Abstract
Introduction
Dexamethasone and vitamin B12 are currently used in the clinic to treat peripheral nerve damage but their mechanisms of action remain incompletely understood. In this study we hypothesized that dexamethasone and vitamin B12 promote the production of endogenous neurotrophic factors, thereby enhancing peripheral nerve repair.
Material and methods
Ninety-six adult male Wistar rats were employed to establish a sciatic nerve injury model. They were then randomly divided into 4 groups to be subjected to different treatment: saline (group A), dexamethasone (group B), vitamin B12 (group C), and dexamethasone combined with vitamin B12 (group D). The walking behavior of rats was evaluated by footprint analysis, and the nerve regeneration was assessed by electrophysiological analysis and ultrastructural examination. The expression of brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor, NT-3 and IL-6 in the injured sciatic nerves was detected by immunohistochemical and RT-PCR analysis.
Results
Dexamethasone and vitamin B12 promoted the regeneration of myelinated nerve fibers and the proliferation of Schwann cells. Furthermore, dexamethasone and vitamin B12 promoted the recovery of sciatic functional index and sensory nerve conduction velocity, and upregulated BDNF expression in the injured sciatic nerves.
Conclusions
Dexamethasone and vitamin B12 promote peripheral nerve repair in a rat model of sciatic nerve injury through the upregulation of BDNF expression. These findings provide new insight into the neurotrophic effects of dexamethasone and vitamin B12 and support the application of these agents in clinical treatment of peripheral nerve injury.
Keywords: dexamethasone, vitamin B12, brain-derived neurotrophic factor, peripheral nerve repair, sciatic nerve
Introduction
Peripheral nerve trauma is a significant cause of morbidity and remains a great challenge in the clinic [1]. After the trauma, the peripheral nerve is hard repair due to the following reasons: the degeneration of motor neurons, the lack of a survival environment for Schwann cells, and the poor ability of the nerve to regenerate [2]. The generation of endogenous neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and glial cell-derived neurotrophic factor (GDNF) is known to play a critical role in supporting axon regeneration during peripheral nerve repair [3, 4]. Dexamethasone and vitamin B12 are currently used in the clinic to treat peripheral nerve damage but their mechanisms of action remain incompletely understood. In this study we hypothesized that dexamethasone and vitamin B12 have synergistic effects to promote the production of endogenous neurotrophic factors, thereby enhancing peripheral nerve repair.
Therefore, we used a rat model of peripheral nerve injury and demonstrated that dexamethasone and vitamin B12 synergistically enhanced the expression of BDNF and promoted the regeneration of myelinated nerve fibers and the proliferation of Schwann cells after injury of the sciatic nerve in rats.
Material and methods
Animal models and experimental groups
Ninety-six adult male Wistar rats (6 weeks old, 180-200 g) were provided by the Animal Center of Liaoning Medical University. The rats were randomly divided into 4 groups (n = 24) and anesthetized with an intraperitoneal injection of 10% chloral hydrate (3 ml/kg body weight), then shaved and washed with antiseptic solution before positioning for surgery. The left sciatic nerve and its two major branches were exposed through a gluteal muscle-splitting incision. A crush injury was created using a fine watchmaker forceps for 10 s on the peroneal nerve at 10 mm from the extensor digitorum longus muscle and complete crush was confirmed by presence of a translucent band across the nerve. The incision was then closed in layers (muscle and skin) with absorbable sutures. All operations were performed on the left limb and the right limb served as a non-operated control. After the surgery, the rats were treated as follows: group A – 0.9% saline was injected into the injured site; group B – dexamethasone (1 mg/kg) was injected into the injured site; group C – vitamin B12 (2 mg/kg) was injected into the injured site; and group D – dexamethasone (1 mg/kg) and vitamin B12 (2 mg/kg) were injected into the injured site. The injection was performed once daily for 28 days. At 1, 3, 7, 14, 21 and 28 days, 4 rats were randomly selected from each group for sciatic functional index (SFI) and sensory nerve conduction velocity (SCV) tests, then the rats were sacrificed and 1 cm of the left sciatic nerve was immediately taken from each rat as experimental samples. In addition, 1 cm of the right sciatic nerve was immediately taken from each rat as experimental samples.
