Abstract
Introduction
Fetuin-A is an important player in the enhancement of insulin resistance. There are very limited data available concerning the relationships between fetuin-A, weight status and features of the metabolic syndrome (Met S) in obese Egyptian subjects, and especially in children. The aim of the study was to evaluate fetuin-A serum level in subjects with obesity and its possible association with other laboratory and clinical variables.
Material and methods
A total of 140 obese subjects and 50 controls aged 10-40 years were recruited. Demographic, anthropometric and biochemical features were collected according to a standard protocol. Serum fetuin-A levels were measured using ELISA and the modified Third Report of the National Cholesterol Education Program (NCEP-ATP III) criteria were adopted to diagnose Met S.
Results
A higher level of serum fetuin-A was detected in obese subjects. Met S cases were also significantly associated with higher serum fetuin-A. Fetuin-A correlated significantly with BMI (r = 0.437), systolic (r = 0.228) and diastolic blood pressure (r = 0.295), waist circumference (r = 0.332), insulin resistance calculated by the homeostasis model (HOMA-IR) (r = 0.295) and high-density lipoprotein (HDL) (r = 0.362).
Conclusions
Fetuin-A levels were higher in adults and children with obesity and Met S. They were related to insulin resistance and to features of the Met S in cross-sectional analyses. Our study demonstrates a novel association between human fetuin-A and the Met S among obese subject. Therefore, fetuin-A might be a new promising link between obesity and its comorbidities.
Keywords: fetuin A, obesity, metabolic syndrome, insulin resistance
Introduction
Fetuin-A, formerly named α2-Heremans-Schmid glycoprotein (AHSG), is an abundant serum protein that is exclusively produced by the liver and placenta [1]. It is an important glycoprotein, involved in vascular pathology and bone metabolism [2]. Fetuin-A acts as a natural inhibitor of the insulin receptor tyrosine kinase in liver and skeletal muscle to induce whole-body insulin resistance [3]. It is not only associated with type 2 diabetes [4] but also affects insulin action in adipocytes [5] and generates an atherogenic lipoprotein profile [6]. Recent evidence suggests a pro-inflammatory role of fetuin-A in obesity. Fetuin-A has been suggested to potentially cause insulin resistance and/or metabolic syndrome [3, 5]. In terms of treatment or prevention of insulin resistance, fetuin-A may be considered as a new therapeutic target. To analyze the role of fetuin-A as a pro-inflammatory marker in obesity we evaluated its serum level in subjects with obesity and its possible association with other laboratory and clinical variables.
Material and methods
Material
The study was approved by the local ethics committee of the National Research Center (NRC). Written informed consent was obtained from adults and all children and their parents.
To study fetuin-A level in obese children, adolescents and adults, a total of 140 obese subjects were included in group 1 (“patient group”; 70 children and 70 adults). They were recruited from the Obesity Clinic at the NRC. The pediatric age group included 70 obese children ranging in age from 10 to 18 years. The adult age group included 70 obese adults ranging in age from 19 to 40 years. Adult obesity is diagnosed with body mass index > 30 kg/m2. A child was obese if BMI > 95th percentile for age and gender percentile curves of growth for our population [7]. The obese subjects were divided into two subgroups: subgroup 1A – patients with metabolic syndrome were diagnosed according to the modified NCEP-ATP III criteria [8], subgroup 1B – patients with uncomplicated obesity. Group 2 (“control group”) included 25 children and 25 adults; they were age-matched healthy subjects.
Exclusion criteria: medical conditions associated with obesity such as cardiac, hepatic or renal diseases, hypothyroidism, Cushing syndrome or Turner syndrome, also obesity with mental retardation, such as Prader-Willi syndrome, Laurence-Moon-Biedl and Cohen syndrome, subjects taking anti-inflammatory drugs and pregnancy.
Methods
The following were performed on the studied groups of subjects:
Full history taking through clinical examination, with emphasis on any complications or medications.
Blood pressure measured according to American Heart Association guidelines.
Anthropometric indices: Body weight measured to the nearest 0.1 kg with a balance scale and height measured to the nearest 0.1 cm. Body mass index was calculated as weight divided by height squared (kg/m2). Waist circumference (WC) and hip circumference (HIP C) were measured and waist to hip ratio (WHR) was calculated. Waist to height ratio (W/Ht) was also calculated.
Abdominal ultrasonography (liver and visceral fat).
Ten millimetres of blood were drawn from the antecubital vein under aseptic precautions from fasting (for 12-14 h) subjects. After centrifugation serum was collected from each subject to evaluate inflammatory markers.
Lipid assays in sera and liver function tests (ALT, AST) were performed by an Olympus AU 400 chemistry analyzer.
