Abstract
The radical SAM (RS) enzymes RlmN and Cfr methylate 23S ribosomal RNA, modifying the C2 or C8 position of adenosine 2503. The methyl groups are installed by a two-step sequence involving initial methylation of a conserved Cys residue (RlmN Cys 355) by SAM. Methyl transfer to the substrate requires reductive cleavage of a second equivalent of SAM. Crystal structures of RlmN and RlmN with SAM show that a single molecule of SAM coordinates the [4Fe-4S] cluster. Residue Cys 355 is S-methylated and located proximal to the SAM methyl group, suggesting that SAM involved in the initial methyl transfer binds at the same site. Thus, RlmN accomplishes its complex reaction with structural economy, harnessing the two most important reactivities of SAM within a single site.
Methyl transfer is essential in the synthesis of cellular metabolites and clinically relevant natural products, and in the modification of RNA, DNA, lipids, and proteins. The majority of methyl transfer reactions employ S-adenosyl-l-methionine (SAM) (1) and proceed by SN2 displacement (2, 3). Until recently, this mechanism was generally accepted as the only means of SAM-dependent methylation (4), even for methyl groups transferred to weakly nucleophilic carbon atoms (5). The identification and characterization of two ribosomal RNA (rRNA) modification enzymes (RlmN and Cfr) (6–10) that use SAM to methylate electrophilic rather than nucleophilic carbon centers has changed this view. RlmN and Cfr catalyze methylation of a 23S rRNA nucleotide (adenosine 2503, A2503) (6–10) ultimately located within the peptidyltransferase center of the 50S subunit of the bacterial ribosome near the entrance to the nascent peptide exit tunnel (11). RlmN methylates the C2 position of A2503 (Fig. S1), a housekeeping modification important in translational fidelity and the nascent peptide response (9, 12, 13). The related protein Cfr, recently found in a hospital isolate of a methicillin resistant strain of Staphylococcus aureus (14, 15), catalyzes a similar modification at C8 of A2503 (6) (Fig. S1) which confers resistance to several classes of antibiotics that target the large subunit of the ribosome (16–18). Inactivation of RlmN in S. aureus and loss of C2 modification is also linked to increased linezolid antibiotic resistance (19, 20).
RlmN and Cfr belong to the radical SAM (RS) superfamily of enzymes (10), catalysts that use SAM as an oxidant to perform difficult and often complex transformations by radical mechanisms (21–23). RS superfamily enzymes employ a [4Fe–4S] cluster to supply the requisite electron for reductive cleavage of SAM, usually to l-methionine and a 5’-deoxyadenosyl 5’-radical (5’-dA•) (22, 24–26). In RlmN and Cfr, SAM is the source of both the 5’-dA• and the appended methyl group (10). Thus, these enzymes activate SAM for two distinct reactions: reductive cleavage to generate 5’-dA• and methyl transfer. Based on all other characterized RS proteins (22, 24–26), the expected role of the 5’-dA• would be to abstract a hydrogen atom from the substrate, in this case the C2 (RlmN) or C8 (Cfr) hydrogen atom from A2503, activating the substrate for subsequent methylation. However, two recent studies suggest that in Cfr and RlmN, 5’-dA• activates the methyl component added to the substrate rather than the substrate itself (7, 27). Yan and Fujimori proposed that 5’-dA• abstracts a hydrogen atom from the methyl substituent of a second simultaneously bound SAM molecule, which then performs a radical addition at C2 of A2503. The resultant adduct is resolved by a hydride shift (7, 27). By contrast, data reported by Grove et al. indicate that both the RlmN and Cfr reactions proceed by a ping-pong mechanism (Fig. S2) (7). The methyl group from one SAM molecule is initially appended to a conserved Cys residue (Cys 355 in RlmN) by a typical SN2 displacement (28). This SAM-derived one-carbon unit is then attached to the RNA by radical addition initiated by a 5’-dA• formed from a second molecule of SAM. Finally, this covalent intermediate is resolved by formation of a disulfide bond between the methyl-carrying Cys (mCys) residue and a second conserved Cys residue (Cys 118 in RlmN).