Walking track analysis
At 1, 3, 7, 14, 21 and 28 days, the rats were put on a walking track (15 cm × 50 cm) darkened at one end. White office paper cut to the appropriate dimensions was placed on the bottom of the track. The rat's hind limbs were dipped in Chinese ink, and the rat was permitted to walk down the track, leaving its hind foot prints on the paper. The SFI value was calculated based on the analysis of walking tracks as described previously [5]. Functional recovery was assessed by calculating SFI ratio with SFI value of 0 set as normal and SFI value of (–) 100 set as complete injury.
Electrophysiological analysis
At 1, 3, 7, 14, 21 and 28 days, the rats were anesthetized with chloral hydrate and the SCV of the left sciatic nerve was measured using a Cantata electromyograph (DANTEC Medical, Denmark) as described previously [6].
Immunohistochemical analysis
The sciatic nerves were fixed in 10% formaldehyde and were paraffin wax-embedded. Subsequently, the tissues were cut into 5 µm thick serial sections, which were washed carefully with phosphate buffered saline (PBS) three times. To block endogenous peroxidases, dewaxed paraffin sections were treated with 3% hydrogen peroxide for 20 min. The sections were blocked with 2% goat serum in PBS for 1 h at room temperature, then incubated at 4°C overnight with rabbit antibody against human BDNF, GDNF, neurotrophin-3 (NT-3), and interleukin-6 (IL-6) (1: 250 dilution, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Afterwards, the sections were washed with PBS and subjected to immunohistochemical staining using a PV6000 kit (Bioss Inc. Beijing, China). Next, the sections were washed with PBS and developed with 3,3’-diaminobenzidine (DAB) for 5 min. Finally the sections were counterstained by hematoxylin and assessed by two independent investigators under light microscopy. The staining density was calculated based on the absorbance (A) value of the nerves using an image analysis system.
RT-PCR analysis
Total RNA was extracted from the sciatic nerves using Trizol (Invitrogen, Carlsbad, CA, USA) following the manufacturer's protocol. cDNA was produced by reverse transcription using an RT kit (Takara, Shiga, Japan) following the manufacturer's protocol. PCR amplification of BDNF, GDNF, NT-3, IL-6 and β-actin was done with Taq Master Mix (Promega, Madison, WI, USA) with cDNA synthesized from the nerves. The primers were synthesized by Takara (Dalian, China) with the following sequences: BDNF 5’-CATGT CTATG AGGGT TCGGC-3’ and 5’-AAGTT GTGCG CAAAT GACTG-3’, amplicon 581 bp; GDNF 5’-CCG GAC GGG ACT CTA AGA TGA-3’ and 5’-GTC AGG ATA ATC TTC GGG CAT ATT G-3’, amplicon 256 bp; NT-3 5’-CGGATGCCATGGTTACTTCT-3’ and 5’-GATATCCGCCTGGATCAACT-3’, amplicon 176 bp; IL-6 5’-CTCCATCTGCCCTTCA-3’ and 5’-CCAGGATAGAGCCACCAAT-3’, amplicon 569 kb; β-actin 5’-GTTCG CCATG GATGA CGATA TC-3’ and 5’-GCCAG ATCTT CTCCA TGTCG TC-3’, amplicon 256 bp. Amplification conditions were as follows: 5 min at 95°C (one cycle) and 40 s at 94°C; 40 s at the annealing temperature (56.8°C for BDNF, 61.1°C for GDNF, 59.2°C for NT-3, 58.3°C for IL-6, and 58.8°C for β-actin); and 1 min at 72°C (35 cycles) and 72°C for 5 min (one cycle). PCR products were analyzed by 1.5% gel electrophoresis followed by densitometric analysis of band intensity. The relative mRNA levels were determined with β-actin as the internal control.