The serum high sensitivity C reactive protein (hs-CRP) and insulin assay were based on the chemiluminescence technique with the Diagnostic Products Immulite 1000 analyzer (Siemens Medical Diagnostics).
Insulin resistance was calculated by the homeostasis model (HOMA-IR) using the following formula: HOMA-IR = fasting insulin (mU/l) × plasma glucose (mmol/l)/22.5.
Adiponectin was measured by ELISA technique using the kit provided from Orgenium Laboratories’ Adiponectin (Acrp30) (AviBion Human Adiponectin ELISA, Finland).
Human fetuin-A was measured using ELISA technique with the kit provided from Adipo Bioscience, Inc (2348 Walsh Ave, Suite C, Santa Clara, CA 95051, USA) [9].
Statistical analysis
The standard computer program SPSS for Windows, release 12.0 (SPSS Inc., USA) was used for data entry and analysis. All numeric variables were expressed as mean ± standard error of mean (SE). Comparison of different variables in various groups was done using Student t test and Mann-Whitney test for normal and nonparametric variables respectively. Pearson's and Spearman's correlation tests (r = correlation coefficient) were used for correlating normal and non-parametric variables respectively. In order to identify predictors of serum fetuin-A, linear regression analyses were performed. For all tests, a probability (p) less than 0.05 (< 0.05) is considered significant.
Results
Our results showed that among the obese individuals, 84 (60%) were females and 56 (40%) were males. There were no significant differences between obese subjects and control groups in terms of age or gender (p > 0.05).
Obese subjects were divided into two subgroups: subgroup 1A – patients with metabolic syndrome (50 subjects, 14 children, 36 adults); subgroup 1B – patients with uncomplicated obesity (90 subjects, 56 children, 34 adults).
Demographic and metabolic features of the obese participants and controls revealed that obese and control children were comparable with regard to age, systolic and diastolic blood pressure (p > 0.05). Waist circumference and visceral fat thickness (VFT) were significantly higher in obese compared to lean children (p < 0.04) but triglyceride was not significantly higher in obese children than controls. Waist circumference, VFT, fasting blood glucose, fasting insulin, HOMA index and triglyceride were significantly higher in obese adults than in controls (p < 0.001). In addition, adiponectin was significantly lower in obese adults than in controls, while it was not significantly lower in obese children than controls (Tables I and II).
Table I.
Characteristics of the studied population (obese, non-obese children and adults)
| Parameter | 1 = obese 2 = not obese | Children group | Adult group | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Mean | Standard deviation | Sig. (2-tailed) | Mean | Standard deviation | Sig. (2-tailed) | ||
| Age [years] | 1 | 11.28 | 3.71 | 0.82 | 27.6 | 7.9 | 0.78 |
|
|
|
||||||
| 2 | 11.45 | 3.44 | 25.6 | 8.2 | |||
|
| |||||||
| SBP [mm Hg] | 1 | 112.65 | 14.37 | 0.12 | 117.56 | 20.74 | 0.02 |
|
|
|
||||||
| 2 | 107.00 | 11.54 | 102.73 | 9.05 | |||
|
| |||||||
| DBP [mm Hg] | 1 | 73.35 | 11.29 | 0.26 | 78.22 | 9.04 | < 0.01 |
|
|
|
||||||
| 2 | 70.23 | 7.32 | 69.55 | 5.68 | |||
|
| |||||||
| Waist C [cm] | 1 | 92.99 | 14.69 | 0.046 | 101.45 | 18.68 | < 0.01 |
|
|
|
||||||
| 2 | 90.52 | 16.30 | 77.57 | 14.57 | |||
|
| |||||||
| Hip C [cm] | 1 | 104.66 | 19.04 | 0.36 | 119.09 | 16.53 | 0.01 |
|
|
|
||||||
| 2 | 100.27 | 14.36 | 100.29 | 15.46 | |||
|
| |||||||
| SFT [cm] | 1 | 1.81 | 0.66 | 0.98 | 1.69 | 0.80 | 0.65 |
|
|
|
||||||
| 2 | 1.81 | 0.69 | 1.50 | 1.59 | |||
|
| |||||||
| VFT [cm] | 1 | 3.96 | 1.65 | 0.04 | 3.38 | 1.40 | 0.06 |
|
|
|
||||||
| 2 | 3.87 | 1.53 | 2.37 | 1.07 | |||
|
| |||||||
| Liver size [cm] | 1 | 15.93 | 1.65 | 0.46 | 16.01 | 1.42 | 0.03 |
|
|
|
||||||
| 2 | 15.52 | 2.08 | 14.64 | 1.64 | |||
SBP – systolic blood pressure, DBP – diastolic blood pressure, BMI – body mass index, Waist C – waist circumference, Hip C – hip circumference, SFT – subcutaneous fat thickness, VFT – visceral fat thickness
Table II.
