Abstract
Metformin is generally recommended as first-line treatment in type 2 diabetes, especially in overweight patients, but in recent years new indications for its use have emerged. Metformin has been found to be safe and efficacious both as monotherapy and in combination with all oral antidiabetic agents and insulins. If metformin use during pregnancy and the lactation period is supported by few data, it could be indicated for women with polycystic ovary syndrome, since it could diminish circulating androgens and insulin resistance, thus ameliorating the ovulation rate. Metformin seems to reduce cancer risk, which appears to be increased in diabetics, and is a promising agent for oncoprevention and chemotherapy combinations. Moreover, metformin could find a place in the treatment of non-alcoholic fatty liver disease. Lactic acidosis could be decreased by avoiding metformin use in patients with hypovolemia, sepsis, renal impairment, hypoxic respiratory diseases and heart failure, in the preoperative period and before intravenous injection of contrast media.
Keywords: metformin, type 2 diabetes, efficacy, safety, cancer
Introduction
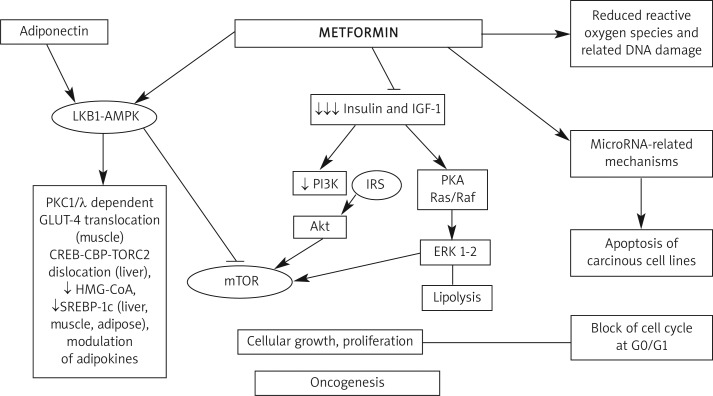
The biguanide metformin, put on the market 50 years ago, is now generally accepted as first-line treatment in type 2 diabetes mellitus (T2DM), especially in overweight patients. It improves peripheral and liver sensitivity to insulin, reduces basal liver glucose production, increases insulin-stimulated uptake and utilization of glucose by peripheral tissues, decreases appetite and causes weight reduction (Figure 1). Both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists and American College of Endocrinology (referred to as AACE) recommend metformin as first-line therapy in T2DM [1]. In recent years, new indications for metformin use in clinical practice have emerged, besides diabetes. The aim of this review is to summarize these new fields according to reports in the medical literature (Table I).
Figure 1.
Metformin mechanism of action
LKB1-AMPK – liver kinase B1-adenosine monophosphate activated protein kinase, PI3K – phosphoinositide 3-kinase inhibitor, IRS – insulin receptor substrate, mTOR – mammalian target of rapamycin, IGF-1 – insulin-like growth factor 1, PKA – protein kinase A, ERK – extracellular-signalregulated kinases, GLUT-4 – glucose transporter type 4, HMG-CoA – 3-hydroxy-3-methyl-glutaryl-CoA reductase, SREBP-1c – sterol regulatory element binding proteins
Table I.
Summary of metformin molecular and metabolic actions
Anti-obesity effects:
|
Anti-hyperglycemic effects:
|
Anti-lipidemic effects:
|
Anti-diabetic protective effects:
|
Hepatoprotective effects:
|
Anti-neoplastic effects:
|
Cardioprotective effects:
|
GLP-1 – glucagon-like peptide-1, AMPK – AMP-activated protein kinase, GLUT-4 – glucose transporter type 4, IGF-1 – insulin-like growth factor 1, PAI-1 – plasminogen activator inhibitor-1
Metformin in type 2 diabetes treatment: what's old and what's new?
Metformin monotherapy has been estimated to reduce glycated hemoglobin (HbA1c) by approximately 1.5% with a dose-dependent glucose lowering effect [2, 3]. Garber et al. [4] and Fujioka et al. [5] described a clear dose-response relationship of treatment with metformin and found that its therapeutic dose is between 1500 mg/day and 2000 mg/day.
Hoffmann et al. [6] showed that metformin 850 mg twice daily and acarbose 100 mg three times a day are equally effective when compared with placebo. In another study [7], 250 patients were randomized to metformin or pioglitazone: a similar reduction in HbA1c and fasting blood glucose was observed, although pioglitazone was significantly more effective in improving insulin sensitivity defined by homeostasis model assessment for insulin sensitivity (HOMA-S).
Previously, the ADOPT (A Diabetes Outcomes Progression Trial) study considered rosiglitazone, metformin and glyburide as the initial treatment of newly diagnosed type 2 diabetics [8]. There was no difference in the proportion of patients reaching the HbA1c target of < 7%, but the incidence of monotherapy failure was higher with glyburide and metformin, despite the fact that metformin-treated patients had significantly lower body weight compared with rosiglitazone-treated ones.
Dipeptidyl peptidase-4 (DPP-4) inhibitors are new drugs for T2DM treatment [9, 10]. Goldstein et al. [11] compared sitagliptin, metformin and placebo: both agents accomplished significant reductions in HbA1c, slightly more pronounced with metformin. Also, Derosa et al. [12] compared sitagliptin or metformin in addition to pioglitazone: both treatments improved HbA1c, but metformin also led to a decrease of body weight and to a faster and better improvement of insulin resistance and inflammatory state parameters. Sitagliptin + metformin also improved β-cell function defined by HOMA-β better than metformin alone [13]. Similar results were found in a placebo-controlled, randomized trial with vildagliptin, another DPP-4 inhibitor [14–16].
The efficacy of metformin treatment was thoroughly evaluated in a Cochrane review considering 29 trials and 5,259 participants [17]. In this paper, metformin was compared with sulfonylureas (13 trials), glitazones (3 trials), meglitinides (2 trials), α-glucosidase inhibitors (2 trials), placebo (12 trials), diet (3 trials) and insulin (2 trials). Metformin monotherapy was associated with significant improvements in weight, lipemia and diastolic blood pressure; glycemic control was better when compared with placebo or diet and modestly better when compared with sulfonylureas.
Metformin can safely and efficaciously be combined with all oral antidiabetic agents and insulins. Metformin-glibenclamide combination caused greater reduction in fasting blood glucose [18], whereas metformin-glyburide combination improved the hypoglycemic effect of this sulfonylurea, leading to lower HbA1c levels [19]. In another study with glimepiride, a reduction in postprandial glycemia was also observed, but the risk of hypoglycemia was increased [20]. Similar results were also reported with glibenclamide and glipizide [21, 22].
Nateglinide and repaglinide are other drugs commonly used in combination with metformin. Metformin-nateglinide combination resulted in better HbA1c levels and modest decrease in fasting glucose levels [23]. Combination with repaglinide caused reduced fasting glucose by 39.6 mg/dl and HbA1c by 1.4% [24]. Metformin-acarbose combination led to a reduction of 20.38 mg/dl in fasting glucose levels and of 1.02% in HbA1c [25], but a potential increase in gastrointestinal side effects should be considered. In a multicenter study, metformin-miglitol combination significantly improved HbA1c, postprandial glucose levels and fasting glucose [26].
Glitazones, another group of antiglycemic agents, have also been adequately studied in combination with metformin [27, 28]. Although rosiglitazone is no longer available in current treatment of T2DM after new evidence suggested an increased cardiovascular risk linked to this drug [29], in previous studies its association with metformin allowed the glycemic target (HbA1c < 7.0%) to be reached in 54-58% of patients [30, 31] and in the Karamanos et al. study [32] this combination significantly increased high-density lipoprotein (HDL) cholesterol levels. Pioglitazone was studied in combination with metformin, as well [33, 34]. Besides improving glycemic values, this combination showed beneficial effects on lipid profile with a significant decrease in triglycerides and a slight increase in HDL levels [35].