Electron microscopy
The sciatic nerves were extracted from different groups of rats, fixed by osmium tetroxide, dehydrated by acetone, and sliced into 0.5 µm ultra thin sections. The sections were dual stained by uranium acetate and lead citrate. The ultrastructure of the nerves was examined by JEM 2000 EX transmission electron microscope (JEOL, Japan).
Statistical analysis
The data were expressed as mean ± standard deviation and analyzed by SPSS 13.0 software. Single factor analysis of variance was used for the comparisons among the groups. Value of p < 0.05 was considered as statistically significant.
Results
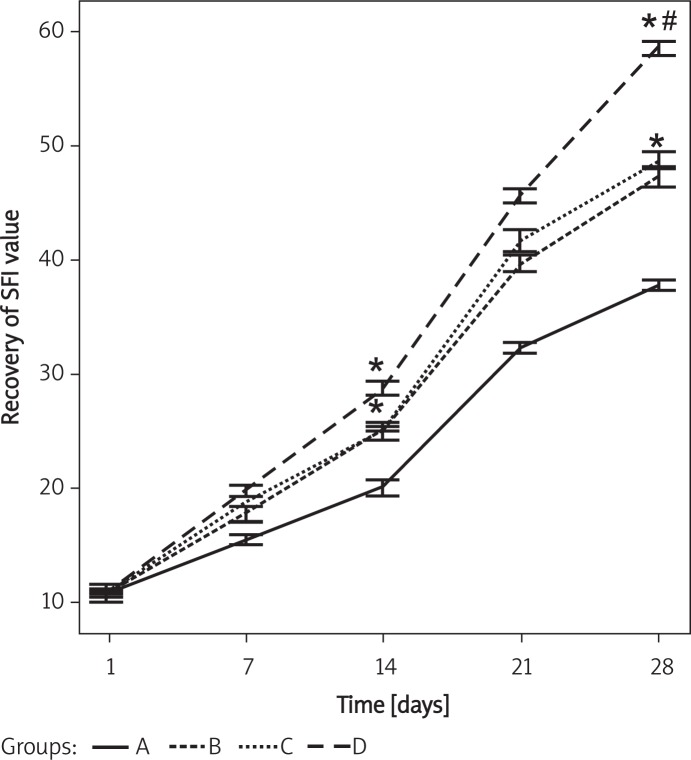
Dexamethasone and vitamin B12 synergistically promote the recovery of SFI in the injured sciatic nerves
Walking track analysis demonstrated that 1 day after the injury, SFI values in different groups showed no significant differences. However, 14 days after the injury, SFI value was recovered by 25.43 ±2.3%, 24.92 ±1.6%, and 28.76 ±2.5% in group B, C and D, respectively, significantly higher than in group A (20.12 ±1.2%) (p < 0.05), but showed no differences among group B, C and D (p > 0.05). Furthermore, 28 days after the injury, SFI value was recovered by 46.79 ±1.6%, 48.43 ±1.7%, and 59.37 ±2.8% in group B, C and D, respectively, significantly higher than in group A (37.83 ±3.1%) (p < 0.05). In addition, at this time the recovery of SFI value was significantly higher in group D than in group B and C (p < 0.05) with no significant difference between group B and C (p > 0.05) (Figure 1). These results suggest that dexamethasone and vitamin B12 synergistically promoted the recovery of SFI in the injured sciatic nerves.
Figure 1.