Laboratory results of obese, non-obese children and adults
| Parameter | 1 = obese 2 = not obese | Children group | Adult group | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Mean | Standard deviation | Sig. (2-tailed) | Mean | Standard deviation | Sig. (2-tailed) | ||
| Fetuin-A [ng/ml) | 1 | 130.17 | 163.65 | 0.030 | 254.29 | 180.08 | < 0.001 |
|
|
|
||||||
| 2 | 50.16 | 55.17 | 80.27 | 32.75 | |||
|
| |||||||
| hs-CRP [mg/l] | 1 | 4.15 | 8.81 | 0.336 | 7.70 | 16.05 | 0.016 |
|
|
|
||||||
| 2 | 2.64 | 3.64 | 2.14 | 2.67 | |||
|
| |||||||
| Cholesterol | 1 | 168.74 | 30.69 | 0.044 | 203.16 | 45.07 | 0.026 |
|
|
|
||||||
| [mg/dl] | 2 | 184.50 | 31.91 | 177.36 | 29.62 | ||
|
| |||||||
| Triglycerides | 1 | 128.37 | 54.68 | 0.159 | 111.64 | 50.82 | 0.045 |
|
|
|
||||||
| [mg/dl] | 2 | 110.26 | 49.83 | 84.73 | 35.13 | ||
|
| |||||||
| HDL [mg/dl] | 1 | 49.61 | 13.78 | 0.018 | 49.72 | 16.56 | 0.615 |
|
|
|
||||||
| 2 | 42.23 | 11.17 | 47.09 | 11.07 | |||
|
| |||||||
| LDL [mg/dl] | 1 | 115.93 | 28.55 | 0.010 | 129.04 | 39.28 | 0.045 |
|
|
|
||||||
| 2 | 96.24 | 30.20 | 113.36 | 17.61 | |||
|
| |||||||
| Insulin [mU/l] | 1 | 7.29 | 4.07 | 0.893 | 7.52 | 6.35 | 0.007 |
|
|
|
||||||
| 2 | 7.49 | 5.83 | 3.98 | 2.62 | |||
|
| |||||||
| FBG [mg/dl] | 1 | 88.39 | 10.48 | 0.281 | 94.46 | 15.46 | 0.013 |
|
|
|
||||||
| 2 | 93.07 | 23.74 | 84.22 | 9.08 | |||
|
| |||||||
| HOMA_IR | 1 | 1.22 | 0.56 | 0.652 | 1.66 | 1.32 | 0.003 |
|
|
|
||||||
| 2 | 1.12 | 0.76 | 0.83 | 0.34 | |||
|
| |||||||
| Adiponectin | 1 | 55.5 | 46.93 | 0.209 | 5.05 | 2.93 | 0.023 |
|
|
|
||||||
| [µg/ml] | 2 | 75.2 | 64.96 | 64.6 | 39.30 | ||
|
| |||||||
| AST [IU/dl] | 1 | 29.36 | 10.41 | 0.694 | 35.07 | 25.02 | 0.250 |
|
|
|
||||||
| 2 | 31.45 | 27.85 | 80.27 | 32.75 | |||
|
| |||||||
| ALT [IU/dl] | 1 | 21.92 | 12.49 | 0.869 | 7.70 | 16.05 | 0.005 |
|
|
|
||||||
| 2 | 22.68 | 24.91 | 2.14 | 2.67 | |||
|
| |||||||
| Comparison of fetuin-A between obese children and adults | Mean [ng/ml] | Standard deviation | Sig. (2-tailed) | ||||
|
| |||||||
| 1 = obese children | 130.16 | 163.65 | 0.001 | ||||
|
| |||||||
| 2 = obese adults | 254.29 | 180.07 | |||||
|
| |||||||
| Comparison of fetuin-A between normal children and adults | Mean | Standard deviation | Sig. (2-tailed) | ||||
|
| |||||||
| 1 = normal children | 90.70 | 155.16 | 0.536 | ||||
|
| |||||||
| 2 = normal adults | 123.58 | 153.24 | |||||
Abbreviations – see Table I
Descriptive statistics and laboratory results of the metabolic versus non-metabolic group (children and adults) showed that there was a highly statistically significant difference in adults regarding WC, HIP C, and VFT, and liver size in both children and adults. Furthermore, laboratory data showed a highly statistically significant difference in insulin and HOMA_IR in both children and adults (Tables III and IV).
Table III.
