Abstract
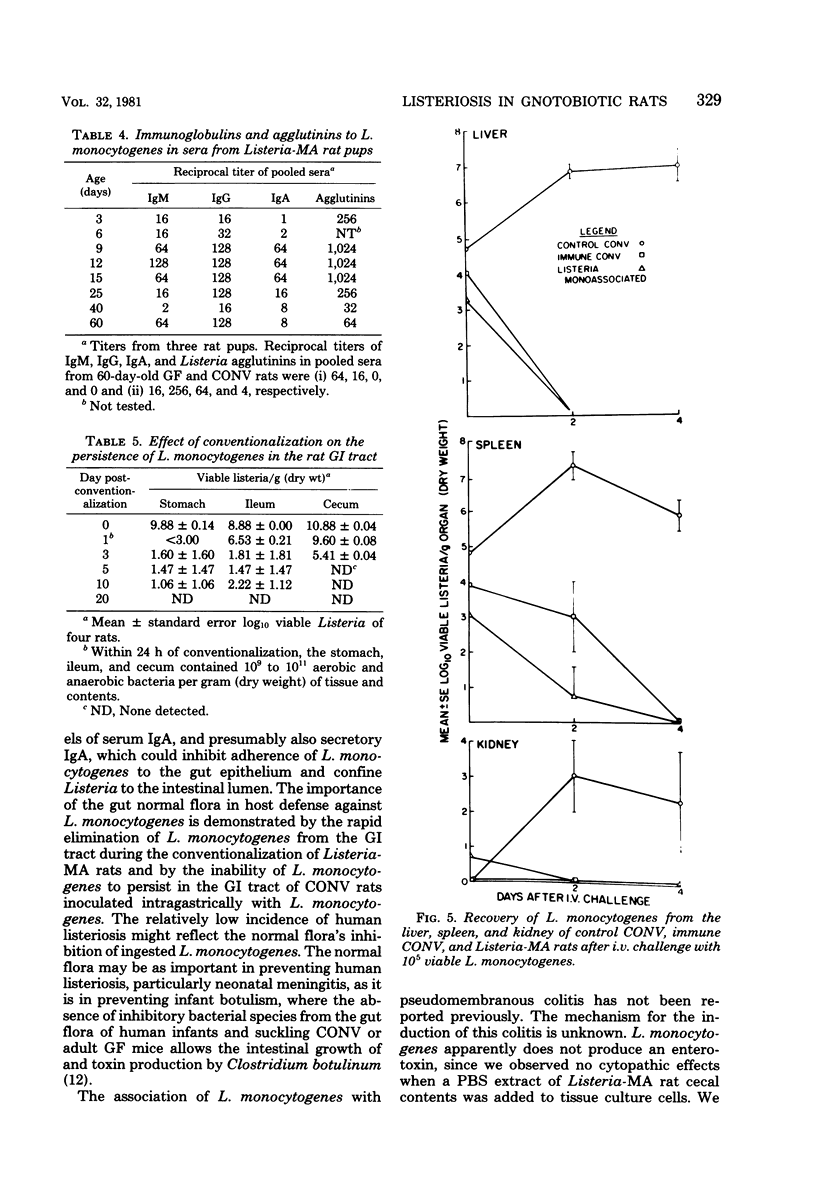
Listeria monocytogenes colonized the gastrointestinal tract of adult germfree rats (10(10) to 10(11)/g, dry weight) within 24 h after oral exposure. Between 3 and 14 days after monoassociation, L. monocytogenes caused a self-limiting pseudomembranous colitis, bacteremia, and infection of the spleen and liver. Monoassociation of rats with Listeria for 8 weeks stimulated 32- and 4-fold increases in serum immunoglobulin A (IgA) and IgG, respectively, whereas serum IgM decreased 2-fold. The normal microbial flora was inhibitory to Listeria colonization, since L. monocytogenes was cleared from the gastrointestinal tract of formerly monoassociated rats within 20 days after conventionalization and did not colonize the gastrointestinal tract of conventional rats after intragastric instillation of 10(8) viable L. monocytogenes. Listeria-monoassociated rats delivered large litters of healthy pups whose gastrointestinal tracts were slowly colonized with L. monocytogenes. between 3 and 60 days of age, Listeria-monoassociated rat pups exhibited eight- and fourfold increases in serum IgG and IgM, respectively; however, serum IgA was elevated (16-fold) only at 9 to 15 days of age. Adult Listeria-monoassociated rats had acquired cellular resistance to intravenous challenge with L. monocytogenes. Prolonged monoassociation of L. monocytogenes in rats attenuated its virulence for conventional rats.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNASON B. G., VAUXST-CYRC DE, RELYVELD E. H. ROLE OF THE THYMUS IN IMMUNE REACTIONS IN RATS. IV. IMMUNOGLOBULINS AND ANTIBODY FORMATION. Int Arch Allergy Appl Immunol. 1964;25:206–224. doi: 10.1159/000229522. [DOI] [PubMed] [Google Scholar]
- Balish E., Yale C. E., Hong R. Serum proteins of gnotobiotic rats. Infect Immun. 1972 Aug;6(2):112–118. doi: 10.1128/iai.6.2.112-118.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J., Rodriguez G. E., Kind P. D., Campbell P. A. Listeria cell wall fraction: a B cell mitogen. J Immunol. 1975 Mar;114(3):1132–1134. [PubMed] [Google Scholar]