Recovery of SFI in injured sciatic nerves in different groups at different times after the injury
Group A – 0.9% saline treatment, group B – dexamethasone (1 mg/kg) treatment, group C – vitamin B12 (2 mg/kg) treatment, group D – dexamethasone (1 mg/kg) and vitamin B12 (2 mg/kg) treatment. *p < 0.05 vs. group A. #p < 0.05 vs. group B and C
Dexamethasone and vitamin B12 synergistically increase SCV of the injured sciatic nerves
Electrophysiological analysis demonstrated that the SCV of the injured sciatic nerves was increased in all groups with the duration of treatment. However, SCV in different groups showed no significant differences at the same time point of 1, 7 or 14 days after the injury. Notably, 21 days after the injury, SCV was increased to 22.85 ±4.35, 23.82 ±2.46, and 26.46 ±3.19 in group B, C and D, respectively, significantly higher than in group A (16.75 ±2.37) (p < 0.05), but showed no differences among group B, C and D (p > 0.05). Furthermore, 28 days after the injury, SCV was increased to 27.79 ±2.87, 28.87 ±1.92 and 36.65 ±3.76 in group B, C and D, respectively, significantly higher than in group A (17.92 ±3.98) (p < 0.05). In addition, at this time the SCV was significantly higher in group D than in group B and C (p < 0.05) with no significant difference between group B and C (p > 0.05) (Table I). These results suggest that dexamethasone and vitamin B12 synergistically increase SCV of the injured sciatic nerves.
Table I.
SCV of the injured sciatic nerves in different groups
| Group | n | 1 d.a.o. | 7 d.a.o. | 14 d.a.o. | 21 d.a.o. | 28 d.a.o. |
|---|---|---|---|---|---|---|
| A | 6 | 12.32 ±3.21 | 13.45 ±3.57 | 15.39 ±4.53 | 16.75 ±2.37 | 17.92 ±3.98 |
| B | 6 | 12.86 ±4.25 | 14.11 ±2.05 | 17.62 ±1.95 | 22.85 ±4.35* | 27.79 ±2.87* |
| C | 6 | 13.37 ±2.64 | 15.74 ±3.32 | 17.29 ±3.36 | 23.82 ±2.46* | 28.87 ±1.92* |
| D | 6 | 11.93 ±4.51 | 15.96 ±2.26 | 20.55 ±3.31 | 26.46 ±3.19* | 36.65 ±3.76* # |
d.a.o. – days after operation. SCV was expressed as x ± SD (m/s). Group A – 0.9% saline treatment, group B – dexamethasone (1 mg/kg) treatment, group C – vitamin B12 (2 mg/kg) treatment, group D – dexamethasone (1 mg/kg) and vitamin B12 (2 mg/kg) treatment.
Value of p < 0.05 vs. group A at 21 day and 28 day.
p < 0.05 vs. group B and C at 28 day
Dexamethasone and vitamin B12 synergistically increase BDNF expression in the injured sciatic nerves
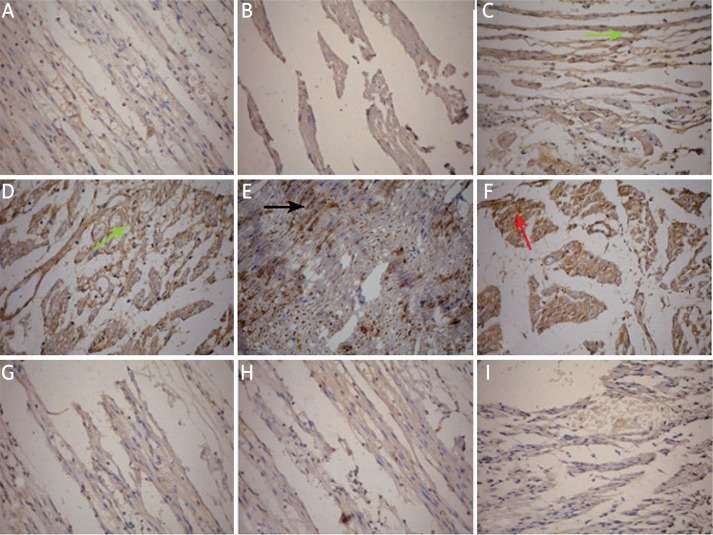
By immunohistochemical staining, the expression of BDNF, GDNF, NT-3 and IL-6 in the injured sciatic nerves (left side) and normal control sciatic nerves (right side) was detected. The results showed that in the control sciatic nerves, the axons were closely arranged in cords and BDNF, NT-3, IL-6 and GDNF were mildly expressed in the cytoplasm (Figure 2). Fourteen days after the injury, the sciatic nerves underwent degeneration and BDNF expression was slightly increased in each group but the staining was stronger in group B, C and D than in group A. However, the expression of NT-3, IL-6 and GDNF showed no significant changes in each group. Twenty-eight days after the injury, in group A the axons remained disarranged and BDNF expression was significantly decreased compared to the normal control (p < 0.05). The arrangement of axons was improved in group B, C and D, especially in group D (Figure 2). BDNF expression was increased in group B, C and D with the highest expression level observed in group D (p < 0.05 compared to group B and C). Similarly, the expression of NT-3, IL-6 and GDNF showed no significant changes in each group (Table II).