Combination of metformin with DPP-4 inhibitors is another therapeutic option that brings a few benefits, such as weight reduction and better glycemic control [9, 10]. In a placebo-controlled study involving diabetics poorly controlled with metformin > 1500 mg/day, the addition of sitagliptin reduced HbA1c and fasting blood glucose, and more patients treated with sitagliptin reached the HbA1c target of < 7% (47%) compared with those receiving placebo (18.3%), with no increase in hypoglycemias. Another study with 780 drug-naïve patients showed that more patients attained HbA1c < 7% with metformin-vildagliptin combination than using each drug alone [36]. A randomized, double-blind, placebo-controlled study considering saxagliptin or placebo addition to metformin 1500-2000 mg/day in 743 patients reported a significant, dose-dependent reduction in HbA1c and fasting blood glucose [37].
Metformin combination with glucagon-like peptide-1 (GLP-1) analogues exenatide and liraglutide was found to be safe and efficacious [38–40]. When comparing liraglutide, metformin, metformin plus glimepiride and metformin plus liraglutide, this last association was the most effective treatment, reducing fasting blood glucose by 70.2 mg/dl and HbA1c by 0.8% [41]. In a study with 150 patients poorly controlled with maximal doses of metformin, the addition of exenatide resulted in mean reduction in HbA1c of 1% which persisted after 82 weeks [42].
Metformin-insulin combinations are commonly used to decrease insulin resistance, reduce insulin need and minimize weight gain. Studies with new analogue insulins showed efficacy of this combination as well, with decreased side effects such as hypoglycemia and weight gain [43].
Metformin in gestational diabetes
Gestational diabetes is an interesting research area for metformin. Insulin treatment is effective and considered safe during pregnancy, but it requires sufficient education and patient skills to provide good compliance and prevent hypoglycemias. Therefore, oral antidiabetic treatments are being researched as more convenient alternatives to insulin during this period and the medical literature reveals many studies about fetal and maternal outcomes after metformin use in gestational diabetes. Available data are mainly from patients with polycystic ovary syndrome (PCOS) during pregnancy.
Non-diabetic women with PCOS who conceived during metformin treatment had a 10-fold reduction of gestational diabetes [44] and prospective studies showed similar results with no increase in congenital defects in newborns or spontaneous abortions [45]. These results supported the idea of using insulin-sensitizers in this group of subjects [46]. Other studies underlined a reduction of complications with metformin use during pregnancy in patients with PCOS [47]. Metformin pharmacokinetics are similar in pregnant and non-pregnant women; metformin easily crosses the placenta so the fetus is exposed to drug concentrations comparable with those of the mother [48]. In 2008 Rowan et al. [49] studied 751 women at 20-33 weeks of pregnancy with gestational diabetes during the MiG trial (Metformin versus Insulin for the treatment of Gestational diabetes). They found that metformin treatment, alone or in association with insulin, was not linked to increased perinatal complications or serious adverse effects.
Although recent studies with larger populations support the safety of metformin during pregnancy [50], the risk of macrosomia [51] and preeclampsia [52] is still not clear and there are also studies with conflicting results [53].
Few studies are available about metformin use during the lactation period. In small studies considering metformin treatment versus formula feeding, no adverse effect was observed on growth, motor-social development and intercurrent illness during the first 6 months of life [54].
So far, evidence for safety of continued therapy throughout gestation is insufficient; published papers are limited in design and might mask fetal toxic outcomes due to metformin therapy. Therefore, patients should be informed about benefits and risks of metformin use during pregnancy before starting a therapy.
Metformin in pre-diabetes and in prevention of cardiovascular disease
Pre-diabetes (defined as impaired glucose tolerance, impaired fasting glucose or both) represents an intermediate state that often progresses to overt T2DM within a few years; therefore it should be increasingly screened for [55]. In addition, pre-diabetes may be associated with a higher risk of microvascular and macrovascular complications [56]. In this context, treatment modalities to revert pre-diabetes to normal are a current challenge for many clinicians [57]. Apart from lifestyle modifications, the use of drugs such as metformin, thiazolidinediones and acarbose is usually needed in high-risk patients [58]. In the analysis of Lilly et al. [59], metformin significantly reduced the rate of conversion of pre-diabetes to diabetes for both high (850 mg twice a day) and low doses (250 mg twice or 3 times a day). Therefore, metformin is important for prevention of T2DM and it is recommended for patients < 60 years of age, individuals with body mass index (BMI) ≥ 35 kg/m2 and those with risk factors such as family history of diabetes in first-degree relatives, elevated triglycerides, reduced HDL cholesterol, hypertension or HbA1c > 6% [60].
Another positive effect of metformin could be cardioprotection. As is well known, the presence of T2DM increases the risk of cardiovascular disease (CVD). Previous studies suggest that metformin monotherapy is associated with a lower death rate when compared with sulfonylureas; death due to CVD is lower in metformin users after adjusting for age, gender, nitrate use and chronic disease score [61, 62]. This difference could be the result of the “healthier” group of patients who use metformin, since metformin is contraindicated in heart failure and renal impairment, or it could be due to additional benefits of metformin on weight gain and lipid profile [63]. Another study showed that, in subjects with MetS without diabetes or CVD, metformin can give a considerable CVD risk reduction together with multifactorial treatment of MetS [64].
Metformin in obesity
Metformin is the first-line treatment for obese type 2 diabetic patients, but a possible role in non-diabetic obese people was also suggested on the basis of its effects on insulin resistance and weight loss. Desilets et al. [65] considered all the studies with metformin concerning weight loss and they reported a significant weight reduction in overweight or obese adults and adolescents without diabetes; positive effects on metabolic parameters such as blood pressure, waist circumference, lipid and glucose/insulin levels were also observed. Many studies have evaluated metformin treatment in patients with weight gain secondary to antipsychotic drug use and the results were encouraging [66, 67]. Beneficial effects on obesity promoted new studies on the pediatric age group, as well [68]; although it has modest, but favorable effects on body weight and glucose homeostasis in insulin-resistant children, more studies are still needed before suggesting metformin use for weight reduction in non-diabetic obese children [69]. However, when compared with other anti-obesity drugs, i.e. orlistat and sibutramine, metformin appeared to be less effective [70]. Its combination with orlistat for obesity treatment did not result in any additional benefit whereas it had a higher risk of gastrointestinal side effects [71]. The association of metformin and thiazolidinediones also compensates the weight gain associated with the use of these drugs [72]. Finally metformin could be a beneficial treatment in patients with impaired fasting blood glucose or impaired glucose tolerance for weight reduction. There is also evidence that metformin has some effects on peptides regulating food intake such as leptin, adiponectin, ghrelin and neuropeptide Y [73, 74].
Metformin in hepatology
Non-alcoholic fatty liver disease (NAFLD) and its end result non-alcoholic steatohepatitis (NASH) are the most common liver disorders worldwide. Their prevalence ranges from 10% to 24% in the general population [75], reaching 60-95% and 28-55% in obese and diabetic patients, respectively [76]. Although the etiology of NAFLD is still unclear, several lines of evidence have indicated a pathogenetic role of insulin resistance in this disorder and it is generally considered as the hepatic component of the metabolic syndrome (MetS). Since NAFLD patients have comorbidities such as obesity, impaired glucose levels, dyslipidemia and hypertension, treatment with an insulin-sensitizing agent may correct several of these aspects. Metformin has beneficial effects on glycemia levels, cardiovascular risk and metabolic complications and improves serum transaminases [77], and weight loss in these patients [78], as also shown in post-hoc analyses of the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) [79] and the Assessing The Treatment Effect in Metabolic syndrome without PercepTible diabetes (ATTEMPT) study [80]. Down-regulation of secretory phospholipase A2 mRNA expression, decrease in serum secretory phospholipase A2, lysophosphatidylcholine and inflammatory response and protection of mitochondrial function are the possible liver protective mechanisms of metformin in NAFLD [81].