The crystal structures of Escherichia coli RlmN in the absence and presence of SAM reveal a monomeric enzyme with two protein molecules in the asymmetric unit. The core of the protein forms an α6/β6 partial barrel (Fig. 1A) harboring a [4Fe-4S] cluster coordinated by a canonical CX3CX2C motif located at the carboxy edge of the barrel and enclosed by two long loop regions. This overall fold and an open coordination site at the [4Fe-4S] cluster, allowing the enzyme to bind a SAM cosubstrate, are structural hallmarks of the RS superfamily (29–35). The RlmN core structure is most similar to that of pyruvate formate-lyase activating enzyme (PFL-AE) (rmsd 2.6 Å for 220 Cα carbon atoms) (Fig. S3), noteworthy because PFL-AE also targets a large macromolecule.
Fig. 1.
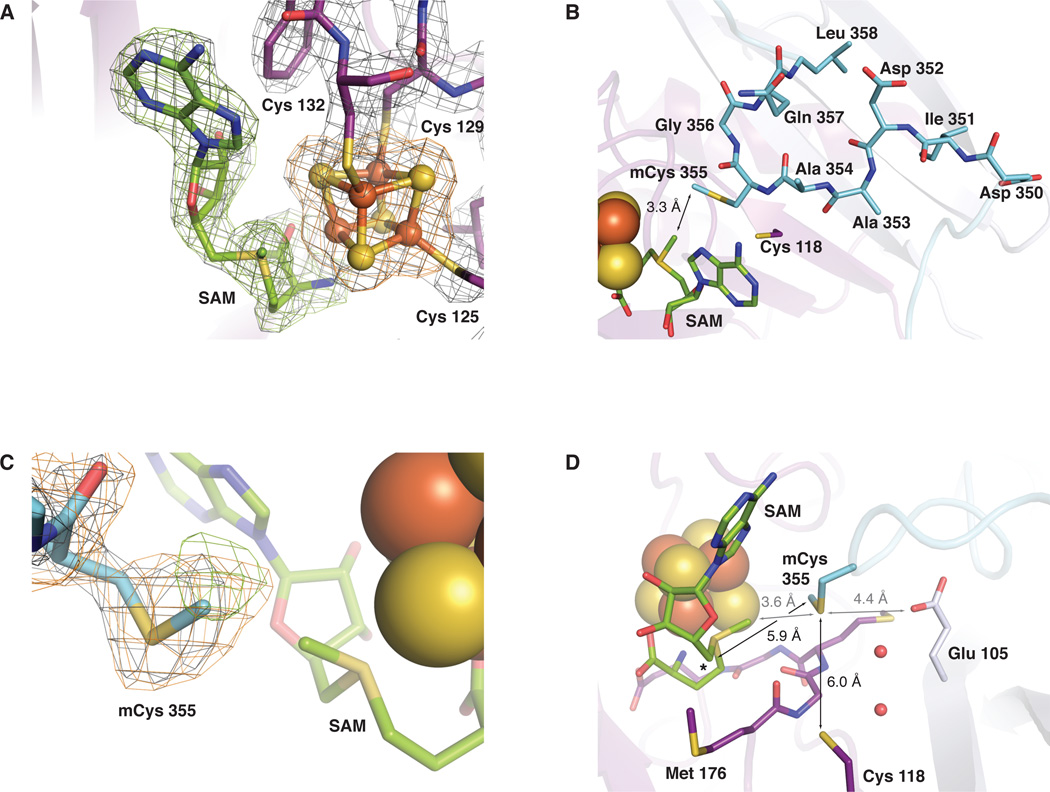
The structure of E. coli RlmN with S-adenosyl-L-methionine (SAM) (A). The α6/β6 partial barrel core is shown in dark purple, a three strand β extension to the core (β’1–3 extension) is shown in light purple, an N-terminal helical accessory domain is shown in green, and a C-terminal extension (β7 extension) culminating in an α-helix is shown in blue. The [4Fe-4S] cluster cofactor is represented as a space-filling model and SAM is shown in stick format colored by atom type. Cysteine (Cys) ligands to the [4Fe-4S] cluster and mechanistically important residues Cys 118 and methylcysteine (mCys) 355 are shown as purple and blue sticks, respectively, and colored by atom type. (B) A top-down view comparing the RlmN active site in the absence (right) and presence (left) of SAM. An unmodeled region (residues 351–360) in the RlmN structure obtained without SAM is represented as a dashed line.