Figure 2.
Immunohistochemical staining of BDNF, GDNF, NT-3 and IL-6 in the sciatic nerves in different groups. A – Mild staining of BDNF in the control uninjured sciatic nerve. Organized arrangement of axons was obvious. B – Weak staining of BDNF in the injured sciatic nerve in group A at 28 day. The axons were disarranged. C – Strong staining of BDNF (indicated by green arrow) in the injured sciatic nerve in group B at 28 day. The axons were better arranged. D – Strong staining of BDNF (indicated by green arrow) in the injured sciatic nerve in group C at 28 day. The axons were better arranged. E – Very strong staining of BDNF (indicated by black arrow) in the injured sciatic nerve in group D at 28 day. The axons were well arranged. F – Strong staining of GDNF (indicated by red arrow) in the injured sciatic nerve in group D at 14day. G-I – Mild staining of GDNF (G), NT-3 (H) and IL-6 (I) in the injured sciatic nerve in group D at 28 day, although the axons were well arranged.
Group A – 0.9% saline treatment, group B – dexamethasone (1 mg/kg) treatment, group C – vitamin B12 (2 mg/kg) treatment, group D – dexamethasone (1 mg/kg) and vitamin B12 (2 mg/kg) treatment. Amplification 200×
Table II.
Scores of immunohistochemical staining of BDNF, NT-3, IL-6 and GDNF in the sciatic nerves in different groups
| Group | BDNF | NT-3 | IL-6 | GDNF |
|---|---|---|---|---|
| Control | 159.35 ±3.49 | 157.44 ±2.98 | 161.79 ±1.88 | 158.85 ±2.77 |
| A 14 day | 161.72 ±2.78 | 155.32 ±1.66 | 158.69 ±1.06 | 167.36 ±2.13 |
| 28 day | 141.59 ±3.62* | 142.52 ±2.21 | 147.35 ±2.36 | 147.04 ±1.69 |
| B 14 day | 168.42 ±2.86 | 156.14 ±3.11 | 159.92 ±1.61 | 169.17 ±2.05 |
| 28 day | 173.25 ±2.23* # | 143.26 ±1.31 | 145.63 ±1.92 | 148.86 ±1.09 |
| C 14 day | 169.26 ±1.98 | 154.29 ±2.28 | 156.81 ±2.09 | 168.06 ±1.38 |
| 28 day | 178.58 ±1.56* # | 146.87 ±2.02 | 144.39 ±1.08 | 149.53 ±2.46 |
| D 14 day | 171.47 ±2.25* | 155.83 ±3.26 | 154.04 ±2.53 | 168.79 ±1.19 |
| 28 day | 191.63 ±1.65* # ** | 143.19 ±2.66 | 146.57 ±1.07 | 145.25 ±1.86 |
The score was expressed as x ± SD (A value). Group A – 0.9% saline treatment, group B – dexamethasone (1 mg/kg) treatment, group C – vitamin B12 (2 mg/kg) treatment, group D – dexamethasone (1 mg/kg) and vitamin B12 (2 mg/kg) treatment.
