 | 9.9 ±3.2 | 0.01 |
| BALP [U/ml] | 63.2 ±25.1 | 38.1 ±12.8 | 0.001 |
| Ntx [mEBCE/mg/ml CR] | 408.0 ±282.1 | 195.6 ±144.7 | 0.001 |
NS – non-significant (p < 0.05, ANOVA)
The following haplotypes of ER-α were found to be present in the subjects studied: XXPP (n = 12), XxPP (n = 21), xxPP (n = 12), XXPp (n = 1), XxPp (n = 7), xxPp (n = 4), XXpp (n = 1), and xxpp (n = 26). Patients with the PP polymorphism were characterized by significantly lower baseline BMD compared to carriers of other polymorphic variants of the PvuII gene (0.771 ±0.10 g/cm2 vs. 0.837 ±0.07 g/cm2 for Pp vs. 0.839 ±0.06 g/cm2 for pp; p = 0.003). No significant differences in other baseline parameters, or parameters determined following 6 months of EP therapy were noted amongst patients with various polymorphisms of XbaI or PvuII genes, or amongst carriers of various haplotypes of ER-α.
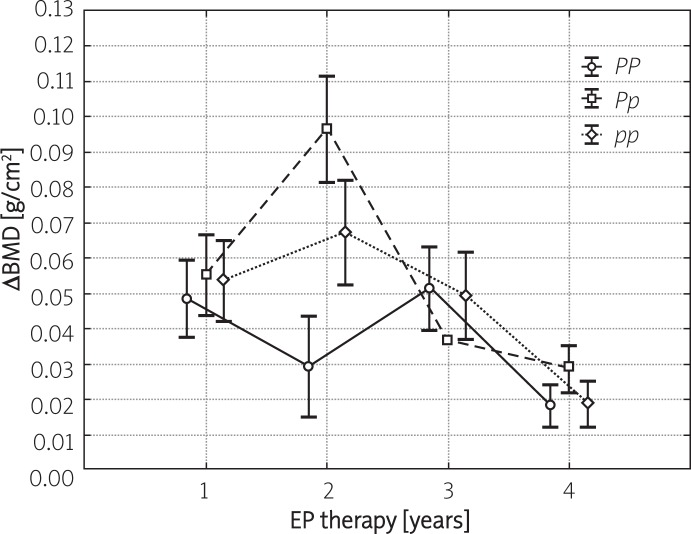
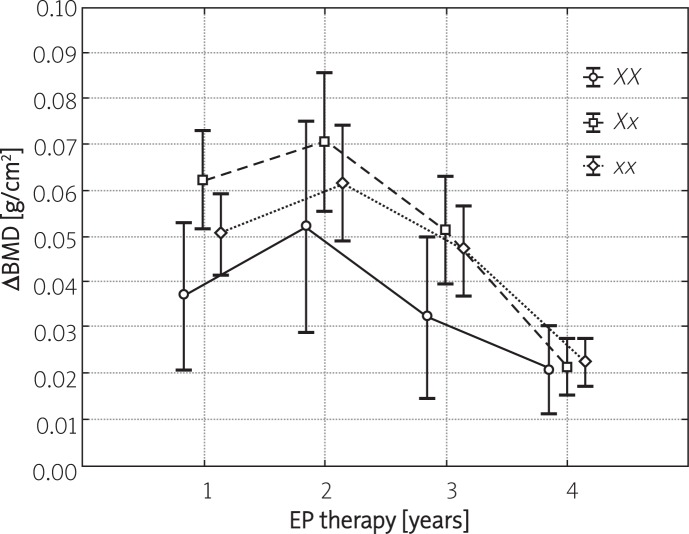
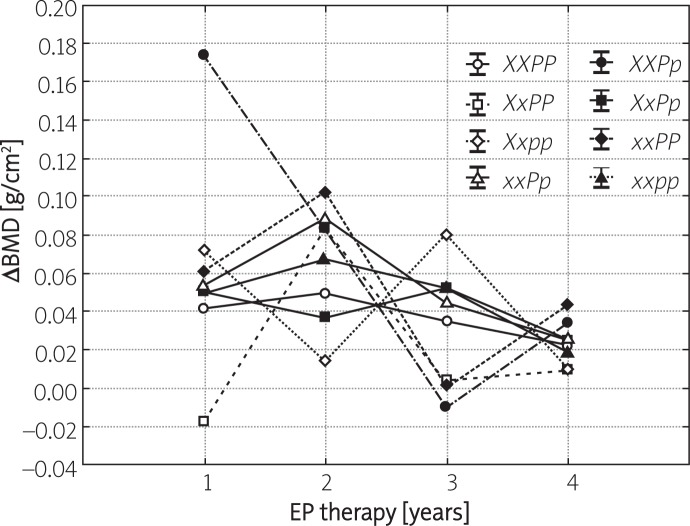
Figures 1 and 2 present the yearly increases in BMD during consecutive years of hormonal therapy, stratified depending on XbaI and PvuII polymorphisms of ER-α. In the case of all studied polymorphisms, a significant increase in BMD was observed throughout the entire therapy period with the most evident changes noted during the initial 2 years of treatment. No significant differences in the yearly dynamics of BMD changes were observed amongst various polymorphic variants of XbaI and PvuII. The only exception pertained to the PP polymorphism, whose percentage increase following 2 years of therapy was significantly lower compared to other polymorphisms of PvuII. Moreover, no significant differences in the dynamics of BMD gain during consecutive years of therapy were noted amongst carriers of various haplotypes of ER-α (Figure 3).
Figure 1.
Yearly increases in BMD (mean ± SD) in FHA patients subjected to 4-year EP treatment (group A) stratified depending on PvuII polymorphisms of ER-α
Figure 2.
Yearly increases in BMD (mean ± SD) in FHA patients subjected to 4-year EP treatment (group A) stratified depending on XbaI polymorphisms of ER-α
Figure 3.
Yearly increases in BMD (mean ± SD) in FHA patients subjected to 4-year EP treatment (group A) stratified depending on haplotypes of ER-α
Despite a constant tendency to increase, following 4 years of hormonal treatment, BMD in group A patients was still significantly lower than in controls (1.005 ±0.10 g/cm2 vs. 1.127 ±0.10 g/cm2; p < 0.001).