Descriptive statistics of metabolic vs. non-metabolic group (children and adults)
| Parameter | 0 = No Met S 1 = Positive Met S | Children group | Adult group | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Mean | Standard deviation | Sig. (2-tailed) | Mean | Standard deviation | Sig. (2-tailed) | ||
| SBP [mm Hg] | 0 | 108.00 | 12.07 | 0.640 | 110.95 | 16.40 | 0.121 |
|
|
|
||||||
| 1 | 110.56 | 16.29 | 119.17 | 15.74 | |||
|
| |||||||
| DBP [mm Hg] | 0 | 71.25 | 10.99 | 0.559 | 73.10 | 8.44 | 0.072 |
|
|
|
||||||
| 1 | 73.89 | 11.40 | 78.33 | 9.24 | |||
|
| |||||||
| Waist C [cm] | 0 | 88.22 | 13.55 | 0.791 | 77.12 | 14.43 | < 0.001 |
|
|
|
||||||
| 1 | 89.67 | 15.60 | 105.33 | 11.83 | |||
|
| |||||||
| Hip C [cm] | 0 | 97.86 | 19.80 | 0.974 | 96.85 | 12.95 | < 0.001 |
|
|
|
||||||
| 1 | 98.11 | 16.48 | 122.89 | 14.84 | |||
|
| |||||||
| SFT [cm] | 0 | 1.65 | 0.55 | 0.066 | 1.25 | 1.23 | 0.050 |
|
|
|
||||||
| 1 | 2.05 | 0.62 | 2.00 | 0.76 | |||
|
| |||||||
| VFT [cm] | 0 | 3.90 | 1.56 | 0.233 | 2.39 | 1.19 | 0.001 |
|
|
|
||||||
| 1 | 4.65 | 1.90 | 4.00 | 1.15 | |||
|
| |||||||
| Liver size [cm] | 0 | 14.17 | 1.89 | 0.030 | 14.53 | 1.37 | < 0.001 |
|
|
|
||||||
| 1 | 15.85 | 1.80 | 16.75 | 1.00 | |||
Abbreviations – see Table I
Table IV.
Laboratory results of metabolic vs. non-metabolic group (children and adults)
| Parameter | 0 = No Met S 1 = Positive Met S | Children group | Adult group | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Mean | Standard deviation | Sig. (2-tailed) | Mean | Standard deviation | Sig. (2-tailed) | ||
| Fetuin-A [mg/dl] | 0 | 96.93 | 35.85 | 0.0489 | 152.43 | 50.75 | 0.0162 |
|
|
|
||||||
| 1 | 158.93 | 55.85 | 226.39 | 69.49 | |||
|
| |||||||
| hs-CRP [mg/l] | 0 | 1.82 | 1.43 | 0.724 | 3.64 | 6.71 | 0.064 |
|
|
|
||||||
| 1 | 2.07 | 2.79 | 14.98 | 26.34 | |||
|
| |||||||
| Cholesterol [mg/dl] | 0 | 172.69 | 32.45 | 0.189 | 188.29 | 49.29 | 0.063 |
|
|
|
||||||
| 1 | 186.92 | 29.04 | 216.33 | 42.24 | |||
|
| |||||||
| Triglycerides [mg/dl] | 0 | 94.15 | 34.77 | 0.010 | 99.95 | 66.71 | 0.309 |
|
|
|
||||||
| 1 | 151.83 | 62.53 | 118.50 | 44.71 | |||
|
| |||||||
| HDL [mg/dl] | 0 | 42.73 | 8.62 | 0.011 | 47.62 | 13.57 | 0.176 |
|
|
|
||||||
| 1 | 35.75 | 6.62 | 42.44 | 9.75 | |||
|
| |||||||
| LDL [mg/dl] | 0 | 111.31 | 31.07 | 0.469 | 120.71 | 33.42 | 0.016 |
|
|
|
||||||
| 1 | 119.17 | 30.03 | 150.28 | 38.29 | |||
|
| |||||||
| Insulin [mU/l] | 0 | 3.46 | 1.65 | 0.002 | 3.67 | 2.17 | 0.004 |
|
|
|
||||||
| 1 | 6.36 | 3.45 | 9.23 | 6.85 | |||
|
| |||||||