p < 0.05 vs. control.
p < 0.05 vs. group A at 28 day.
p < 0.05 vs. group B and C at 28 day
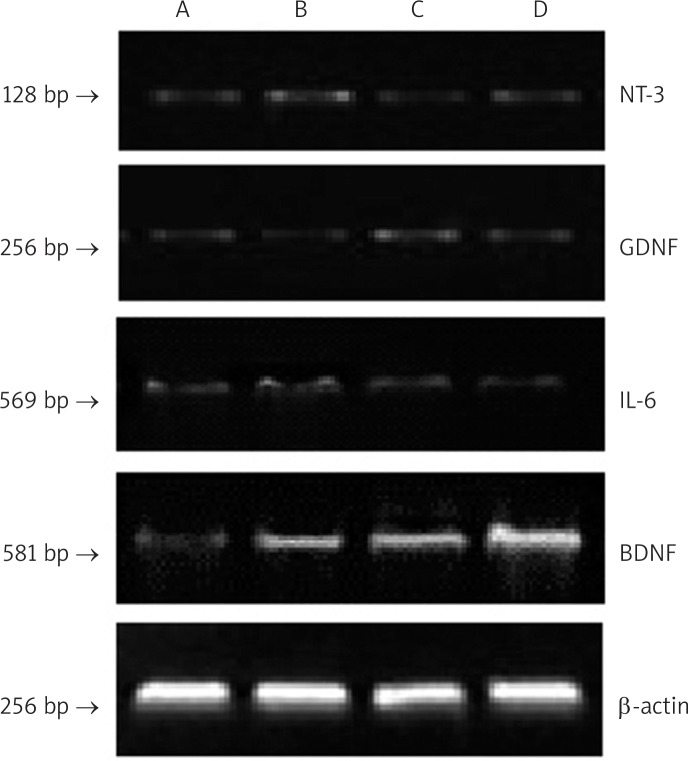
To confirm that dexamethasone and vitamin B12 specially increase BDNF expression in the injured sciatic nerves, we performed RT-PCR analysis on the sciatic nerves collected from different groups on day 28 (Figure 3). The results showed that mRNA level of BDNF was significantly increased in group B, C and D compared to group A (p < 0.05). In addition, mRNA level of BDNF was significantly higher in group D than in group B and C (p < 0.05). However, mRNA levels of NT-3, IL-6 and GDNF showed no significant changes in each group (Table III). Taken together, both immunohistochemistry and RT-PCR analysis demonstrated that dexamethasone and vitamin B12 specially increase BDNF expression in the injured sciatic nerves.
Figure 3.
RT-PCR analysis of BDNF, GDNF, NT-3 and IL-6 mRNA levels in the sciatic nerves in different groups at 28 day. The analysis was performed in 3 in dependent experiments and representative images are shown. β-actin was the internal control
Group A – 0.9% saline treatment, group B – dexamethasone (1 mg/kg) treatment, group C – vitamin B12 (2 mg/kg) treatment, group D – dexamethasone (1 mg/kg) and vitamin B12 (2 mg/kg) treatment
Table III.
mRNA level of BDNF, NT-3, IL-6 and GDNF in the sciatic nerves in different groups at 28 day
| Group | BDNF | NT-3 | IL-6 | GDNF |
|---|---|---|---|---|
| A | 0.03 ±0.01 | 0.15 ±0.03 | 0.07 ±0.02 | 0.12 ±0.05 |
| B | 0.26 ±0.05* | 0.12 ±0.02 | 0.05 ±0.01 | 0.09 ±0.01 |
| C | 0.29 ±0.03* | 0.14 ±0.05 | 0.04 ±0.03 | 0.08 ±0.04 |
| D | 0.43 ±0.12* # | 0.11 ±0.03 | 0.02 ±0.02 | 0.06 ±0.03 |
The mRNA level was expressed as x ± SD with mRNA level of β-actin set as 1. Group A – 0.9% saline treatment, group B – dexamethasone (1 mg/kg) treatment, group C – vitamin B12 (2 mg/kg) treatment, group D – dexamethasone (1 mg/kg) and vitamin B12 (2 mg/kg) treatment.