Unlike PFL-AE, which represents a minimal architecture among RS enzymes (30, 35), RlmN contains several extensions to the core structure (Fig. 1A). An N-terminal accessory domain is connected to the core via three additional β-strands (β’1–3 extension) that extend the barrel laterally. The sixty-residue N-terminal domain is composed of four short α-helices and differs in structure from accessory domains in other crystallographically characterized RS superfamily members (29–35). The domain is a variant of the HhH2 fold (Fig. S4), a structure that mediates substrate recognition in proteins that target nucleic acids (36, 37). While typically involved in DNA recognition, a similar domain is also found in the MraW family of methyltransferases (37), enzymes recently demonstrated to methylate rRNA (38). In the RlmN core, the loop between α1 and β2 is extended by 10 residues as compared to PFL-AE and adopts different conformations in each molecule in the asymmetric unit (Figs. S3, S5). The β7 strand extends across the open side of the barrel (β7 extension) and interacts with the β’1–3 extension, terminating in a ten residue region (residues 351–360) (Fig 1B, left), disordered in the absence of SAM and not included in the model in the RlmN structure (Fig. 1B, right). The sequence of this region is strictly conserved among RlmN and Cfr homologs and includes residue Cys 355, a key catalytic residue that is methylated in the first step of the proposed mechanism (7). Residues 361–374 comprise a helix that forms no contacts with the rest of the chain (nearest neighbor ~10 Å) in the absence of SAM and interacts instead with a symmetry related molecule in the crystal lattice (Fig. S6).
In the 2.05 Å resolution structure of RlmN with SAM bound (RlmN+SAM), SAM is coordinated to the [4Fe-4S] cluster and several structural changes near the enzyme active site are observed. The SAM coordination mode, via its α-amino and α-carboxy moieties (Figs. 2A, S7), is typical of all structurally characterized RS enzymes (29–34), as is the distance between the SAM sulfonium atom and the unique iron site of the cluster (3.2 Å). This distance is shorter than that to the nearest bridging μ-sulfido ion (3.7 Å), consistent with a mechanism for SAM cleavage originally proposed by Frey and coworkers (39–41). The other interactions between SAM and the surrounding protein include packing of the SAM adenine ring into a hydrophobic cleft and hydrogen bonding interactions, largely to ordered solvent (Fig. S8). The sole direct hydrogen bonding interaction involving the adenine ring, between its exocylic amine and the backbone carbonyl of Asn 312, causes a ~2.5 Å shift of the backbone, which moves the Asn side chain over the face of the adenine ring (Fig. S9). A hydrogen bonding network involving the SAM adenine Hoogsteen face, ordered solvent, and Asn 312 extends to the region encompassing residues 351–360 (Fig. S8), resulting in observable electron density that could be modeled for one of the two molecules in the asymmetric unit (Fig. S10).
Fig. 2.
Selected views of the RlmN active site. (A) 2Fo-Fc electron density map (gray mesh, 1.5σ) for residues 125–132 (containing the CX3CX2C motif), the [4Fe-4S] cluster, and the SAM cosubstrate in the RlmN+SAM structure. An omit map contoured at 3.0σ for SAM (green mesh) and the [4Fe-4S] cluster (orange mesh) is superimposed. (B) A top-down view of the highly conserved 351–360 linker region as modeled in the RlmN+SAM structure. The linker is represented in stick format colored by atom type. A loop (residues 310–320) located in front of the SAM cofactor is omitted for clarity. (C) 2Fo-Fc electron density map (gray mesh, 1.0σ) for residue 355 in the RlmN+SAM structure. Omit maps for the S-methyl group of residue 355 (green mesh, 2.2σ) and the entire residue (orange mesh, 1.9σ) are superimposed. (D) A side view of the active site. The conserved MGMGE sequence motif in the α6/β6 core is shown in stick format and colored by atom type. Selected conserved residues and ordered water molecules are shown as sticks and spheres, respectively. The 5’ position of the SAM cosubstrate is denoted by an asterisk. Distances relevant to the methyl transfer reaction are drawn as gray lines and those relevant to mCys 355 activation and disulfide formation are shown as black lines.