| FBG [mg/dl] | 0 | 85.69 | 8.93 | 0.287 | 87.47 | 11.87 | 0.039 |
|
|
|
||||||
| 1 | 89.67 | 13.51 | 96.61 | 13.29 | |||
|
| |||||||
| HOMA_IR | 0 | 0.77 | 0.36 | 0.004 | 0.69 | 0.37 | < 0.001 |
|
|
|
||||||
| 1 | 1.33 | 0.69 | 2.24 | 1.46 | |||
|
| |||||||
| Adiponectin [µg/ml] | 0 | 57.94 | 44.28 | 0.237 | 54.38 | 53.82 | 0.882 |
|
|
|
||||||
| 1 | 78.97 | 67.96 | 82.53 | 87.40 | |||
Abbreviations – see Table 1
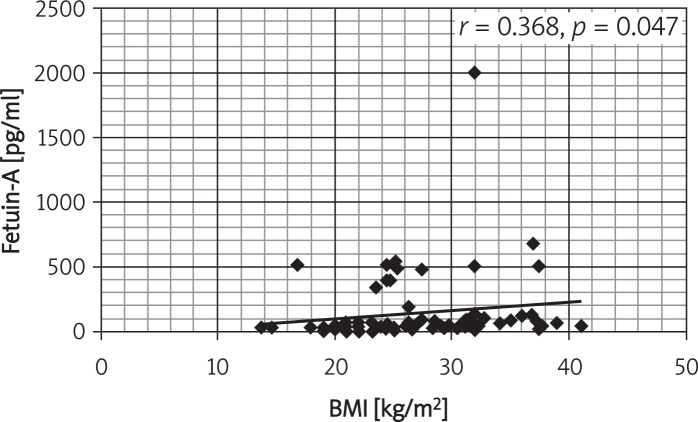
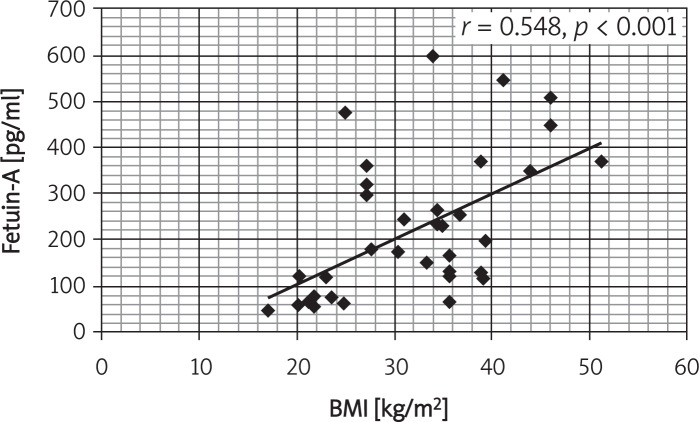
Correlations between serum fetuin-A and the studied variables in obese subjects showed positive correlations with BMI, SBP, DBP, hs-CRP, HDL, HOMA_IR and WC (Table V). Figures 1 and 2 show the correlation between fetuin-A and BMI in controls and obese children and adults.
Table V.
Correlations between fetuin-A and the studied variables in obese subjects
| BMI | SBP | DBP | hs-CRP | TG | LDL | HDL | Waist C | HOMA_IR | Sex | |
|---|---|---|---|---|---|---|---|---|---|---|
| Fetuin-A | 0.437** | 0.228** | 0.295** | 0.194* | 0.085 | 0.166 | 0.362* | 0.332** | 0.262* | –0.061 |
| Sig. (2-tailed) | < 0.001 | 0.010 | 0.001 | 0.023 | 0.326 | 0.053 | 0.020 | < 0.001 | 0.03 | 0.476 |
Correlation is significant at the 0.01 level (2-tailed);
correlation is significant at the 0.05 level (2-tailed). Abbreviations – see Table I
Figure 1.
Correlation between fetuin-A and BMI in controls and obese children
Figure 2.
Correlation between fetuin-A and BMI in controls and obese adults
Stepwise multiple regression analysis of Met S (dependable variable) versus predictor variables (HOMA_IR, fetuin-A, triglyceride, HDL, waist C) in obese children is demonstrated in Table VI.
Table VI.
Stepwise multiple regression analysis of Met S (dependent variable) versus predictor variables
| Dependent variable | Predictor variables | Coefficients β | Constant | Adjusted R 2 | Value of p |
|---|---|---|---|---|---|
| Metabolic syndrome | Waist C | 0.249 | −1.127 | 0.453 | 0.031 |
|
|
|
||||
| HDL | −0.210 | 0.046 | |||
|
|
|
||||
| Triglycerides | 0.287 | 0.006 | |||
|
|
|
||||
| Fetuin-A | 0.379 | 0.001 | |||
|
|
|
||||
| HOMA_IR | 0.238 | 0.028 | |||
Value of p < 0.05 is significant
Discussion
To the best of our knowledge, this is the first study analyzing the cross-sectional relationships between fetuin-A, obesity, insulin resistance, and other markers of the Met S in childhood and adults in Egypt. We are aiming to study fetuin-A levels in obese children, adolescents and adults and to investigate the association of serum fetuin-A and metabolic syndrome (Met S) in obese children and adults.