Another potential area of interest is the use of metformin during viral hepatitis treatment, but the currently available small studies about hepatitis C patients are still conflicting [82, 83].
Metformin in polycystic ovary syndrome
Polycystic ovary syndrome (PCOS) is a complex and heterogeneous syndrome with increased risk of cardiovascular morbidities and diabetes involving 6.6-6.8% of the women of reproductive age [84]. Insulin resistance and hyperinsulinism have been known as pathogenetic mechanisms for the last 15 years, present in 50-70% of these women, whereas MetS prevalence is higher than in age and weight-matched controls [85]. Even in the absence of obesity or MetS, these patients may have insulin resistance and increased cardiovascular risk [86]. High insulin levels affect the hypothalamus-pituitary-ovarian function, as well as glucose utilization in peripheral tissues [87]. This syndrome was particularly considered in the evaluation of the use of metformin in adolescent patients and in pediatric clinical practice [88].
Metformin reduces circulating androgens and insulin resistance, thus ameliorating the ovulation rate [89]. In the analysis of Tang et al. the ovulation rate was improved by metformin compared with placebo (Pooled OR 2.12, 95% CI: 1.50-3.0) and by metformin plus clomiphene versus clomiphene alone (Pooled OR = 3.46, 95% CI: 1.97-6.07) [90]. The medical literature agrees that metformin alone is not sufficient to restore regular menstruation and ovulation in PCOS patients, and if treatment fails with clomiphene citrate, metformin will not be effective, because it is beneficial only in combination and in obese and/or diabetic/prediabetic PCOS patients [91]. Since women with PCOS constitute a very heterogeneous group of patients, responses to metformin may also be different; in the small but interesting study of Tomova et al. the patients who responded to metformin treatment by restoring regular menstruation and decreasing anti-Mullerian hormone levels (which represent folliculogenesis and quantity and quality of the follicle pool) were significantly overweight, with higher BMI, waist circumference, body fat and blood pressure compared with non-responders [92]. This finding shows that metformin's beneficial effects in PCOS are mainly observed in obese and highly insulin-resistant patient subgroups. Therefore, treatment should be addressed to specific metabolic or reproductive problems and insulin sensitizing drugs are not always the optimum therapy to restore ovulation or reduce hyperandrogenism.
Another review showed that metformin may affect ovulation induction, menstrual irregularities, fertility and hirsutism, as well as lipids, markers of atherosclerosis and inflammation, obesity parameters and quality of life in PCOS women. Metformin seems to improve these features, although conflicting results were also reported [93]. There are also many studies supporting the continuation of metformin throughout pregnancy in PCOS patients but more data are needed, as explained above.
Metformin and cancer
Diabetes itself increases the risk of cancer; although T1DM emerges at an earlier age, intrinsic hyperinsulinemia probably causes higher risk of cancer in type 2 diabetics compared with type 1 diabetic patients. Obesity in type 2 diabetics may be a predisposing factor, as well as high insulin and insulin-like growth factor 1 (IGF-1) levels because of the proliferative effects of insulin; some studies have suggested extrinsic insulin preparations to be another possible cause [94]. Insulin and the IGF-1 axis, indeed, function in an integrated fashion to promote cell growth and survival; chronic exposure to these growth factors enhances carcinogenesis, so factors that influence bioactive IGF-1 will affect cancer risk. Despite the increase in cancer risk in diabetics, patients on metformin show a reduction in cancer risk by nearly 40% [95, 96].
Through antiglycemic actions such as enhancing insulin receptor activation and downstream signaling, biguanides were found to impair mitochondrial adenosine-5’-triphosphate (ATP) production, which resulted in activation of the liver kinase B (LKB1)-5’ adenosine monophosphate-activated protein kinase (AMPK) signaling pathway. This pathway is central for the regulation of cellular energy homeostasis and its activation in conditions of energy stress leads to a physiologic down-regulation of energy consuming processes, such as protein and fatty acid synthesis, restoring ATP levels [97]. Moreover, LKB1 has been recognized as a tumor suppressor gene [98]. In vitro studies showed that metformin activates the LKB1-AMPK pathway, resulting in inhibition of mammalian target of rapamycin (mTOR) and protein synthesis (consistent with the need to reduce energy expenditure) and thereby reducing proliferation [99, 100]. In addition, metformin exhibited an opposite effect on tumor cells with regard to its insulin-sensitizing action: it inhibited insulin-stimulated mTOR activation and proliferation in an AMPK-dependent manner [101].
Therefore, metformin exerts both an indirect effect by decreasing insulin resistance and IGF-1-related proliferative pathways, and a direct one at the cellular level by reducing the production of endogenous reactive oxygen species and associated DNA damage [102], inhibiting cell proliferation, invasion and migration with the up-regulation of miR-26 expression, increasing cell apoptosis in some cancer cell lines [103, 104] and blocking the cell cycle in G0/G1 in vitro and in vivo [105].
In a recent study with 2,763 pancreas cancer patients taken from the UK-based General Practice Research Database, long-term use of metformin was linked to a decrease in pancreas cancer prevalence in women, whereas the use of sulfonylureas and insulin was highly associated with that pathology. In another study with 341 ovarian cancer patients, among whom 28 were diabetic and 16 were on metformin therapy, metformin improved progression-free survival [106]. In the study of He et al. [107] considering 1,983 patients with HER2+ breast cancer, analyses showed that metformin and thiazolidinediones were associated with longer survival and decreased breast cancer-induced mortality. Despite some contradictory results, a recent meta-analysis by Zhang et al. [108] conducted on 107,961 patients with T2DM revealed that metformin treatment was related to a lower risk of colorectal cancers (0.63 [0.47-0.84]; p = 0.002). Metformin-induced beneficial effects were also observed in prostate cancer patients receiving androgen deprivation therapy [109].
Metformin may also be proven useful in lung cancer therapy due to its apoptosis-inducing effect via activation of the JNK/p38 MAPK pathway and GADD153 [110]. In trastuzumab-resistant breast cancer cell lines which were also resistant to rapamycin-induced changes in mTOR activity and cell growth, metformin could still be effective via inhibition of erbB2/IGF-1 receptor interactions [111].
In a study with a doxorubicin-resistant thyroid cancer cell line, metformin showed an antimitogenic effect, as well [112]. Studies with melanoma cell lines also revealed a potential p53 suppressor effect of metformin, in addition to the apoptotic effects on these cells [113].
Metformin was related to improved survival in diabetic patients with the diagnosis of cancer compared with the use of other antidiabetic drugs and with non-diabetic patients [114]. In a study that analyzed hospital discharge records from 2.5 million individuals in the Netherlands, metformin was found to be associated with a lower risk of cancer in general with a hazard ratio of 0.90 (95% CI: 0.88-0.91) compared with the use of sulfonylurea derivatives [115]. In the analysis of DeCensi et al. [116], overall a 31% decrease in cancer incidence was found with metformin compared with other antidiabetic treatments; the highest reductions were seen in colon and pancreatic cancers.
Inhibition of cell proliferation and modulation of mTOR may potentiate the effects of chemotherapeutic drugs [117]. In this context, metformin may be a promising agent for oncoprevention and chemotherapy combinations [118, 119].
