Abstract
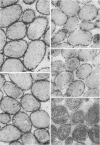
The distribution of characterized antigens in extracts of representative strains of Brucella abortus biotypes 1 to 9 was investigated by hemagglutination and precipitation reactivity. An antigen designated X was present in high concentrations in sodium dodecyl sulfate extracts of all smooth strains examined, but in low concentrations in the rough strain 45/20 extract. Antigen beta was demonstrable in 8 out of 12 smooth strains of B. abortus, but antigen gamma was detected in only 3 of these strains. Vaccine strain 19 extract contained beta antigen but no detectable gamma antigen, whereas both these antigens were present in rough strain 45/20 at slightly lower concentrations that in strain 544/W extract. Immunoenzyme histochemical studies performed with an electron microscope showed that antigens beta, gamma, and X were all situated in the outer membrane envelope of the cell wall of B. abortus 544/W.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baughn R. E., Freeman B. A. Antigenic structure of Brucella suis spheroplasts. J Bacteriol. 1966 Nov;92(5):1298–1303. doi: 10.1128/jb.92.5.1298-1303.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson H. E., Hurvell B., Lindberg A. A. Enzyme-linked immunosorbent assay (ELISA) for titration of antibodies against Brucella abortus and Yersinia enterocolitica. Acta Pathol Microbiol Scand C. 1976 Jun;84(3):168–176. doi: 10.1111/j.1699-0463.1976.tb00016.x. [DOI] [PubMed] [Google Scholar]
- Corbel M. J., Day C. A. Assessment of indirect haemagglutination procedures for the serological diagnosis of bovine brucellosis. Br Vet J. 1973 Sep-Oct;129(5):480–492. doi: 10.1016/s0007-1935(17)36389-3. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE PETRIS S., KARLSBAD G., KESSEL R. W. THE ULTRASTRUCTURE OF S AND R VARIANTS OF BRUCELLA ABORTUS GROWN ON A LIFELESS MEDIUM. J Gen Microbiol. 1964 Jun;35:373–382. doi: 10.1099/00221287-35-3-373. [DOI] [PubMed] [Google Scholar]
- Diaz R., Jones L. M., Leong D., Wilson J. B. Surface antigens of smooth brucellae. J Bacteriol. 1968 Oct;96(4):893–901. doi: 10.1128/jb.96.4.893-901.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubray G. Etude ultrastructurale des bactéries de colonies lisses (S) et rugueuses (R) du genre "Brucella". Ann Inst Pasteur (Paris) 1972 Aug;123(2):171–193. [PubMed] [Google Scholar]
- Dubray G., Plommet M. Structure et constituants des Brucella. Caractérisation des fractions et propriétés biologiques. Dev Biol Stand. 1976;31:68–91. [PubMed] [Google Scholar]
- Freeman B. A., Baughn R. E., McGhee J. R. Some physical, chemical, and taxonomic features of the soluble antigens of the Brucellae. J Infect Dis. 1970 May;121(5):522–527. doi: 10.1093/infdis/121.5.522. [DOI] [PubMed] [Google Scholar]
- Hatten B. A., Brodeur R. D. Soluble antigens of virulent and attenuated biotypes of Brucella abortus. Infect Immun. 1978 Dec;22(3):956–962. doi: 10.1128/iai.22.3.956-962.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinsdill R. D., Berman D. T. Antigens of Brucella abortus. I. Chemical and immunoelectrophoretic characterization. J Bacteriol. 1967 Feb;93(2):544–549. doi: 10.1128/jb.93.2.544-549.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARLSBAD G., KESSEL R. W., DE PETRIS S., MONACO L. ELECTRON MICROSCOPE OBSERVATIONS OF BRUCELLA ABORTUS GROWN WITHIN MONOCYTES IN VITRO. J Gen Microbiol. 1964 Jun;35:383–390. doi: 10.1099/00221287-35-3-383. [DOI] [PubMed] [Google Scholar]