Discussion
This study revealed that the 4-year hormonal treatment is reflected by significant increase in estrogen levels and BMD in girls with functional hypothalamic amenorrhea. Even after only half a year of therapy, estradiol levels in the studied group returned to their normal values. Additionally, there was a marked decrease in levels of both bone turnover markers, and average BALP concentrations were not significantly different compared to baseline values of controls. These positive changes in the aforementioned laboratory parameters were reflected by BMD values, which increased gradually following subsequent years of treatment. The lack of increase in BALP, being a marker of bone synthesis, may seem to be a surprising finding. We previously observed this phenomenon in patients with Turner syndrome who underwent EP therapy [15]. In this aforementioned study, an increase in BALP was noted no earlier than after a year of therapy. This suggests that the inhibition of bone resorption is the primary effect of estrogen therapy, and that enhanced osteogenesis takes place during the later stages of treatment.
According to our knowledge, this study is the first of its kind to confirm the effectiveness of estrogen therapy in pubertal girls with functional amenorrhea. Previous studies did not confirm the efficiency of EP therapy in FHA girls with anorexia nervosa [18, 19], athletic amenorrhea patients [20], or FHA females who were ballet dancers [21]. However, the duration of hormonal treatment in these aforementioned studies was significantly shorter than in our study, and did not exceed 2 years in length in any of the experiments. In turn, our results suggest that the positive influence of hormonal therapy on bone mineral density becomes evident predominantly after 2 years of therapy. Additionally, participants of these previous studies differed from girls taking part in this experiment in terms of higher bone mass depletion at the onset of therapy (particularly in the case of anorexia nervosa patients) and older age. It should be remembered that besides the duration of hormonal treatment, patient age at initiation of therapy seems to be another crucial factor for achieving therapeutic goals. According to current knowledge, the rate of bone mineralization decreases 2 to 4 years after menarche and peak bone mass characteristic for adult women should be achieved by the 5th year after menarche [22].