Metformin in other areas of clinical use
New-onset diabetes after solid-organ transplantations is one of the major complications. Metformin may be a good alternative to meglitinides with beneficial effects on MetS components, lipid-lowering properties, and anti-neoplastic and cardiovascular protection potential [120].
Beneficial effects of metformin on thyroid nodules are another area of discussion in the medical literature, but larger studies are still needed to make any comment [121].
Metformin and safety
Metformin is one of the most frequently used drugs worldwide, with an estimated 40 million prescriptions in the USA alone in the year 2008, its safety allowing a large area of use [122]. The most common adverse effect of metformin is gastrointestinal upset, including diarrhea, cramps, nausea, vomiting and increased flatulence. However, the main concern about safety is focused on the risk of lactic acidosis, and cases are still seen in medical practice [123]. Metformin-associated lactic acidosis (MALA) is a severe metabolic failure with high related mortality; in severe cases patients may need renal replacement therapy [124]. However, MALA risk could be decreased by avoiding metformin use in patients with high risk of hypovolemia, sepsis, renal impairment, reduced renal capacity (such as the elderly), hypoxic respiratory diseases, and in heart failure [125]. However, a recent review commenting on the relationship between metformin and heart failure mentions that metformin may even reduce the risk of heart failure morbidity and mortality in diabetics [126].
Moreover, metformin administration should be avoided in the 24-78 h of the preoperative period as well as before intravenous injection of contrast media, since its use may increase the risk of nephropathy. However, the recent evaluation of MALA cases from 347 trials by Salpeter et al. [127] showed that the risk of lactic acidosis with metformin was not significantly increased compared with other anti-glycemic agents.
Another important but generally underestimated issue during metformin treatment is the risk of vitamin B12 deficiency [128]. Since diabetes itself exerts a risk of peripheral polyneuropathy, vitamin B12 deficiency-related neuropathy may confuse clinicians in long-term metformin-treated patients [129]. This decrease in vitamin levels could also cause higher homocysteine levels [130]. The amount of vitamin B12 recommended by the Institute of Medicine (IOM) (2.4 µg/day) and the amount available in general multivitamins (6 µg) may not be enough to correct the deficiency in subjects with diabetes [131].
Conclusions
New indications for metformin use are emerging in the medical literature, mainly related to its beneficial effects on insulin resistance and weight loss. Treatment with metformin in pregnant women and in cancer patients is promising, but more data are needed in order to assess drug efficacy and safety in such specific populations.
Acknowledgments
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
References
- 1.Goldman-Levine JD. Beyond metformin: initiating combination therapy in patients with type 2 diabetes mellitus. Pharmacotherapy. 2011;31:44S–53S. doi: 10.1592/phco.31.12.44S. [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM, Buse JB, Davidson MB, Management of hyperglycaemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy A Consensus Statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2006;49:1711–21. doi: 10.1007/s00125-006-0316-2. [DOI] [PubMed] [Google Scholar]
- 3.Scarpello JHB. Optimal dosing strategies for modelling the clinical response to metformin in type 2 diabetes. Br J Diabetes Vasc Dis. 2001;1:28–36. [Google Scholar]
- 4.Garber AJ, Duncan TG, Goodman AM, Mills DJ, Rohlf JL. Efficacy of metformin in type II diabetes: result of a double-blind, placebo-controlled, dose-response trial. Am J Med. 1997;103:491–7. doi: 10.1016/s0002-9343(97)00254-4. [DOI] [PubMed] [Google Scholar]
- 5.Fujioka K, Brazg RL, Raz I, et al. Efficacy, dose-response relationship and safety of once-daily extended release metformin (Glucophage XR) in type 2 diabetic patients with inadequate glycemic control despite prior treatment with diet and exercise: results from two double-blind, placebo-controlled studies. Diabetes Obes Metab. 2005;7:28–39. doi: 10.1111/j.1463-1326.2004.00369.x. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann J, Spengler M. Efficacy of 24-week monotherapy with acarbose, metformin and placebo in dietary-treated NIDDM patients: the Essen-II study. Am J Med. 1997;103:483–90. doi: 10.1016/s0002-9343(97)00252-0. [DOI] [PubMed] [Google Scholar]
- 7.Pavo I, Jermendy G, Varkonyi TT, et al. Effect of pioglitazone compared with metformin on glycemic control and indicators of insulin sensitivity in recently diagnosed patients with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:1637–45. doi: 10.1210/jc.2002-021786. [DOI] [PubMed] [Google Scholar]
- 8.Kahn SE, Haffner SM, Heise MA, et al. ADOPT Study Group. Glycemic durability of rosiglitazone, metformin or glyburide monotherapy. N Eng J Med. 2006;335:2427–43. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 9.Derosa G, Maffioli P. Dipeptidyl peptidase-4 inhibitors: 3 years of experience. Diabetes Technol Ther. 2012;14:350–64. doi: 10.1089/dia.2011.0204. [DOI] [PubMed] [Google Scholar]
- 10.Derosa G, Maffioli P. Patient considerations and clinical utility of a fixed dose combination of saxagliptin/metformin in the treatment of type 2 diabetes. Diabetes Metab Syndr Obes. 2011;4:263–71. doi: 10.2147/DMSO.S16361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE. Sitagliptin 036 Study Group. Effect of initial combination therapy with sitagliptin, a dipeptidyl-peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007;30:1979–87. doi: 10.2337/dc07-0627. [DOI] [PubMed] [Google Scholar]
- 12.Derosa G, Maffioli P, Salvadeo SA, et al. Effects of sitagliptin or metformin added to pioglitazone monotherapy in poorly controlled type 2 diabetes mellitus patients. Metabolism. 2010;59:887–95. doi: 10.1016/j.metabol.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Derosa G, Carbone A, Franzetti I, et al. Effects of a combination of sitagliptin plus metformin vs metformin monotherapy on glycemic control, beta-cell function and insulin resistance in type 2 diabetic patients. Diabetes Res Clin Pract. 2012 doi: 10.1016/j.diabres.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 14.Schweizer A, Counturier A, Foley JE, Dejager S. Comparison between vildagliptin and metformin to sustain reductions in HbA1c over 1 year in drug-naïve patients with type 2 diabetes. Diabet Med. 2007;24:955–61. doi: 10.1111/j.1464-5491.2007.02191.x. [DOI] [PubMed] [Google Scholar]
- 15.Derosa G, Maffioli P, Ferrari I, et al. Effects of one year treatment of vildagliptin added to pioglitazone or glimepiride in poorly controlled type 2 diabetic patients. Horm Metab Res. 2010;42:663–9. doi: 10.1055/s-0030-1255036. [DOI] [PubMed] [Google Scholar]
