Abstract
BACKGROUND
Osteochondral allografts are an increasingly popular treatment for the repair of articular cartilage lesions. Current tissue bank protocols require bacteriological testing that takes from 21 to 28 days to process. During this time, tumor necrosis factor-alpha TNF-α (a pro-apoptotic cytokine) is upregulated resulting in loss of chondrocyte viability. To date, etanercept (a cytokine inhibitor) has not been studied in the current storage paradigm with the intention of preserving cell viability.
HYPOTHESIS/PURPOSE
To assess whether or not the addition of Etanercept can improve the chondrocytic viability of osteochondral allograft during storage.
STUDY DESIGN
Controlled, randomized and blinded in vitro laboratory study.
METHODS
Osteochondral allografts were harvested from eight Boer goat femurs and placed into storage media and stored at 4°C for 28 days. The experimental group was supplemented with 10 µg/mL of Etanercept. After storage, cell viability was assessed by live/dead staining and confocal microscopy. Specimens were also analyzed histologically and underwent histomorphological analysis. TNF-α expression was measured with semi-quantitative PCR.
RESULTS
At 28 days, the percent viability of the superficial zone in etanercept-treated allografts was maintained at significantly higher levels than those measured in the untreated group (69.3 ± 9.4 compared to 47.8 ± 19.1, p=0.01). No difference was found histologically between the etanercept and the untreated group (i.e. safranin-O staining for GAG expression). Histomorphologic assessment showed no difference in indentation stiffness or roughness between groups. TNF-α expression was significantly decreased in the etanercept group compared to the untreated group.
CONCLUSION
Etanercept was able to maintain cell viability of osteochondral allografts significantly better than the current storage paradigm after 28 days storage.
CLINICAL RELEVANCE
Maintaining the viability of the superficial zone will benefit outcomes by facilitating joint articulation via improved lubrication. Additionally, maintaining the cellular viability for increased periods of time may allow a greater window of time in which a suitable recipient may be found.
KEY TERMS: Osteochondral, Allograft, Cartilage, Etanercept
INTRODUCTION
Osteochondral allografting has developed from an experimental surgical procedure performed at a few centers, to a more widespread and accepted procedure with networks of tissue banks available to provide tissue to many surgeons throughout the country. Currently, the main limitation to the procedure is not deficiencies in indications, but rather a limitation of graft supply. Increasing the availability of grafts would allow for treatment of greater numbers of patients who have few other cartilage repair options.
Fresh osteochondral allograft resurfacing allows implantation of geographically matched, mature articular cartilage with viable chondrocytes while avoiding donor site morbidity. There has been extensive clinical experience with this procedure. Lexer18 first described osteochondral allografting in 1908, transplanting isolated articular surfaces and whole joints for reconstruction after resection for sarcomas, osteomyelitis, and tuberculosis.1 Musculoskeletal tumor surgeons later relied on this technique in the reconstructive phase of tumor resection, using frozen grafts. Bulk frozen allografts continue to be used for reconstruction in tumor surgery.21,23,39 However, freezing intact articular cartilage, even in the presence of cryopreservative agents such as dimethyl sulfoxide or with techniques such as controlled-rate freezing, was found to result in either complete or near complete loss of chondrocytic viability.20,24,38 Lack of chondrocyte viability has been demonstrated in surgically implanted frozen allografts with breakdown in the acellular cartilage matrix within 5 years of the index procedure.12
During the past half century, the indications for osteochondral allografting have expanded to include isolated osteochondral defects secondary to osteochondritis dissecans and traumatic injury. Since the 1970s, osteochondral allografting using fresh tissue for various indications has become routine at certain institutions, with 5-year clinical success rates greater than 75%.4, 5, 7, 14,26
One of the primary limitations of osteochondral allografting is donor tissue availability. Traditionally, the procedure could only be done in areas with a tissue bank equipped to procure and process tissue. Initially, grafts were stored in lactated Ringer’s solution at 4°C and rapidly implanted after harvesting. Currently, transplantation usually occurs several weeks following procurement as the required microbial testing and other safety measures have become more rigorous. There is a need, therefore, to optimization storage conditions to best preserve the cartilage viability for the longest possible duration.