Our previous results [11], along with studies published by other authors [18–21], suggest that patients with BMD depletion do not respond homogeneously to hormonal treatment. This is not a surprising finding, taking into account the complex etiology of BMD deficits observed in pubertal girls. Besides hormonal dysfunction, these deficits may also be a direct consequence of poor diet, impaired metabolism and/or genetic predispositions. They may also result from various interactions between these aforementioned factors [23]. Paradoxically, in our previous studies of FHA patients we identified higher baseline BMD and lower baseline Ntx levels as factors predicting a delayed response to hormonal treatment [11]. The XXPP haplotype of the estrogen receptor gene was in turn revealed as a predictor of a poorer response to hormonal treatment in our study of Turner syndrome patients [15]. The prognostic value of ER-α genes, however, was not fully confirmed in this current study. Although patients carrying the PP genotype of the PvuII gene were characterized by lower baseline BMD and a slower response of this parameter to treatment, these relationships were not confirmed when entire haplotypes of ER-α were considered during analysis. Also, other authors have not concretely confirmed the role of estrogen receptor genotypes and haplotypes in determining bone mineral density and changes in this parameter following hormonal treatment. Studies on postmenopausal women subjected to hormonal therapy did not reveal which genetic variants of ER-α were predictors of poor BMD response to treatment. Depending on the study, either the xxPP haplotype [24, 25] or the XX genotype of XbaI [26, 27] and PP genotype of PvuII [27] were revealed as negative predictive factors. Other authors, in turn, did not observe significant relationships between ER-α and the response to hormonal treatment [28–30].
The small sample size is one potential weakness of this study. Nonetheless, it revealed the effectiveness of estroprogestagen treatment in BMD depletion associated with functional hypothalamic amenorrhea. Therefore, we have confirmed that this treatment, being the protocol of choice in this primary disorder, is also potent in attenuating other consequences of hypothalamic-pituitary-gonadal axis disorders. This finding seems important not only from the viewpoint of current clinical procedures, but also in the context of the long-term prevention of osteoporosis [31–34]. However, the complex etiology of both FHA and decreased BMD should be kept in mind. Hormonal therapy should therefore be accompanied by appropriate education of patients and their parents, along with proper nutrition and modification of physical activity levels. Providing this multimodal therapeutic attitude, puberty is still the optimal time period for modifying environmental factors that are associated with bone mass gain.
In conclusion, EP therapy is effective in the treatment of BMD disorders associated with FHA, and treatment results do not depend on PvuII and XbaI polymorphisms of ER-α.
References
- 1.Liu JH, Bill AH. Stress-associated or functional hypothalamic amenorrhea in the adolescent. Ann N Y Acad Sci. 2008;1135:179–84. doi: 10.1196/annals.1429.027. [DOI] [PubMed] [Google Scholar]
- 2.Bomba M, Gambera A, Bonini L, et al. Endocrine profiles and neuropsychologic correlates of functional hypothalamic amenorrhea in adolescents. Fertil Steril. 2007;87:876–85. doi: 10.1016/j.fertnstert.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Patton GC, Hibbert ME, Carlin J, et al. Menarche and the onset of depression and anxiety in Victoria, Australia. J Epidemiol Community Health. 1996;50:661–6. doi: 10.1136/jech.50.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slap GB. Menstrual disorders in adolescence. Best Pract Res Clin Obstet Gynaecol. 2003;17:75–92. doi: 10.1053/ybeog.2002.0342. [DOI] [PubMed] [Google Scholar]