- 16.Derosa G, Ragonesi PD, Carbone A, et al. Vildagliptin added to metformin on beta-cell function after a euglycemic hyperinsulinemic and hyperglycemic clamp in type 2 diabetes patients. Diabetes Technol Ther. 2012;14:475–484. doi: 10.1089/dia.2011.0278. [DOI] [PubMed] [Google Scholar]
- 17.Saenz A, Fernandez-Esteban I, Mataix A, Ausejo M, Roque M, Moher D. Metformin monotherapy for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;3:CD002966. doi: 10.1002/14651858.CD002966.pub3. [DOI] [PubMed] [Google Scholar]
- 18.Hermann LS, Schersten B, Melander A. Antihyperglycemic efficacy, response prediction and dose response relations of treatment with metformin and sulfonylurea, alone and in primary combination. Diabet Med. 1994;11:953–60. doi: 10.1111/j.1464-5491.1994.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 19.DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin dependent diabetes mellitus. The Multicenter Metformin Study Group. New Eng J Med. 1995;333:541–9. doi: 10.1056/NEJM199508313330902. [DOI] [PubMed] [Google Scholar]
- 20.Charpentier G, Fleury F, Kabir M, Vaur L, Halimi S. Improved glycemic control by addition of glimepiride to metformin monotherapy in type 2 diabetic patients. Diabet Med. 2001;18:828–34. doi: 10.1046/j.1464-5491.2001.00582.x. [DOI] [PubMed] [Google Scholar]
- 21.Marre M, Howlett H, Lehert P, Allavoine T. Improved glycaemic control with metformin-glibenclamide combined tablet therapy (Glucovance) in type 2 diabetic patients inadequately controlled on metformin. Diabet Med. 2002;19:673–80. doi: 10.1046/j.1464-5491.2002.00774.x. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein BJ, Pans M, Rubin CJ. Multicenter, randomized, double masked parallel-group assessment of simultaneous glipizide/metformin as second line pharmacologic treatment for patients with type 2 diabetes mellitus that is inadequately controlled by a sulfonylurea. Clin Ther. 2003;25:890–903. doi: 10.1016/s0149-2918(03)80112-1. [DOI] [PubMed] [Google Scholar]
- 23.Marre M, Van Gaal L, Usadel KH, Ball M, Whatmough I, Guitard C. Nateglinide improves glycemic control when added to metformin monotherapy: results of a randomized trial with type 2 diabetes patients. Diabetes Obes Metab. 2002;4:177–86. doi: 10.1046/j.1463-1326.2002.00196.x. [DOI] [PubMed] [Google Scholar]
- 24.Moses R. Repaglinide in combination therapy. Diabetes Nutr Metab. 2002;15:33–8. [PubMed] [Google Scholar]
- 25.Philips R, Karrasch J, Scott R, Wilson D, Moses R. Acarbose improves glycemic control in overweight type 2 diabetic patients insufficiently treated with metformin. Diabetes Care. 2003;26:269–73. doi: 10.2337/diacare.26.2.269. [DOI] [PubMed] [Google Scholar]
- 26.Chiasson JL, Naditch L, Miglitol Canadian University Investigator Group The synergistic effect of miglitol plus metformin combination therapy in the treatment of type 2 diabetes. Diabetes Care. 2001;24:989–94. doi: 10.2337/diacare.24.6.989. [DOI] [PubMed] [Google Scholar]
- 27.Derosa G, Maffioli P. Thiazolidinediones plus metformin association on body weight in patients with type 2 diabetes. Diabetes Res Clin Pract. 2011;91:265–70. doi: 10.1016/j.diabres.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Derosa G, Maffioli P, Salvadeo SA, et al. Direct comparison among oral hypoglycemic agents and their association with insulin resistance evaluated by euglycemic hyperinsulinemic clamp: the 60's study. Metabolism. 2009;58:1059–66. doi: 10.1016/j.metabol.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Derosa G. Pioglitazone is a valid alternative to rosiglitazone. Am J Cardiovasc Drugs. 2011;11:357–62. doi: 10.2165/11595990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Bailey CJ, Bagdonas A, Rubes J, et al. Rosiglitazone/ metformin fixed dose combination compared with uptitrated metformin alone n type 2 diabetes mellitus: a 24 week, multicenter, randomized, double-blind, parallel-group study. Clin Ther. 2005;27:1548–61. doi: 10.1016/j.clinthera.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Garber A, Klein E, Bruce S, Sankoh S, Mohideen P. Metformin-glibenclamide vs metformin plus rosiglitazone in patients with type 2 diabetes inadequately controlled on metformin monotherapy. Diabetes Obese Metab. 2006;8:156–63. doi: 10.1111/j.1463-1326.2005.00570.x. [DOI] [PubMed] [Google Scholar]
- 32.Karamanos B, Thanopoulou A, Drossinos V, Charalampidou E, Sourmeli S, Archimandritis A. Hellenic ECLA Study Group. Study comparing the effect of pioglitazone in combination with either metformin or sulfonylureas. Curr Med Res Opin. 2011;27:303–13. doi: 10.1185/03007995.2010.542081. [DOI] [PubMed] [Google Scholar]
- 33.Derosa G, D'Angelo A, Ragonesi PD, et al. Metabolic effects of pioglitazone and rosiglitazone in patients with diabetes and metabolic syndrome treated with metformin. Intern Med J. 2007;37:79–86. doi: 10.1111/j.1445-5994.2007.01238.x. [DOI] [PubMed] [Google Scholar]
- 34.Derosa G, D'angelo A, Ragonesi PD, et al. Effects of rosiglitazone and pioglitazone combined with metformin on the prothrombotic state of patients with type 2 diabetes mellitus and metabolic syndrome. J Int Med Res. 2006;34:545–55. doi: 10.1177/147323000603400513. [DOI] [PubMed] [Google Scholar]
- 35.Einhorn D, Rendell M, Rosenzweig J, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride in combination with metformin in the treatment of type 2 diabetes mellitus: a randomized, placebo-controlled study. The Pioglitazone 027 Study Group. Clin Ther. 2000;22:1395–409. doi: 10.1016/s0149-2918(00)83039-8. [DOI] [PubMed] [Google Scholar]
- 36.Schweizer A, Coutrier A, Foley JA, Dejager S. Comparison between vildagliptin and metformin to sustain reductions in HbA1c over 1 year in drug-naïve patients with type 2 diabetes. Diabet Med. 2007;24:955–61. doi: 10.1111/j.1464-5491.2007.02191.x. [DOI] [PubMed] [Google Scholar]
- 37.DeFronzo RA, Hissa MN, Garber AJ, et al. Saxagliptin 014 Study Group. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes on metformin alone. Diabetes Care. 2009;32:1649–55. doi: 10.2337/dc08-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Derosa G, Maffioli P. GLP-1 agonists exenatide and liraglutide: a review about their safety and efficacy. Curr Clin Pharmacol. 2012;7:214–28. doi: 10.2174/157488412800958686. [DOI] [PubMed] [Google Scholar]
- 39.Derosa G, Putignano P, Bossi AC, et al. Exenatide or glimepiride added to metformin on metabolic control and on insulin resistance in type 2 diabetic patients. Eur J Pharmacol. 2011;666:251–6. doi: 10.1016/j.ejphar.2011.05.051. [DOI] [PubMed] [Google Scholar]
- 40.Derosa G, Franzetti IG, Querci F, et al. Exenatide plus metformin compared with metformin alone on beta-cell function in patients with type 2 diabetes. Diabet Med. 2012 doi: 10.1111/j.1464-5491.2012.03699.x. [DOI] [PubMed] [Google Scholar]
- 41.Nauck MA, Hompesch M, Filipczak R, Le TD, Zdravkovic M, Gumprecht J, NN2211-1499 Study Group Five-weeks of treatment with the GLP-1 analogue liraglutide improves glycaemic control and lowers body weight in subjects with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2006;114:417–23. doi: 10.1055/s-2006-924230. [DOI] [PubMed] [Google Scholar]