Residues 351–360 lie across the top of the carboxy end of the barrel in the RlmN+SAM structure forming a short loop that dips into the active site (Fig. 2B) and contain a conserved motif (IDAACGQL, residues 351–358) preceded by an additional conserved glycine (Gly 348) and aspartate (Asp 350) (8). The conserved glycines and alanines may confer conformational variability. The region remains flexible even in the presence of SAM, as evidenced by temperature factors of 40–50 Å2 relative to ~20 Å2 for rest of the model and weak backbone connectivity upon contouring the 2Fo-Fc map above 1σ. The side chain of residue 355 is at the tip of the loop and protrudes into the active site in close proximity to the methionine moiety (3.3 Å) (Fig. 2B) of the coordinated SAM molecule. Modeling this residue as a cysteine resulted in positive difference electron density adjacent to the side chain sulfur atom, eliminated by inclusion of a methyl group appended to the side chain (S-methylcysteine, mCys) (Fig. 2C). The mCys 355 sulfur atom is located ~6 Å from both the sidechain of Cys 118 and the 5’ position of the SAM deoxyadenosine group (Figs. 2D, S11). The structure thus represents the binding of the second SAM and strongly supports key downstream steps in the proposed mechanism (7).
The active site contains a conserved MGMGE sequence (residues 176–180) on the strand adjacent to that containing Cys 118 (Fig. 2D). This sequence aligns with a GGE motif conserved in RS enzymes and found near the SAM binding pocket (30, 35). The N-terminal methionine, Met 176, is contiguous to the SAM cosubstrate. Binding of SAM results in a peptide flip in the backbone carbonyl of Met 176, moving it out of a canonical β-strand backbone configuration (Fig. S12). Consequently, the Met 176 carbonyl oxygen atom points towards the mCys 355 side chain and the SAM methyl substituent. Cys 118 adopts a different rotamer, the adjacent residues shift slightly, and the sulfur atom of Cys 118 is brought closer to the SAM cofactor and mCys 355 compared to its location in the RlmN structure without SAM.
Observation of the mCys 355 modification in the RlmN+SAM crystal structure (Fig. 2C) supports the proposed ping-pong mechanism (7). Furthermore, the structure suggests that SAM-dependent methyl transfer to Cys 355 could occur from a SAM bound to the [4Fe-4S] cluster. The position of mCys 355, 3.6 Å from the SAM methyl substituent (Figs. 2D, S13) may permit methyl transfer via SN2 displacement (7). Additional structural aspects that would be favorable for this reaction include positioning of a conserved residue Glu 105 (~4 Å away) to deprotonate Cys 355, critical in reaction initiation, and hydrogen bond acceptors (Met 176 backbone carbonyl oxygen (42) and [4Fe-4S] μ-sulfido atoms (43)) within 3.5 Å of the SAM methyl group, potentially important in stabilizing the transition state or product methyl configuration (Figs. 2D, S13). The observation that SAM is bound to the [4Fe–4S] cluster similarly to other structurally characterized RS enzymes, the absence of any other clear SAM binding site (structurally or via bioinformatic analysis), and the proximity of Cys 355 and the activated methyl group of SAM all suggest that RlmN and Cfr, in accordance with the principle of “economy in the evolution of binding sites (44),” evolved a single SAM binding pocket to facilitate two different reactions.
In the mechanism proposed by Grove et al. (7), 5’-dA• abstracts a hydrogen from the methyl group of mCys 355 and the resulting Cys-appended methyl radical attacks the substrate adenine ring. Methyl transfer to C2 results in a covalent adduct between the substrate and Cys 355, resolved by formation of a disulfide bond between Cys 355 and Cys 118. The location of mCys 355 in the structure, 6 Å equidistant from the 5’ position on the deoxyadenosine moiety of SAM and the side chain of Cys 118 (Fig. 2D), is consistent with this mechanism. Conserved charged residues, including Glu 105 and Lys 94, and ordered solvent molecules (Fig. 2D), are well positioned to orient the substrate and/or facilitate acid/base chemistry during the reaction (45). The location of mCys 355 in a flexible loop likely confers mobility to facilitate its role in methyl acquisition and substrate addition. The structural homology of this loop to analogous regions in the glycyl radical enzymes pyruvate formate-lyase (30, 46) (Fig. S14) and class III ribonucleotide reductase (47, 48) is striking and underscores that these systems perform fundamentally similar transformations, the generation of a carbon-centered radical at a specific site on a protein side chain with a requirement for controlled mobility of the resulting radical within an enzyme/substrate complex.