- Kellerman G. D., Foster J. W., Badakhsh F. F. Comparison of Chemical Components of Cell Walls of Brucella abortus Strains of Low and High Virulence. Infect Immun. 1970 Sep;2(3):237–243. doi: 10.1128/iai.2.3.237-243.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulshrestha R. C., Ramachandran M. The use of various extracts of Brucella abortus for haemagglutination test in brucellosis. Indian Vet J. 1971 Jun;48(6):564–569. [PubMed] [Google Scholar]
- MACRAE R. M., SMITH H. THE CHEMICAL BASIS OF THE VIRULENCE OF BRUCELLA ABORTUS. VI. STUDIES ON IMMUNITY AND INTRACELLULAR GROWTH. Br J Exp Pathol. 1964 Dec;45:595–603. [PMC free article] [PubMed] [Google Scholar]
- McGhee J. R., Freeman B. A. Fractionation of Phenol Extracts from Brucella suis: Separation of Multiple Biologically Active Components. Infect Immun. 1970 Sep;2(3):244–249. doi: 10.1128/iai.2.3.244-249.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Pitt M. W., Jones L. M., Schurig G. G., Berman D. T. Purification and characterization of smooth and rough lipopolysaccharides from Brucella abortus. J Bacteriol. 1979 May;138(2):361–369. doi: 10.1128/jb.138.2.361-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy L. K. Studies on the precipitin content of bovine sera prepared against various biotypes of Brucella abortus. Immunology. 1967 Apr;12(4):463–474. [PMC free article] [PubMed] [Google Scholar]
- Plackett P., Cottew G. S., Best S. J. An indirect haemolysis test (IHLT) for bovine brucellosis. Aust Vet J. 1976 Mar;:136–140. doi: 10.1111/j.1751-0813.1976.tb05448.x. [DOI] [PubMed] [Google Scholar]
- Raybould T. J., Chantler S. Serological differentiation between infected and vaccinated cattle by using purified soluble antigens from Brucella abortus in a hemagglutination system. Infect Immun. 1980 Aug;29(2):435–441. doi: 10.1128/iai.29.2.435-441.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybould T. J., Chantler S. Serological differentiation between infected and vaccinated cattle by visual and quantitative immunofluorescence using Brucella abortus antigen coupled sepharose beads. J Immunol Methods. 1979;30(1):37–46. doi: 10.1016/0022-1759(79)90271-0. [DOI] [PubMed] [Google Scholar]
- Renoux M. A passive hemagglutination test for the detection of Brucella infection. J Immunol Methods. 1980;32(4):349–355. doi: 10.1016/0022-1759(80)90027-7. [DOI] [PubMed] [Google Scholar]
- SMITH H., FITZGEORGE R. B. THE CHEMICAL BASIS OF THE VIRULENCE OF BRUCELLA ABORTUS. V. THE BASIS OF INTRACELLULAR SURVIVAL AND GROWTH IN BOVINE PHAGOCYTES. Br J Exp Pathol. 1964 Apr;45:174–186. [PMC free article] [PubMed] [Google Scholar]
- Saunders G. C., Clinard E. H. Rapid micromethod of screening for antibodies to disease agents using the indirect enzyme-labeled antibody test. J Clin Microbiol. 1976 Jun;3(6):604–608. doi: 10.1128/jcm.3.6.604-608.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurig G. G., Jones L. M., Speth S. L., Berman D. T. Antibody response to antigens distinct from smooth lipopolysaccharide complex in Brucella infection. Infect Immun. 1978 Sep;21(3):994–1002. doi: 10.1128/iai.21.3.994-1002.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. A., Walker P. D. The location of bacterial antigens on sections of Bacillus cereus by use of the soluble peroxidase--anti-peroxidase complex and unlabelled antibody. J Gen Microbiol. 1975 Jul;89(1):93–101. doi: 10.1099/00221287-89-1-93. [DOI] [PubMed] [Google Scholar]
- Smith H. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977 Jun;41(2):475–500. doi: 10.1128/br.41.2.475-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemshorn B., Nielsen K. The bovine immune response to Brucella abortus I. A water soluble antigen precipitated by sera of some naturally infected cattle. Can J Comp Med. 1977 Apr;41(2):152–159. [PMC free article] [PubMed] [Google Scholar]