- 5.Katz N, Mazer NA. The impact of opioids on the endocrine system. Clin J Pain. 2009;25:170–5. doi: 10.1097/AJP.0b013e3181850df6. [DOI] [PubMed] [Google Scholar]
- 6.Kostrzak A, Warenik-Szymankiewicz A, Meczekalski B. The role of serum PRL bioactivity evaluation in hyperprolactinaemic women with different menstrual disorders. Gynecol Endocrinol. 2009;25:799–806. doi: 10.3109/09513590903209329. [DOI] [PubMed] [Google Scholar]
- 7.Li XF, Bowe JE, Kinsey-Jones JS, Brain SD, Lightman SL, O'Byrne KT. Differential role of corticotrophin-releasing factor receptor types 1 and 2 in stress-induced suppression of pulsatile luteinising hormone secretion in the female rat. J Neuroendocrinol. 2006;18:602–10. doi: 10.1111/j.1365-2826.2006.01450.x. [DOI] [PubMed] [Google Scholar]
- 8.Golden NH, Carlson JL. The pathophysiology of amenorrhea in the adolescent. Ann N Y Acad Sci. 2008;1135:163–78. doi: 10.1196/annals.1429.014. [DOI] [PubMed] [Google Scholar]
- 9.Syed F, Khosla S. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun. 2005;328:688–96. doi: 10.1016/j.bbrc.2004.11.097. [DOI] [PubMed] [Google Scholar]
- 10.Bonjour JP, Chevalley T, Ferrari S, Rizzoli R. The importance and relevance of peak bone mass in the prevalence of osteoporosis. Salud Publica Mex. 2009;51(Suppl 1):S5–17. doi: 10.1590/s0036-36342009000700004. [DOI] [PubMed] [Google Scholar]
- 11.Sowińska-Przepiera E, Chełstowski K, Friebe Z, Syrenicz A. Bone mineral density in girls with functional hypothalamic amenorrhea subjected to estroprogestagen treatment – a 4-year prospective study. Gynecol Endocrinol. 2011;27:966–70. doi: 10.3109/09513590.2011.569605. [DOI] [PubMed] [Google Scholar]
- 12.Willing M, Sowers M, Aron D, et al. Bone mineral density and its change in white women: estrogen and vitamin D receptor genotypes and their interaction. J Bone Miner Res. 1998;13:695–705. doi: 10.1359/jbmr.1998.13.4.695. [DOI] [PubMed] [Google Scholar]
- 13.Gennari L, Becherini L, Masi L, et al. Vitamin D and estrogen receptor allelic variants in Italian postmenopausal women: evidence of multiple gene contribution to bone mineral density. J Clin Endocrinol Metab. 1998;83:939–44. doi: 10.1210/jcem.83.3.4649. [DOI] [PubMed] [Google Scholar]
- 14.Sowers M, Jannausch ML, Liang W, Willing M. Estrogen receptor genotypes and their association with the 10-year changes in bone mineral density and osteocalcin concentrations. J Clin Endocrinol Metab. 2004;89:733–39. doi: 10.1210/jc.2003-030691. [DOI] [PubMed] [Google Scholar]
- 15.Sowińska-Przepiera E, Andrysiak-Mamos E, Chełstowski K, Adler G, Friebe Z, Syrenicz A. Association between ER-alpha polymorphisms and bone mineral density in patients with Turner syndrome subjected to estroprogestagen treatment – a pilot study. J Bone Miner Metab. 2011;29:484–92. doi: 10.1007/s00774-010-0247-3. [DOI] [PubMed] [Google Scholar]
- 16.Tanner JM, Whitehouse RH, Marshall WA, Carter BS. Prediction of adult height from height, bone age, and occurrence of menarche, at ages 4 to 16 with allowance for midparent height. Arch Dis Child. 1975;50:14–26. doi: 10.1136/adc.50.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carling T, Rastad J, Kindmark A, Lundgren E, Ljunghall S, Akerström G. Estrogen receptor gene polymorphism in postmenopausal primary hyperparathyroidism. Surgery. 1997;122:1101–5. doi: 10.1016/s0039-6060(97)90214-2. [DOI] [PubMed] [Google Scholar]
- 18.Munoz MT, Morandé G, García-Centenera JA, Hervás F, Pozo J, Argente J. The effects of estrogen administration on bone mineral density in adolescents with anorexia nervosa. Eur J Endocrinol. 2002;146:45–50. doi: 10.1530/eje.0.1460045. [DOI] [PubMed] [Google Scholar]
- 19.Gordon CM, Grace E, Emans SJ, et al. Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab. 2002;87:4935–41. doi: 10.1210/jc.2002-020545. [DOI] [PubMed] [Google Scholar]