- 42.Ratner RE, Maggs D, Nielsen LL, et al. Long-term effects of exenatide therapy over 82 weeks on glycemic control and weight in lower weight metformin-treated patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2006;8:419–28. doi: 10.1111/j.1463-1326.2006.00589.x. [DOI] [PubMed] [Google Scholar]
- 43.Papanas N, Maltezos E. Metformin: a review of its use in the treatment of type 2 diabetes. Clin Med Therapeut. 2009;1:1367–81. [Google Scholar]
- 44.Glueck CJ, Wang P, Kobayashi S, Philips H, Sieve-Smith L. Metformin therapy throughout pregnancy reduces the development of gestational diabetes in women with polycystic ovary syndrome. Fertil Steril. 2002;77:520–5. doi: 10.1016/s0015-0282(01)03202-2. [DOI] [PubMed] [Google Scholar]
- 45.Glueck CJ, Wang P, Goldenberg N, Sieve-Smith L. Pregnancy outcomes among women with polycystic ovary syndrome treated with metformin. Hum Reprod. 2002;17:2858–64. doi: 10.1093/humrep/17.11.2858. [DOI] [PubMed] [Google Scholar]
- 46.Checa MA, Requena A, Salvador C, et al. Reproductive Endocrinology Interest Group of the Spanish Society of Fertility. Insulin-sensitizing agents: use in pregnancy and as therapy in polycystic ovary syndrome. Hum Reprod Update. 2005;11:375–90. doi: 10.1093/humupd/dmi015. [DOI] [PubMed] [Google Scholar]
- 47.Vanky E, Salvesen KA, Heimstad R, Fougner KJ, Romundstad P, Carlsen SM. Metformin reduces preganancy complications without affecting androgen levels in pregnant polycystic ovary syndrome women: results of a randomized study. Human Reprod. 2004;19:1734–40. doi: 10.1093/humrep/deh347. [DOI] [PubMed] [Google Scholar]
- 48.Charles B, Norris R, Xiao X, Hague W. Population pharmacokinetics of metformin inlate pregnancy. Drug Monit. 2006;28:67–72. doi: 10.1097/01.ftd.0000184161.52573.0e. [DOI] [PubMed] [Google Scholar]
- 49.Rowan JA, Hague WM, Gao W, Battin MR, Moore MP, MiG Trial Investigators Metformin versus insulin for the treatment of gestational diabetes. N Eng J Med. 2008;358:2003–15. doi: 10.1056/NEJMoa0707193. [DOI] [PubMed] [Google Scholar]
- 50.Goh JE, Sadler L, Rowan J. Metformin for gestational diabetes in routine clinical practice. Diabet Med. 2011;28:1082–7. doi: 10.1111/j.1464-5491.2011.03361.x. [DOI] [PubMed] [Google Scholar]
- 51.Nagai T, Imamura M, Mori M. Metformin use in an obese type 2 diabetic patient from weeks 1 to 21 of pregnancy. J Med. 2003;34:163–8. [PubMed] [Google Scholar]
- 52.Hellmuth E, Damm P, Mølsted-Pedersen L. Oral hypoglycaemic agents in 118 diabetic pregnancies. Diabet Med. 2000;17:507–11. doi: 10.1046/j.1464-5491.2000.00314.x. [DOI] [PubMed] [Google Scholar]
- 53.Vanky E, Stridsklev S, Heimstad R, et al. Metformin versus placebo from first trimester to delivery in polycystic ovary syndrome: a randomized, controlled multicenter study. J Clin Endocrinol Metab. 2010;95:E448–55. doi: 10.1210/jc.2010-0853. [DOI] [PubMed] [Google Scholar]
- 54.Glueck CJ, Wang P. Metformin before and during pregnancy and lactation in polycystic ovary syndrome. Expert Opin Drug Safety. 2007;6:191–8. doi: 10.1517/14740338.6.2.191. [DOI] [PubMed] [Google Scholar]
- 55.Chatterjee R, Narayan KM, Limbscomb J, Philips LS. Screening adults for pre-diabetes and diabetes may be cost-saving. Diabetes Care. 2010;33:1484–90. doi: 10.2337/dc10-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hopper I, Billah B, Skiba M, Krum H. Prevention of diabetes and reduction in major cardiovascular events in studies of subjects with prediabetes: meta-analysis of randomized scontrolled trials. Eur J Cardiovasc Prev Rehab. 2011;18:813–23. doi: 10.1177/1741826711421687. [DOI] [PubMed] [Google Scholar]
- 57.Sullivan SD, Ratner ER. Should the metabolic syndrome patient with prediabetes be offered pharmacotherapy? Curr Diab Rep. 2011;11:91–8. doi: 10.1007/s11892-010-0170-y. [DOI] [PubMed] [Google Scholar]
- 58.Sharma MD, Garber AJ. What is the best treatment for prediabetes? Curr Diab Rep. 2009;9:335–41. doi: 10.1007/s11892-009-0053-2. [DOI] [PubMed] [Google Scholar]
- 59.Lilly M, Godwin M. Treating prediabetes with metformin: systematic review and meta-analysis. Can Fam Physician. 2009;55:363–9. [PMC free article] [PubMed] [Google Scholar]
- 60.Nathan DM, Davidson MB, DeFronzo RA, et al. American Diabetes Association. Impaired fasting glucose and impaired glucose tolerance. Diabetes Care. 2007;30:753–9. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- 61.Johnson JA, Majumdar SR, Simpson SH, Toth EL. Decreased mortality associated with the use of metformin compared with sulfonylurea monotherapy in type 2 diabetes. Diabetes Care. 2002;25:2244–8. doi: 10.2337/diacare.25.12.2244. [DOI] [PubMed] [Google Scholar]
- 62.Turner RC, Holman RR. Metformin and risk of cardiovascular disease. Cardiology. 1999;91:203–4. doi: 10.1159/000006910. [DOI] [PubMed] [Google Scholar]
- 63.Sasali A, Leahy JL. Is metformin cardioprotective? Diabetes Care. 2003;26:243–4. doi: 10.2337/diacare.26.1.243. [DOI] [PubMed] [Google Scholar]
- 64.Athyros VG, Ganotakis E, Kolovou GD, et al. Assessing The Treatment Effect in Metabolic Syndrome Without Perceptible Diabetes (ATTEMPT) Collaborative. Assessing the treatment effect in metabolic syndrome without perceptible diabetes (ATTEMPT): a prospective-randomized study in middle aged men and women. Curr Vasc Pharmacol. 2011;9:647–57. doi: 10.2174/157016111797484080. [DOI] [PubMed] [Google Scholar]
- 65.Desilets AR, Dhakal-Karki S, Dunican KC. Role of metformin for weight management in patients without type 2 diabetes. Ann Pharmacother. 2008;42:817–26. doi: 10.1345/aph.1K656. [DOI] [PubMed] [Google Scholar]
- 66.Miller LJ. Management of atypical antipsychotic drug-induced weight gain: focus on metformin. Pharmacotherapy. 2009;29:725–35. doi: 10.1592/phco.29.6.725. [DOI] [PubMed] [Google Scholar]
- 67.Khan AY, Macaluso M, McHale RJ, Dahmen MM, Girrens K, Ali F. The adjunctive use of metformin to treat or prevent atypical antipsychotic-induced weight gain: a review. J Psychiatr Pract. 2010;16:289–96. doi: 10.1097/01.pra.0000388624.91039.a3. [DOI] [PubMed] [Google Scholar]
- 68.Park MH, Kinra S, Ward KJ, White B, Viner RM. Metformin for obesity in children and adolescents: a systematic review. Diabetes Care. 2009;32:1743–5. doi: 10.2337/dc09-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yanovski JA, Krakoff J, Salaita CG, et al. Effects of metformin on body weight and body composition in obese, insulin-resistant children: a randomized clinical trial. Diabetes. 2011;60:477–85. doi: 10.2337/db10-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gokcel A, Gumurdulu Y, Karakose H, et al. Evaluation of the safety and efficacy of sibutramine, orlistat and metformin in the treatment of obesity. Diabetes Obes Metab. 2002;4:49–55. doi: 10.1046/j.1463-1326.2002.00181.x. [DOI] [PubMed] [Google Scholar]