Insulin sensitivity is influenced by several circulating proteins such as leptin, adiponectin, retinol binding protein 4, tumor necrosis factor α, interleukin-6 and fetuin-A [10, 11]. Our results showed that fetuin-A serum concentrations were significantly higher in obese children and adults as compared to controls. Because fetuin-A knockout mice are resistant to weight gain on a high-fat diet [12] one could speculate that high fetuin-A levels lead to obesity. The hypothesis that obesity leads to increased fetuin-A levels in obese individuals is supported by animal studies. In a rat model of diet-induced obesity, an increase in fetuin-A mRNA expression was observed in the liver [13]. Conversely, other study [14] reported that obese children demonstrated similar fetuin-A concentrations compared with normal weight children. Fetuin-A levels decreased after substantial weight loss, demonstrating the reversibility of the increased fetuin-A concentrations in humans, and pointing toward increased fetuin-A levels as a consequence rather than a cause of obesity. We also found that the fetuin-A levels of obese adults we studied were significantly higher than those of obese children, while there was no difference between children and adult controls similar to those of obese adults [15, 16]. In conjunction with existing adult data, fetuin-A concentrations were independent of gender [16, 17].
Obesity is the most common risk factor for the metabolic syndrome, a cluster of increased waist circumference, dyslipidemia, impaired glucose metabolism, hypertension, and atherosclerosis [18]. Because nonalcoholic fatty liver disease (NAFLD) and Met S often begin in childhood or young adulthood [19], studies in this age group are important. We detected a significant correlation between fetuin-A and insulin resistance in obese adults. Thomas and Christian reported that fetuin-A levels decreased significantly and in a parallel manner to the decrease of insulin resistance in obese children who reduced their overweight substantially due to a lifestyle intervention in contrast to obese children without substantial weight loss in the course of 1 year [14].
Furthermore, the cross-sectional significant relationships between serum fetuin-A level and features of the Met S in our study support the hypothesis that fetuin-A is probably involved in the pathogenesis of insulin resistance and Met S in humans. Prior research relating fetuin-A to insulin resistance in animal studies suggests that fetuin-A interferes with insulin action at peripheral tissues through its interaction with the insulin receptor [20]. We were able to show that obese children with Met S demonstrated higher fetuin-A concentrations than obese children without Met S and healthy controls, in concordance with a study in adults [21]. Fetuin-A correlated with many features of the Met S such as blood pressure, waist circumference, and HDL cholesterol, in concordance with studies in adults [21, 22]. These correlations were also found in longitudinal analyses in obese children [14]. Interestingly, the fetuin-A gene in humans localizes to a site previously linked to the Met S quantitative trait locus [23]. Recently, polymorphisms in the gene encoding human fetuin-A were found not only to be associated with type 2 diabetes [24], but also to affect insulin action in adipocytes [25].
These findings support the hypothesis that fetuin-A is probably involved in the pathogenesis of insulin resistance and Met S in humans. Fetuin-A seems to interfere with insulin action at peripheral tissues through its interaction with the insulin receptor [26]. Studies evaluating whether this effect is mediated through tissue-specific actions of the inhibitory effect of fetuin-A on the insulin receptor tyrosine kinase or through alternative pathways in humans could yield novel insights into the regulatory mechanisms of dyslipidemia, hypertension, and disturbed glucose metabolism. Unfortunately, there are limited data available on the role of fetuin-A as a regulator of insulin sensitivity in humans. Mori et al. did not find a significant association between fetuin-A and insulin resistance in type 2 diabetic subjects [15]. In contrast, other studies demonstrated a relationship between fetuin-A and insulin resistance in adults without type 2 diabetes [3, 26, 27]. Furthermore, fetuin-A has been demonstrated as an independent risk factor of type 2 diabetes [26]. Ix et al. [27] suggested that the relationship between fetuin-A and the Met S may be a result of fetuin-A induced suppression of adiponectin production. In fact, Hennige et al. [28] recently demonstrated that fetuin-A represses adiponectin production in animals and humans. Adiponectin is an adipocytokine, and represents an important determinant of whole body sensitivity and cardiovascular disease [29]. In addition, fetuin-A induced low grade inflammation [28], which is also associated with the Met S and an atherogenic lipid profile [1, 5]. Recently, Stępien et al. aimed to estimate the association between anthropometric obesity parameters, serum concentrations of ghrelin, resistin, leptin, adiponectin and homeostasis model assessment (HOMA-IR) in obese non-diabetic insulin-sensitive and insulin-resistant patients. They concluded that waist circumference, adiponectin, leptin and ghrelin are associated with insulin resistance and may be predictors of this pathology [11]. Also, insulin resistance and leptin may be important pathogenic factors in hypertensive patients with severe obesity. Indices of abdominal obesity (WC, WHR) correlate better than BMI with HOMA-IR, insulin, adiponectin and leptin serum levels in hypertensive obese patients [30].