The RlmN structures also provide insight into the nature and location of the rRNA binding site. The electrostatic surface potential (49) for RlmN (Figs. 3A, 3B) suggests the substrate may approach the active site from the bottom of the barrel (Figs. 3A, S15). This extensive surface involves the core barrel, its extensions, and the extra domain, implicating the accessory elements in substrate interaction. The N-terminal domain displays a distinct band of positive charge (Fig. 3B), a feature observed in the specificity domain for an rRNA assembly helicase that binds helices 90–92 in domain V (50), a portion of the ribosome necessary for RlmN activity (10). A large interaction surface for an rRNA polyanion is consistent with the observation that RlmN most effectively methylates its substrate base in the context of large fragments of 23S rRNA (10).
Fig. 3.
Electrostatic surface potential (A, B) and Cfr sequence conservation (C, D) maps of the RlmN structure. A Electrostatic surface calculated with APBS (47) contoured at -15 kBT (red, negative) and 15 kBT (blue, positive). (B) View rotated 90° about the vertical axis and 90° about the horizontal axis from view (A). (C) Map of sequence conservation between E. coli RlmN and S. aureus Cfr using a pairwise alignment. Strictly conserved residues are shown in orange, neutral substitutions are shown in tan, and variable regions are shown in white. Residues 344–350 from the β7 extension (which includes conserved residues Arg 344, Gly 348, and Asp 350) are omitted to afford a better view of the active site cavity. (D) View rotated 90° about the vertical axis and 90° about the horizontal axis from view (C).
Finally, the RlmN structures have important implications for the evolution of Cfr, which confers antibiotic resistance by methylating C8 of A2503 (6). The two enzymes have similar mechanisms, and RlmN is proposed to be an evolutionary precursor to Cfr (7, 8). To understand how specificity for the C8 position might have evolved in Cfr, residues conserved among both enzymes in a pairwise alignment of E. coli RlmN and S. aureus Cfr were mapped onto the RlmN structure (Figs. 3C, 3D, S15). The catalytic residues in the active site (7) are strictly conserved as are most of the surrounding residues within the core of the barrel, supporting the proposal that the enzymes use a common mechanism for C-methylation. The high degree of sequence conservation near the active site (Figs. S16, S17) suggests that methylation site specificity during the reaction may be controlled in part by more distant structural elements. In Cfr, two large conformationally flexible regions in the RlmN structure (Fig. S18) are absent. These include the extended loop between α1 and β2, located between the active site cavity and N-terminal accessory domain and the C-terminal helix connected to the mechanistically critical 351–360 linker. If loss of these motifs allows Cfr to modify a different site, their distance from the active site is noteworthy and leaves open the possibility that the enzyme/substrate complex uses an extended interaction surface to fine tune control of substrate binding and site selectivity.
Supplementary Material
Acknowledgments
This work has been supported by NIH grants GM58518 (A. C. R.), GM63847 (S. J. B.), and an NRSA fellowship to A.K.B. Use of the Advanced Photon Source was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. Use of the LS-CAT Sector 21 was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor (Grant 085P1000817). GM/CA CAT has been funded in whole or in part with Federal funds from the National Cancer Institute (Y1-CO-1020) and the National Institute of General Medical Science (Y1-GM-1104). Coordinates and structure factors have been deposited in the Protein Data Bank with accession codes 3RF9 (RlmN) and 3RFA (RlmN+SAM).