- 71.Sari R, Balci MK, Coban E, Yazicioglu G. Comparison of the effect of orlistat vs orlistat plus metformin on weight loss and insulin resistance in obese women. Int J Obes Relat Metab Disord. 2004;28:1059–63. doi: 10.1038/sj.ijo.0802707. [DOI] [PubMed] [Google Scholar]
- 72.Derosa G, Tinelli C, Maffioli P. Effects of pioglitazone and rosiglitazone combined with metformin on body weight in people with diabetes. Diabetes Obes Metab. 2009;11:1091–9. doi: 10.1111/j.1463-1326.2009.01087.x. [DOI] [PubMed] [Google Scholar]
- 73.Katsiki N, Mikhailidis DP, Gotzamani-Psarrakou A, Yovos JG, Karamitsos D. Effect of various treatments on leptin, adiponectin, ghrelin and neuropeptide Y in patients with type 2 diabetes mellitus. Expert Opin Ther Targets. 2011;15:401–20. doi: 10.1517/14728222.2011.553609. [DOI] [PubMed] [Google Scholar]
- 74.Weickert MO, Hodges P, Tan BK, Randeva HS. Neuroendocrine and endocrine dysfunction in the hyperinsulinemic PCOS patient: the role of metformin. Minerva Endocrinol. 2012;37:25–40. [PubMed] [Google Scholar]
- 75.Alwis NMW, Day CP. Nonalcoholic fatty liver disease: the mistgradually clears. J Hepatol. 2008;48:S104–12. doi: 10.1016/j.jhep.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 76.Angulo P. Medical progress non-alcoholic fatty liver disease. N Eng J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 77.Sofer E, Boaz M, Matas Z, Mashavi M, Shargorodsky M. Treatment with insulin sensitizer metformin improves arterial properties, metabolic parameters, and liver function in patients with non-alcoholic fatty liver disease: a randomized, placebo-controlled trial. Metabolism. 2011;60:1278–84. doi: 10.1016/j.metabol.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 78.Mazza A, Fruci B, Garinis GA, Giuliano S, Malaguarnera R, Belfiore A. The role of metformin in the management of NAFLD. Exp Diabetes Res. 2012;2012:716404. doi: 10.1155/2012/716404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Athyros VG, Tziomalos K, Gossios TD, et al. GREACE Study Collaborative Group. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376:1916–22. doi: 10.1016/S0140-6736(10)61272-X. [DOI] [PubMed] [Google Scholar]
- 80.Athyros VG, Giouleme O, Ganotakis ES, et al. Safety and impact on cardiovascular events of long-term multifactorial treatment in patients with metabolic syndrome and abnormal liver function tests: a post hoc analysis of the randomised ATTEMPT study. Arch Med Sci. 2011;7:796–805. doi: 10.5114/aoms.2011.25554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang Y, Fu JF, Shi HB, Liu LR. Metformin prevents non-alcoholic fatty liver disease in rats: role of phospholipase A2/lysophosphatidylcholine lipoapoptosis pathway in hepatocytes. Zhonghua Er Ke Za Zhi. 2011;49:139–45. [PubMed] [Google Scholar]
- 82.Wang CC, Kao JH. Metformin improves sustained virologic response in difficult-to-cure hepatitis C. More questions than answers. Hepatology. 2010;51:1082–3. doi: 10.1002/hep.23546. [DOI] [PubMed] [Google Scholar]
- 83.Yilmaz Y, Yonal O, Imeryuz N. Metformin, hepatitis C, and insulin resistance: sufficient evidence? Hepatology. 2009;50:2054–5. doi: 10.1002/hep.23359. [DOI] [PubMed] [Google Scholar]
- 84.Diamanti-Kandarakis E, Christakou CD, Kandaraki E, Economou FN. Metformin an old medication of new fashion: evolving new molecular mechanisms and clinical implications in polycystic ovary syndrome. Eur J Endocrinol. 2010;162:193–212. doi: 10.1530/EJE-09-0733. [DOI] [PubMed] [Google Scholar]
- 85.Dokras A, Bochner M, Hollinrake E, Markham S, Vanvoorhis B, Jagasia DH. Screening women with polycystic ovary syndrome for metabolic syndrome. Obstet Gynecol. 2005;106:131–7. doi: 10.1097/01.AOG.0000167408.30893.6b. [DOI] [PubMed] [Google Scholar]
- 86.Ercan EA, Ertek S, Is G, et al. Factors associated with increased carotid intima-media thickness and being non-dipper in non-obese and normotensive young patients affected by PCOS. Angiology. 2011;62:543–8. doi: 10.1177/0003319711400183. [DOI] [PubMed] [Google Scholar]
- 87.Genazzani AD, Ricchieri F, Lanzoni C. Use of metformin in the treatment of polycystic ovary syndrome. Women's Health. 2010;6:577–93. doi: 10.2217/whe.10.43. [DOI] [PubMed] [Google Scholar]
- 88.Geller DH, Pacaud D, Gordon CM, Misra M. Drug and Therapeutics Committee of the Pediatric Endocrine Society. State of the art review: emerging therapies: the use of insulin sensitizers in the treatment of adolescents with polycystic ovary syndrome (PCOS) Int J Pediatr Endocrinol. 2011;2011:9–27. doi: 10.1186/1687-9856-2011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Motta DA. Metformin in the treatment of polycystic ovary syndrome. Curr Pharm Res. 2008;14:2121–5. doi: 10.2174/138161208785294609. [DOI] [PubMed] [Google Scholar]
- 90.Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin sensitizing drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligomenorrhea and subfertility. Cochrane Database Syst Rev. 2009;7:CD003053. doi: 10.1002/14651858.CD003053.pub3. [DOI] [PubMed] [Google Scholar]
- 91.Duranteau L, Lefevre P, Jeandidier N, Simon T, Christin-Maitre S. Should physicians prescribe metformin to women with polycystic ovary syndrome (PCOS)? Ann Endocrinol. 2010;71:25–7. doi: 10.1016/j.ando.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 92.Tomova A, Deepinder F, Robeva R, Kirilov G, Mechandjiev Z, Kumanov P. Antimullerian hormone in women with polycystic ovary syndrome before and after therapy with metformin. Horm Metab Res. 2011;43:723–7. doi: 10.1055/s-0031-1286307. [DOI] [PubMed] [Google Scholar]
- 93.Katsiki N, Hatzitolios AI. Insulin-sensitizing agents in the treatment of polycystic ovary syndrome: an update. Curr Opin Obstet Gynecol. 2010;22:466–76. doi: 10.1097/GCO.0b013e32833e1264. [DOI] [PubMed] [Google Scholar]
- 94.Giovannucci E. Nutrition, insulin, insulin-like growth factors and cancer. Horm Metab Res. 2003;35:694–704. doi: 10.1055/s-2004-814147. [DOI] [PubMed] [Google Scholar]
- 95.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60:207–21. doi: 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]
- 96.Jonhson A, Pollak M. Insulin, glucose and increased risk of cancer in patients with type 2 diabetes. Diabetologia. 2010;53:2086–8. doi: 10.1007/s00125-010-1855-0. [DOI] [PubMed] [Google Scholar]
- 97.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 98.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: the metabolism and growth control in tumor suppression. Nat Rev Cancer. 2009;9:563–75. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–12. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 100.Shaw RJ, Bardeesy N, Manning BD, et al. The LKB-1 tumor suppressor negatively regulates mTOR signalling. Cancer Cell. 2004;6:91–9. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 101.Engelman JA, Cantley LC. Chemoprevention meets glucose control. Cancer Prev Res. 2010;3:1049–52. doi: 10.1158/1940-6207.CAPR-10-0178. [DOI] [PubMed] [Google Scholar]
- 102.Algire C, Moiseeva O, Descheênes-Simard X, et al. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev Res. 2012;5:536–43. doi: 10.1158/1940-6207.CAPR-11-0536. [DOI] [PubMed] [Google Scholar]