p < 0.05 vs. group A.
p < 0.05 vs. group B and C
Dexamethasone and vitamin B12 synergistically promote regeneration of injured sciatic nerves
Finally we performed morphological analysis on the injured sciatic nerves in different groups on day 28. In group A, the myelin sheath was seriously damaged and many vacuoles were formed in the middle of the axons. In group B and C, a number of Schwann cells were formed. In addition, neovascularization and the formation of myelin sheath with thin walls were observed. In group D, the proliferation of Schwann cells and the formation of myelinated nerve fibers were obvious. The regeneration of myelinated fibers in the injured sciatic nerves in the different groups on day 28 is summarized in Table IV.
Table IV.
Regeneration of myelinated fibers in the injured sciatic nerves in different groups at 28 day
| Group | Myelinated fiber number | Axon diameter [µm] | Myelin sheath thickness [µm] |
|---|---|---|---|
| Control | 106.3 ±12.5 | 2.96 ±0.15 | 3.54 ±0.41 |
| A | 46.4 ±18.2 | 0.97 ±0.06 | 2.58 ±0.23 |
| B | 73.5 ±9.4* | 1.96 ±0.27* | 3.36 ±0.58 |
| C | 78.4 ±8.7* | 1.78 ±0.35* | 3.41 ±0.33 |
| D | 97.2 ±14.88# | 2.56 ±0.38* # | 3.43 ±0.47 |
Data are expressed as x ± SD. Group A – 0.9% saline treatment, group B – dexamethasone (1 mg/kg) treatment, group C – vitamin B12 (2 mg/kg) treatment, group D – dexamethasone (1 mg/kg) and vitamin B12 (2 mg/kg) treatment.
p < 0.05 vs. group A.
p < 0.05 vs. group B and C
Discussion
Dexamethasone and vitamin B12 are used for the clinical treatment of nerve injury. Dexamethasone is a synthetic steroid hormone frequently used in the treatment of nervous system injury and inflammation due to its strong anti-inflammatory effects, while vitamin B12 coenzyme is involved in a variety of main metabolic reactions. The mechanisms underlying the neurotrophic effects of dexamethasone and vitamin B12 remain elusive.
Neural regeneration is a very complex process and Schwann cells play an important role in the promotion of regeneration after nerve injury [7]. After peripheral nerve injury, the distal axon myelin degenerates, and the degenerated axons and myelin undergo phagocytosis by Schwann cells or macrophages [8]. Schwann cells not only secrete neurotrophins but also produce basement membrane and myelin to promote the regeneration of axon myelination [9]. Numerous recent studies have focused on the differentiation of stem cells to Schwann cells as a promising approach for neural regeneration [10–12].
In this study we employed a variety of approaches to perform morphological and functional analysis on rat sciatic nerves subjected to injury and treatment by dexamethasone or vitamin B12. Morphologically, by electron microscopy we observed that compared to treatment with dexamethasone or vitamin B12 alone, treatment with both agents led to a much larger number of Schwann cells and myelinated nerve fibers. In addition, the diameter of the axons was larger. These results suggest that dexamethasone or vitamin B12 promoted the regeneration of myelinated nerve fibers and the proliferation of Schwann cells. Functionally, we confirmed that treatment with dexamethasone and vitamin B12 led to the recovery of SFI and SCV in the injured sciatic nerves. Furthermore, we demonstrated that treatment with dexamethasone and vitamin B12 led to the increased expression of BDNF in the injured sciatic nerves at both mRNA and protein levels, without significant effects on the expression of GDNF, NT-3 and IL-6. Interestingly, a recent study reported that adipose-derived stem cells stimulated the regeneration of peripheral nerves through the secretion of BDNF, which promoted nerve healing and axon growth [13]. Based on our results we postulate that dexamethasone and vitamin B12 promote the regeneration and functional recovery of the injured sciatic nerves via the upregulation of BDNF expression. Nevertheless, further studies are necessary to elucidate the mechanism by which dexamethasone and vitamin B12 could upregulate BDNF expression in peripheral nerves.