References and Notes
- 1.Markham GD. Encyclopedia of Life Sciences. John Wiley & Sons, Inc; 2010. [Google Scholar]
- 2.Woodard RW, Tsai MD, Floss HG, Crooks PA, Coward JK. J. Biol. Chem. 1980;255:9124. [PubMed] [Google Scholar]
- 3.Hegazi MF, Borchardt RT, Schowen RL. J. Am. Chem. Soc. 1979;101:4359. [Google Scholar]
- 4.Frey PA, Hegeman AD. Enzymatic Reaction Mechanisms. Oxford University Press; New York: 2007. [Google Scholar]
- 5.Iwig DF, Grippe AT, McIntyre TA, Booker SJ. Biochemistry. 2004;43:13510. doi: 10.1021/bi048692h. [DOI] [PubMed] [Google Scholar]
- 6.Giessing AM, et al. RNA. 2009;15:327. doi: 10.1261/rna.1371409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grove TL, et al. Science. 2011 in press. [Google Scholar]
- 8.Kaminska KH, et al. Nucleic Acids Res. 2010;38:1652. doi: 10.1093/nar/gkp1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toh SM, Xiong L, Bae T, Mankin AS. RNA. 2008;14:98. doi: 10.1261/rna.814408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan F, et al. J Am Chem Soc. 2010;132:3953. doi: 10.1021/ja910850y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ban N, et al. Cell. 1998;93:1105. doi: 10.1016/s0092-8674(00)81455-5. [DOI] [PubMed] [Google Scholar]
- 12.Kowalak JA, Bruenger E, McCloskey JA. J. Biol. Chem. 1995;270:17758. doi: 10.1074/jbc.270.30.17758. [DOI] [PubMed] [Google Scholar]
- 13.Vázquez-Laslop N, Ramu H, Klepacki D, Kannan K, Mankin AS. EMBO J. 2010;29:3108. doi: 10.1038/emboj.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarz S, Werckenthin C, Kehrenberg C. Antimicrob. Agents Chemother. 2000;44:2530. doi: 10.1128/aac.44.9.2530-2533.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toh SM, et al. Mol. Microbiol. 2007;64:1506. doi: 10.1111/j.1365-2958.2007.05744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kehrenberg C, Schwarz S, Jacobsen L, Hansen LH, Vester B. Mol. Microbiol. 2005;57:1064. doi: 10.1111/j.1365-2958.2005.04754.x. [DOI] [PubMed] [Google Scholar]
- 17.Long KS, Poehlsgaard J, Kehrenberg C, Schwarz S, Vester B. Antimicrob. Agents Chemother. 2006;50:2500. doi: 10.1128/AAC.00131-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith LK, Mankin AS. Antimicrob. Agents Chemother. 2008;52:1703. doi: 10.1128/AAC.01583-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaMarre JM, Howden BP, Mankin AS. Antimicrob. Agents Chemother. 2011 doi: 10.1128/AAC.00183-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao W, et al. PLoS Path. 2010;6:e1000944. doi: 10.1371/journal.ppat.1000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Booker SJ. Curr. Opin. Chem. Biol. 2009;13:58. doi: 10.1016/j.cbpa.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frey PA, Hegeman AD, Ruzicka FJ. Crit. Rev. Biochem. Mol. Biol. 2008;43:63. doi: 10.1080/10409230701829169. [DOI] [PubMed] [Google Scholar]
- 23.Sofia HJ, Chen G, Hetzler BG, Reyes-Spindola JF, Miller NE. Nucleic Acids Res. 2001;29:1097. doi: 10.1093/nar/29.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fontecave M, Mulliez E, Ollagnier-de-Choudens S. Curr. Opin. Chem. Biol. 2001;5:506. doi: 10.1016/s1367-5931(00)00237-4. [DOI] [PubMed] [Google Scholar]
- 25.Frey PA, Booker SJ. Adv. Protein Chem. 2001;58:1. doi: 10.1016/s0065-3233(01)58001-8. [DOI] [PubMed] [Google Scholar]
- 26.Jarrett JT. Curr. Opin. Chem. Biol. 2003;7:174. doi: 10.1016/s1367-5931(03)00022-x. [DOI] [PubMed] [Google Scholar]
- 27.Yan F, Fujimori DG. Proc. Natl. Acad. Sci. U S A. 2011;108 doi: 10.1073/pnas.1017781108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Methylation of Cys 355 (mCys 355) was discovered upon observation that incubation of RlmN with deuterium labeled SAM ([methyl-2H3]-SAM) under single turnover conditions yielded unlabeled C2-methyladenosine (7). The Cys 355 methyl modification was not identified in the study by Yan and Fujimori (27), perhaps due to differences in enzyme preparation that result in either a low yield of mCys 355 or low intrinsic enzyme activity that functionally manifests as multiple turnover conditions in their assay. Yan and Fujimori do report small amounts of unlabeled product that could be attributed to methyl transfer from mCys355 present in the as-isolated enzyme.