- 103.Li W, Yuan Y, Huang L, Qiao M, Zhang Y. Metformin alters the expression profiles of microRNAs in human pancreatic cancer cells. Diabetes Res Clin Pract. 2012;96:187–95. doi: 10.1016/j.diabres.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 104.Bao B, Wang Z, Ali S, et al. Metformin inhibits cell proliferation, migration and invasion by attenuating CSC function mediated by deregulating miRNAs in pancreatic cancer cells. Cancer Prev Res. 2012;5:355–64. doi: 10.1158/1940-6207.CAPR-11-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kato K, Gong J, Iwama H, et al. The antidiabetic drug metformin inhibits gastric cancer cell proliferation in vivo and in vitro. Mol Cancer Ther. 2012;11:549–60. doi: 10.1158/1535-7163.MCT-11-0594. [DOI] [PubMed] [Google Scholar]
- 106.Romero IL, McCormick A, McEwen KA, et al. Relationship of type II diabetes and metformin use to ovarian cancer progression, survival and chemosensitivity. Obstet Gynecol. 2012;119:61–7. doi: 10.1097/AOG.0b013e3182393ab3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.He X, Esteva FJ, Ensor J, Hortobagyi GN, Lee MH, Yeung SC. Metformin and thiazolidinediones are associated with improved breast cancer-specific survival of diabetic women with HER 2+ breast cancer. Ann Oncol. 2012;23:1771–80. doi: 10.1093/annonc/mdr534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang ZJ, Zheng ZJ, Kan H, et al. Reduced risk of colorectal cancer with metformin therapy in patients with type 2 diabetes: a meta-analysis. Diabetes Care. 2011;34:2323–8. doi: 10.2337/dc11-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nobes JP, Langley SE, Klopper T, Russell-Jones D, Laing RW. A prospective, randomized pilot study evaluating the effects of metformin and lifestyle intervention on patients with prostate cancer receiving androgen deprivation therapy. BJU Int. 2012;109:1495–502. doi: 10.1111/j.1464-410X.2011.10555.x. [DOI] [PubMed] [Google Scholar]
- 110.Wu N, Gu C, Gu H, Hu H, Han Y, Li Q. Metformin induces apoptosis of lung cancer cells through activating JNK/p38 MAPK pathway and GADD153. Neoplasma. 2011;58:482–90. doi: 10.4149/neo_2011_06_482. [DOI] [PubMed] [Google Scholar]
- 111.Liu B, Fan Z, Edgerton SM, Yang X, Lind SE, Thor AD. Potent anti-proliferative effects of metformin on trastzumab-resistant breast cancer cells via inhibition of erbB2/IGF-1 receptor interactions. Cell Cycle. 2011;10:2959–66. doi: 10.4161/cc.10.17.16359. [DOI] [PubMed] [Google Scholar]
- 112.Chen G, Xu S, Renko K, Derwahl M. Metformin inhibits growth of thyroid carcinoma cells, suppresses self renewal of derived cancer stem cells, and potentiates the effect of chemotherapeutic agents. J Clin Endocrinol Metab. 2012;97:E510–20. doi: 10.1210/jc.2011-1754. [DOI] [PubMed] [Google Scholar]
- 113.Janjetovic K, Harhaji-Trajkovic L, Misirkic-Marjanovic M, et al. In vitro and in vivo anti-melanoma action of metformin. Eur J Pharmacol. 2011;668:373–82. doi: 10.1016/j.ejphar.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 114.Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012;35:299–304. doi: 10.2337/dc11-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ruiter R, Visser LE, van Herk-Sukel MP, et al. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care. 2012;35:119–24. doi: 10.2337/dc11-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Decensi A, Puntoni M, Goodwin P, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res. 2010;3:1451–61. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 117.Hanna RK, Zhou C, Malloy KM, et al. Metformin potentiates the effects of paclitaxel in endometrial cancer cells through inhibition of cell proliferation and modulation of mTOR pathyway. Gynecol Oncol. 2012;125:458–69. doi: 10.1016/j.ygyno.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vakana E, Platanias LC. AMPK in BCR-ABL expressing leukemias: regulatory effects and therapeutic implications. Oncotarget. 2011;2:1322–8. doi: 10.18632/oncotarget.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Papanas N, Maltezos E, Mikhailidis DP. Metformin and cancer: licence to heal? Expert Opin Investig Drugs. 2010;19:913–7. doi: 10.1517/13543784.2010.499122. [DOI] [PubMed] [Google Scholar]
- 120.Sharif A. Should metformin be our antiglycemic agent of choice post-transplantation? Am J Transplant. 2011;7:1376–81. doi: 10.1111/j.1600-6143.2011.03550.x. [DOI] [PubMed] [Google Scholar]
- 121.Rezzónico J, Rezzónico M, Pusiol E, Pitoia F, Niepomniszcze H. Metformin treatment for small benign thyroid nodules in patients with insulin resistance. Metab Syndr Related Disord. 2011;9:69–75. doi: 10.1089/met.2010.0026. [DOI] [PubMed] [Google Scholar]
- 122.Pollak M. Metformin and other biguanides in oncology: advancing the research agenda. Cancer Prev Res (Phila) 2010;3:1060–5. doi: 10.1158/1940-6207.CAPR-10-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roche C, Nau A, Peytel E, Moalic JL, Oliver M. Severe lactic acidosis due to metformin: report of 3 cases. Ann Biol Clin (Paris) 2011;69:705–11. doi: 10.1684/abc.2011.0619. [DOI] [PubMed] [Google Scholar]
- 124.Keller G, Cour M, Hernu R, Illinger J, Robert D, Argaud L. Management of metformin-associated lactic acidosis by continuous renal replacement therapy. PLos One. 2011;6:e23200. doi: 10.1371/journal.pone.0023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nye HJ, Herrington WG. Metformin: the safest hypoglycaemic agent in chronic kidney disease? Nephron Clin Pract. 2011;118:c380–3. doi: 10.1159/000323739. [DOI] [PubMed] [Google Scholar]
- 126.Papanas N, Maltezos E, Mikhailidis DP. Metformin and heart failure: never say never again. Expert Opin Pharmacother. 2012;13:1–8. doi: 10.1517/14656566.2012.638283. [DOI] [PubMed] [Google Scholar]
- 127.Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;1:CD002967. doi: 10.1002/14651858.CD002967.pub3. [DOI] [PubMed] [Google Scholar]
- 128.Kos E, Liszek MJ, Emanuele MA, Durazo-Arvizu R, Camacho P. The effect of metformin therapy on vitamin D and B12 levels in patients with diabetes mellitus type 2. Endocr Pract. 2011;22:1–16. doi: 10.4158/EP11009.OR. [DOI] [PubMed] [Google Scholar]
- 129.Bell DS. Metformin-induced vitamin B12 deficiency presenting as a peripheral neuropathy. South Med J. 2010;103:265–7. doi: 10.1097/SMJ.0b013e3181ce0e4d. [DOI] [PubMed] [Google Scholar]
- 130.Palomba S, Falbo A, Giallauria F, et al. Effects of metformin with or without supplementation with folate on homocysteine levels and vascular endothelium of women with polycystic ovary syndrome. Diabetes Care. 2010;33:246–51. doi: 10.2337/dc09-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Reinstatler L, Qi YP, Williamson RS, Garn JV, Oakley GP., Jr Association of biochemical B12 deficiency with metformin therapy and vitamin B12 supplements: the national health and nutrition examination survey, 1999-2006. Diabetes Care. 2012;35:327–33. doi: 10.2337/dc11-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]