- Corbeil L. B., Wunderlich A. C., Ito J. I., McCutchan J. A. Plaque assay for measuring serum bactericidal activity against gonococci. J Clin Microbiol. 1978 Nov;8(5):618–620. doi: 10.1128/jcm.8.5.618-620.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon H. A., Pesti L. The gnotobiotic animal as a tool in the study of host microbial relationships. Bacteriol Rev. 1971 Dec;35(4):390–429. doi: 10.1128/br.35.4.390-429.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. L., Killinger A. H. Listeria monocytogenes and listeric infections. Bacteriol Rev. 1966 Jun;30(2):309–382. doi: 10.1128/br.30.2.309-382.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemenes F. Proceedings: Pathogenicity of Listeria monocytogenes in the rat. Acta Microbiol Acad Sci Hung. 1972;19(4):429–432. [PubMed] [Google Scholar]
- MacDonald T. T., Carter P. B. Cell-mediated immunity to intestinal infection. Infect Immun. 1980 May;28(2):516–523. doi: 10.1128/iai.28.2.516-523.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Koster F. T., Mackaness G. B. The short lived small lymphocyte as a mediator of cellular immunity. Nature. 1970 Nov 28;228(5274):855–856. doi: 10.1038/228855a0. [DOI] [PubMed] [Google Scholar]
- Moberg L. J., Sugiyama H. Microbial ecological basis of infant botulism as studied with germfree mice. Infect Immun. 1979 Aug;25(2):653–657. doi: 10.1128/iai.25.2.653-657.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSEBOLD J. W., INOUYE T. Pathogenesis of Listeria monocytogenes infections in natural host. I. Rabbit studies. J Infect Dis. 1954 Jul-Aug;95(1):52–66. doi: 10.1093/infdis/95.1.52. [DOI] [PubMed] [Google Scholar]
- OSEBOLD J. W., INOUYE T. Pathogenesis of Listeria monocytogenes infections in natural hosts. II. Sheep studies. J Infect Dis. 1954 Jul-Aug;95(1):67–78. doi: 10.1093/infdis/95.1.67. [DOI] [PubMed] [Google Scholar]
- Owens W. E., Berg R. D. Bacterial translocation from the gastrointestinal tract of athymic (nu/nu) mice. Infect Immun. 1980 Feb;27(2):461–467. doi: 10.1128/iai.27.2.461-467.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlock D. M., Deibel R. H. Detection of Salmonella enterotoxin using rabbit ileal loops. Can J Microbiol. 1978 Mar;24(3):268–273. doi: 10.1139/m78-046. [DOI] [PubMed] [Google Scholar]
- Zachar Z., Savage D. C. Microbial interference and colonization of the murine gastrointestinal tract by Listeria monocytogenes. Infect Immun. 1979 Jan;23(1):168–174. doi: 10.1128/iai.23.1.168-174.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]