Limitations – There was a relatively small number of cases and we did not study the effect of losing weight on fetuin-A serum level.
In conclusion, fetuin-A concentrations are higher in obese children and adults as compared to controls. Since fetuin-A is significantly related to insulin resistance and other features of the Met S such as increased waist circumference, increased blood pressure, and decreased HDL cholesterol levels, these findings support the hypothesis of a functionally relevant relationship between fetuin-A and Met S in obesity. Further prospective research is necessary to clarify the role of fetuin-A in the pathogenesis of insulin resistance, especially in obese humans, as well as related molecular pathways leading to the development of Met S.
References
- 1.Denecke B, Graber S, Schafer C, Heiss A, Woltje M, Jahnen-Dechent W. Tissue distribution and activity testing suggest a similar but not identical function of fetuin-B. Biochem J. 2003;376:135–45. doi: 10.1042/BJ20030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briana DD, Boutsikou M, Gourgiotis D, et al. Alpha2-HS-glycoprotein in human pregnancies with normal and restricted fetal growth. J Matern Fetal Neonatal Med. 2008;21:826–30. doi: 10.1080/14767050802326255. [DOI] [PubMed] [Google Scholar]
- 3.Mathews ST, Chellam N, Srinivas PR, et al. Alpha2-HSG, a specific inhibitor of insulin receptor autophosphorylation, interacts with the insulin receptor. Mol Cell Endocrinol. 2000;164:87–98. doi: 10.1016/s0303-7207(00)00237-9. [DOI] [PubMed] [Google Scholar]
- 4.Siddiq A, Lepretre F, Hercberg S, Froguel P, Gibson F. A synonymous coding polymorphism in the alpha2-Heremans-schmid glycoprotein gene is associated with type 2 diabetes in French Caucasians. Diabetes. 2005;54:2477–81. doi: 10.2337/diabetes.54.8.2477. [DOI] [PubMed] [Google Scholar]
- 5.Dahlman I, Eriksson P, Kaaman M, et al. Alpha2-Heremans-Schmid glycoprotein gene polymorphisms are associated with adipocyte insulin action. Diabetologia. 2004;47:1974–9. doi: 10.1007/s00125-004-1556-7. [DOI] [PubMed] [Google Scholar]
- 6.Chan D.C, Barrett HP, Watts GF. Dyslipidemia in visceral obesity: mechanisms, implications, and therapy. Am J Cardiovasc Drugs. 2004;4:227–46. doi: 10.2165/00129784-200404040-00004. [DOI] [PubMed] [Google Scholar]
- 7.Egyptian growth curves. Diabetes Endocrine Metabolism Pediatric Unit Cairo University Childern's Hospital. 2009. Available at: http://dempuegypt.blogspot.com Accessed on 10 October 2011.
- 8.Pergher RNQ, de Melo ME, Halpern A, Mancini MC, Infantil LO. Is a diagnosis of metabolic syndrome applicable to children? J Pediatr (Rio J) 2010;86:101–8. doi: 10.2223/JPED.1983. [DOI] [PubMed] [Google Scholar]
- 9.Tsukumo DM, Carvalho-Filho MA, Carvalheira JBC, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–98. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 10.Stefan N, Hennige A, Staiger H, et al. Alpha2-Heremans-Schmid glycoprotein/fetuin-a is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care. 2006;29:853–7. doi: 10.2337/diacare.29.04.06.dc05-1938. [DOI] [PubMed] [Google Scholar]
- 11.Stępień M, Wlazeł RN, Paradowski M, et al. Serum concentrations of adiponectin, leptin, resistin, ghrelin and insulin and their association with obesity indices in obese normo- and hypertensive patients – pilot study. Arch Med Sci. 2012;8:431–6. doi: 10.5114/aoms.2012.29397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathews ST, Singh GP, Ranalletta M, et al. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes. 2002;51:2450–8. doi: 10.2337/diabetes.51.8.2450. [DOI] [PubMed] [Google Scholar]
- 13.Lin X, Braymer HD, Bray GA, York DA. Differential expression of insulin receptor tyrosine kinase inhibitor (fetuin) gene in a model of dietinduced obesity. Life Sci. 1998;63:145–53. doi: 10.1016/s0024-3205(98)00250-1. [DOI] [PubMed] [Google Scholar]