- 29.Berkovitch F, Nicolet Y, Wan JT, Jarrett JT, Drennan CL. Science. 2004;303:76. doi: 10.1126/science.1088493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vey JL, et al. Proc. Natl. Acad. Sci. U S A. 2008;105:16137. doi: 10.1073/pnas.0806640105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Layer G, Moser J, Heinz DW, Jahn D, Schubert WD. EMBO J. 2003;22:6214. doi: 10.1093/emboj/cdg598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanzelmann P, Schindelin H. Proc. Natl. Acad. Sci. U S A. 2004;101:12870. doi: 10.1073/pnas.0404624101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanzelmann P, Schindelin H. Proc. Natl. Acad. Sci. U S A. 2006;103:6829. doi: 10.1073/pnas.0510711103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicolet Y, et al. J. Biol. Chem. 2008;283:18861. doi: 10.1074/jbc.M801161200. [DOI] [PubMed] [Google Scholar]
- 35.Nicolet Y, Drennan CL. Nucleic Acids Res. 2004;32:4015. doi: 10.1093/nar/gkh728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shao XG, Grishin NV. Nucleic Acids Res. 2000;28:2643. doi: 10.1093/nar/28.14.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller DJ, et al. Protein Sci. 2003;12:1432. doi: 10.1110/ps.0302403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimura S, Suzuki T. Nucleic Acids Res. 2010;38:1341. doi: 10.1093/nar/gkp1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicolet Y, Amara P, Mouesca JM, Fontecilla-Camps JC. Proc. Natl. Acad. Sci. U S A. 2009;106:14867. doi: 10.1073/pnas.0904385106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cosper NJ, Booker SJ, Ruzicka F, Frey PA, Scott RA. Biochemistry. 2000;39:15668. doi: 10.1021/bi0022184. [DOI] [PubMed] [Google Scholar]
- 41.Kampmeier JA. Biochemistry. 2010;49:10770. doi: 10.1021/bi101509u. [DOI] [PubMed] [Google Scholar]
- 42.Allen BD, O'Leary DJ. J. Am. Chem. Soc. 2003;125:9018. doi: 10.1021/ja034242l. [DOI] [PubMed] [Google Scholar]
- 43.Westler WM, Lin IJ, Perczel A, Weinhold F, Markley JL. J. Am. Chem. Soc. 2011;133:1310. doi: 10.1021/ja1049059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frey PA. In: The Enzymes: Mechanisms of Catalysis. Sigman DS, Boyer PD, editors. vol. 20. The Academic Press, Inc.; San Diego: 1992. pp. 142–187. [Google Scholar]
- 45.The finding that methylation of C2 of A2503 proceeds without exchange of solvent hydrons into the product suggests that a monoprotic base abstracts the proton at C2 and returns it to the methyl group. The structure is consistent with Glu 105, located 4 Å away from mCys 355, as a candidate for the monoprotic base. Substitution of the corresponding residue in Cfr (Glu 91) to alanine abrogates methylation, but causes a stop at A2503 in reverse transcription of the resulting RNA. Elimination of the base that removes the proton at C2 (Fig. S2, step 5) might result in a covalent adduct between the protein and the RNA substrate, which could manifest as a stop in reverse transcriptase assays. The proposed role for Glu 105 as a general base in both methyl transfer to Cys 355 and to the substrate adenine, in combination with its central location in the active site and potential for multiple hydrogen bonding interactions with the backbone of the 351-360 linker (Figs. S8, S13), suggests this residue may be multifunctional in RlmNmediated catalysis.
- 46.Becker A, et al. Nat. Struct. Biol. 1999;6:969. doi: 10.1038/13341. [DOI] [PubMed] [Google Scholar]
- 47.Logan DT, Andersson J, Sjöberg BM, Nordlund P. Science. 1999;283:1499. doi: 10.1126/science.283.5407.1499. [DOI] [PubMed] [Google Scholar]
- 48.Larsson KM, Andersson J, Sjöberg BM, Nordlund P, Logan DT. Structure. 2001;9:739. doi: 10.1016/s0969-2126(01)00627-x. [DOI] [PubMed] [Google Scholar]
- 49.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Proc. Natl. Acad. Sci. U S A. 2001;98:10037. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hardin JW, Hu YX, McKay DB. J. Mol. Biol. 2010;402:412. doi: 10.1016/j.jmb.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.