In conclusion, in this study we demonstrated that dexamethasone and vitamin B12 promote peripheral nerve repair in a rat model of sciatic nerve injury through the upregulation of BDNF expression. These findings provide new insight into the neurotrophic effects of dexamethasone and vitamin B12 and support the application of these agents in clinical treatment of peripheral nerve injury.
Acknowledgments
Hongzhi Sun and Tao Yang contributed equally.
This study was supported by the following grants: Fund from Department of Science and Technology of Liaoning Province (No. 2010225034) and Fund from the Department of Science and Technology of Jinzhou (No. 10A1E37).
References
- 1.Robinson LR. Traumatic injury to peripheral nerves. Muscle Nerve. 2000;23:863–73. doi: 10.1002/(sici)1097-4598(200006)23:6<863::aid-mus4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Deumens R, Bozkurt A, Meek MF, et al. Repairing injured peripheral nerves: bridging the gap. Prog Neurobiol. 2010;92:245–76. doi: 10.1016/j.pneurobio.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Gordon T. The role of neurotrophic factors in nerve regeneration. Neurosurg Focus. 2009;26:E3. doi: 10.3171/FOC.2009.26.2.E3. [DOI] [PubMed] [Google Scholar]
- 4.Terenghi G. Peripheral nerve regeneration and neurotrophic factors. J Anat. 1999;194:1–14. doi: 10.1046/j.1469-7580.1999.19410001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu GB, Cheng YX, Feng YK, et al. Adipose-derived stem cells promote peripheral nerve repair. Arch Med Sci. 2011;7:592–6. doi: 10.5114/aoms.2011.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bai YH, Takemitsu M, Atsuta Y, et al. Peripheral mononeuropathy induced by loose ligation of the sciatic nerve in the rat: behavioral, electrophysiological and histopathologic studies. Exp Anim. 1999;48:87–94. doi: 10.1538/expanim.48.87. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs WB, Fehlings MG. The molecular basis of neural regeneration. Neurosurgery. 2003;53:943–50. doi: 10.1227/01.neu.0000083592.74383.b1. [DOI] [PubMed] [Google Scholar]
- 8.Chen ZY, Chai YF, Cao L, et al. Glial cell line-derived neurotrophic factor enhances axonal regeneration following sciatic nerve transection in adult rats. Brain Res. 2001;902:272–6. doi: 10.1016/s0006-8993(01)02395-2. [DOI] [PubMed] [Google Scholar]
- 9.Baptista AF, Gomes JR, Oliveira JT, et al. High-and low-frequency transcutaneous electrical nerve stimulation delay sciatic nerve regeneration after crush lesion in the mouse. J Peripher Nerv Syst. 2008;13:71–80. doi: 10.1111/j.1529-8027.2008.00160.x. [DOI] [PubMed] [Google Scholar]
- 10.Kashani IR, Golipoor Z, Akbari M, et al. Schwann-like cell differentiation from rat bone marrow stem cells. Arch Med Sci. 2011;7:45–52. doi: 10.5114/aoms.2011.20603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Gong P, Liao D. In vitro neural/glial differentiation potential of periodontal ligament stem cells. Arch Med Sci. 2010;6:678–85. doi: 10.5114/aoms.2010.17080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao D, Gong P, Li X, et al. Co-culture with Schwann cells is an effective way for adipose-derived stem cells neural transdifferentiation. Arch Med Sci. 2010;6:145–51. doi: 10.5114/aoms.2010.13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopatina T, Kalinina N, Karagyaur M, et al. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS One. 2011;6:e17899. doi: 10.1371/journal.pone.0017899. [DOI] [PMC free article] [PubMed] [Google Scholar]