- 14.Reinehr T, Christian LR. Fetuin-A and its relation to metabolic syndrome and fatty liver disease in obese children before and after weight loss. Clin Endocrinol Metab. 2008;93:4479–85. doi: 10.1210/jc.2008-1505. [DOI] [PubMed] [Google Scholar]
- 15.Mori K, Emoto M, Yokoyama H, et al. Association of serum fetuin-A with insulin resistance in type 2 diabetic and nondiabetic subjects. Diabetes Care. 2006;29:468. doi: 10.2337/diacare.29.02.06.dc05-1484. [DOI] [PubMed] [Google Scholar]
- 16.Axelsson J, Wang X, Ketteler M, et al. Is fetuin-A/alpha2-Heremans-Schmid glycoprotein associated with the metabolic syndrome in patients with chronic kidney disease? Am J Nephrol. 2008;28:669–76. doi: 10.1159/000121358. [DOI] [PubMed] [Google Scholar]
- 17.Lorant DP, Grujicic M, Hoebaus C, et al. Fetuin-A levels are increased in patients with type 2 diabetes and peripheral arterial disease. Diabetes Care. 2011;34:156–61. doi: 10.2337/dc10-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ix JH, Shlipak MG, Brandenburg VM, Ali S, Ketteler M, Whooley M. Association between human and the metabolic syndrome. Circulation. 2006;113:1760–7. doi: 10.1161/CIRCULATIONAHA.105.588723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.l'Allemand D, Wiegand S, Reinehr T, et al. APV-Study Group Cardiovascular risk in 26,008 European overweight children as established by a multicenter database. Obesity (Silver Spring) 2008;16:1672–9. doi: 10.1038/oby.2008.259. [DOI] [PubMed] [Google Scholar]
- 20.Kalabay L, Chavin K, Lebreton JP, Robinson KA, Buse MG, Arnaud P. Human recombinant 2-HS glycoprotein is produced in insect cells as a full length inhibitor of the insulin receptor tyrosine kinase. Horm Metab Res. 1998;30:1–6. doi: 10.1055/s-2007-978822. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y, Xu M, Bi Y, et al. Serum fetuin-A is correlated with metabolic syndrome in middle-aged and elderly Chinese. Atherosclerosis. 2011;216:180–6. doi: 10.1016/j.atherosclerosis.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 22.Reinehr T, de Sousa G, Toschke M, Andler W. Long-term follow-up of cardiovascular disease risk factors in obese children after intervention. Am J Clin Nutr. 2006;84:490–6. doi: 10.1093/ajcn/84.3.490. [DOI] [PubMed] [Google Scholar]
- 23.Mathews ST, Chellam N, Srinivas PR, et al. Alpha2-HSG, a specific inhibitor of insulin receptor autophosphorylation, interacts with the insulin receptor. Mol Cell Endocrinol. 2000;164:87–98. doi: 10.1016/s0303-7207(00)00237-9. [DOI] [PubMed] [Google Scholar]
- 24.Gu D, Reynolds K, Wu X, et al. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet. 2005;365:1398–405. doi: 10.1016/S0140-6736(05)66375-1. [DOI] [PubMed] [Google Scholar]
- 25.Xiang J, Li XY, Xu M, et al. Zinc transporter-8 gene (SLC30A8) is associated with type 2 diabetes in Chinese. J Clin Endocrinol Metab. 2008;93:4107–12. doi: 10.1210/jc.2008-0161. [DOI] [PubMed] [Google Scholar]
- 26.Vionnet N, Hani EH, Dupont S, et al. Genome wide search for type 2 diabetes-susceptibility genes in French whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2-diabetes locus on chromosome 1q21-q24. Am J Hum Genet. 2000;67:1470–80. doi: 10.1086/316887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ix JH, Chertow GM, Shlipak MG, Brandenburg VM, Ketteler M, Whooley MA. Fetuin-A and kidney function in persons with coronary artery disease: data from the Heart and Soul Study. Nephrol Dial Transplant. 2006;21:2144–251. doi: 10.1093/ndt/gfl204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hennige AM, Staiger H, Wicke C, et al. Fetuin-A induces cytokine expression and suppresses adiponectin production. PLoS ONE. 2008;3:1. doi: 10.1371/journal.pone.0001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–92. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stepien M, Rosniak-Bak K, Paradowski M, et al. Waist circumference, ghrelin and selected adipose tissue-derived adipokines as predictors of insulin resistance in obese patients: preliminary results. Med Sci Monit. 2011;17:PR13–8. doi: 10.12659/MSM.882030. [DOI] [PMC free article] [PubMed] [Google Scholar]