- 20.Gibson JH, Mitchell A, Reeve J, Harries MG. Treatment of reduced bone mineral density in athletic amenorrhea: a pilot study. Osteoporos Int. 1999;10:284–9. doi: 10.1007/s001980050228. [DOI] [PubMed] [Google Scholar]
- 21.Warren MP, Brooks-Gunn J, Fox RP, et al. Persistent osteopenia in ballet dancers with amenorrhea and delayed menarche despite hormone therapy: a longitudinal study. Fertil Steril. 2003;80:398–404. doi: 10.1016/s0015-0282(03)00660-5. [DOI] [PubMed] [Google Scholar]
- 22.Loud KJ, Gordon CM. Adolescent bone health. Arch Pediatr Adolesc Med. 2006;160:1026–32. doi: 10.1001/archpedi.160.10.1026. [DOI] [PubMed] [Google Scholar]
- 23.Havill LM, Mahaney MC, Binkley T, Specker BL. Effects of genes, sex, age, and activity on BMC, bone size, and areal and volumetric BMD. J Bone Miner Res. 2007;22:737–46. doi: 10.1359/jbmr.070213. [DOI] [PubMed] [Google Scholar]
- 24.Albagha OM, McGuigan FE, Reid DM, Ralston SH. Estrogen receptor alpha gene polymorphisms and bone mineral density: haplotype analysis in women from the United Kingdom. J Bone Miner Res. 2001;16:128–34. doi: 10.1359/jbmr.2001.16.1.128. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi N, Fujino T, Shirogane T, et al. Estrogen receptor alpha polymorphism as a genetic marker for bone loss, vertebral fractures and susceptibility to estrogen. Maturitas. 2002;41:193–201. doi: 10.1016/s0378-5122(01)00287-0. [DOI] [PubMed] [Google Scholar]
- 26.Seremak-Mrozikiewicz A, Drews K, Bartkowiak-Wieczorek J, et al. PvuII genetic polymorphism of estrogen receptor alpha in the group of postmenopausal women with osteopenia and osteoporosis [Polish] Ginekol Pol. 2005;76:679–86. [PubMed] [Google Scholar]
- 27.Brodowska A, Starczewski A, Brodowski J, Szydłowska I, Nawrocka-Rutkowska J. The bone mass density in postmenopausal women using hormonal replacement therapy in relation to polymorphism in vitamin D receptor and estrogen receptor genes. Gynecol Endocrinol. 2009;25:315–23. doi: 10.1080/09513590802630138. [DOI] [PubMed] [Google Scholar]
- 28.Bandrés E, Pombo I, González-Huarriz M, Rebollo A, López G, García-Foncillas J. Association between bone mineral density and polymorphisms of the VDR, ERalpha, COL1A1 and CTR genes in Spanish postmenopausal women. J Endocrinol Invest. 2005;28:312–21. doi: 10.1007/BF03347196. [DOI] [PubMed] [Google Scholar]
- 29.Qin YJ, Zhang ZL, Huang QR, et al. Association of vitamin D receptor and estrogen receptor-alpha gene polymorphism with peak bone mass and bone size in Chinese women. Acta Pharmacol Sin. 2004;25:462–8. [PubMed] [Google Scholar]
- 30.Silvestri S, Thomsen AB, Gozzini A, Bagger Y, Christiansen C, Brandi ML. Estrogen receptor alpha and beta polymorphisms: is there an association with bone mineral density, plasma lipids, and response to postmenopausal hormone therapy? Menopause. 2006;13:451–61. doi: 10.1097/01.gme.0000182804.14385.a2. [DOI] [PubMed] [Google Scholar]
- 31.Sypniewska G, Sobanska I, Pater A, Kedziora-Kornatowska K, Nowacki W. Does serum osteoprotegerin level relate to fragility fracture in elderly women with low vitamin D status? Med Sci Monit. 2010;16:CR96–101. [PubMed] [Google Scholar]
- 32.Bączyk G, Opala T, Kleka P. Quality of life in postmenopausal women with reduced bone mineral density: psychometric evaluation of the Polish version of QUALEFFO-41. Arch Med Sci. 2011;7:476–85. doi: 10.5114/aoms.2011.23415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panico A, Lupoli GA, Marciello F, et al. Teriparatide vs. alendronate as a treatment for osteoporosis: changes in biochemical markers of bone turnover, BMD and quality of life. Med Sci Monit. 2011;17:CR442–8. doi: 10.12659/MSM.881905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stojanovic OI, Lazovic M, Lazovic M, Vuceljic M. Association between atherosclerosis and osteoporosis: the role of vitamin D. Arch Med Sci. 2011;7:179–88. doi: 10.5114/aoms.2011.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]