During storage of osteochondral allografts, primary considerations are the preservation of chondrocyte viability to allow for maintenance of cartilage matrix and remodeling after transplantation and the preservation of matrix biochemical and biomechanical properties.10 Several studies have described detrimental changes within stored osteochondral allografts as a function of time; finding that cell viability significantly declines as implantation time approaches or exceeds 14 days.1, 42 In an attempt to improve storage conditions and maintain viability, Ball et al.2 found that storing osteochondral allografts in culture media, as compared with lactated Ringers, significantly improved cell viability at two weeks. Pennock et al32 found that adding 10% fetal bovine serum to the storage media further increased cell viability of stored grafts. Additionally, Pallante et al31 found that storing allografts at 37°C, instead of 4°C further enhanced cell viability. The current storage paradigm employed by tissue banks includes storing intact osteochondral specimens at 4°C in allograft storage media (similar to tissue culture media) supplemented with 10% fetal bovine serum.
A potential method to further improve chondrocyte viability of stored grafts is by modulating the apoptotic pathway1. While the complex apoptotic process is not fully understood, it is well established that inflammatory mediators, reactive oxygen species, and pro-apoptotic cytokines play an important role in programmed cell death15, 19. Robinson et al33 studied the apoptotic gene expression in stored osteochondral allografts and found many apoptotic genes were significantly upregulated with prolonged storage, including TNF-α expression.
TNF-α is a potent pro-apoptotic cytokine involved in systemic inflammation and the acute phase reaction. TNF-α causes apoptotic cell death, cellular proliferation, differentiation, inflammation, tumorigenesis, and viral replication. Dysregulation and, in particular, overproduction of TNF-α have been implicated in a variety of human diseases including rheumatoid and psoriatic arthritis. The application of TNF-α inhibitors, such as the pharmaceutical etanercept, has been clinically successful in decreasing inflammation in a variety of rheumatologic diseases.6, 13, 36
Since TNF-α is upregulated in stored osteochondral allografts, it is hypothesized that the inhibition of TNF-α through the addition of etanercept to standard storage media will enhance chondrocyte viability by interrupting the apoptotic pathway. No study to date has examined the in vitro effects of etanercept on stored osteochondral allografts prior to surgical implantation, i.e. at 28 days. The purpose of this study is to assess the chondrocytic viability of osteochondral allograft plugs stored in allograft storage media with and without inhibition of TNF-α expression by Etanercept at this time point. The aim is to assess chondrocyte viability by zone, matrix production through glycosaminoglycan (GAG) quantification, cartilage integrity through histomorphometric assessment, and the TNF-α expression by reverse transcriptase PCR.
MATERIALS AND METHODS
Sixteen femoral condyles were obtained from eight mature (2–3 year old) Boer goats (Thomas Morris Farm, Reisterstown MD) within 48 hours of animal sacrifice. The goat has been used successfully as a model for the evaluation of cartilage defects and has been recommended for evaluation of chondral defects3, 31 and is recommended for chondral evaluation as it approximates the low range of human cartilage thickness.9 The condyles were exposed using sterile technique and examined for any gross abnormalities.
Six osteochondral plugs, 10 mm in diameter including 10 mm of subchondral bone, were harvested from each femoral condyle under sterile conditions using Arthrex coring instruments (Arthrex, Inc, Naples, FL). These plugs were then placed into aliquots of allograft storage media (Dulbecco's modified Eagle's medium with supplemental fetal bovine serum, L-glutamine, ascorbic acid, streptomycin, penicillin, and amphotericin) before being stored at 4°C. The experimental group additionally received 10 µg/ml of etanercept. The storage solution was changed weekly. Plugs were removed from storage at 28 days after harvesting and assessed as described below.
Chondrocyte Viability
At each data point, full-thickness articular cartilage was dissected free from the subchondral bone and sectioned with a scalpel into approximately 0.5-mm coronal slices. These slices then were placed into a solution containing 2',7'-Bis(2-carboxyethyl)-5(6)-carboxyfluorescein, acetoxymethylester (BCECF-AM), which stains viable cells green, and propidium iodide, which stains nonviable cells red. BCECF-AM is a fluorescein derivative that is metabolized by nonspecific esterases in living cell membranes to a green fluorescent product. Propidium iodide usually is excluded from living cells by the intact cell membrane but penetrates nonviable cells and intercalates with the DNA and fluoresces red.
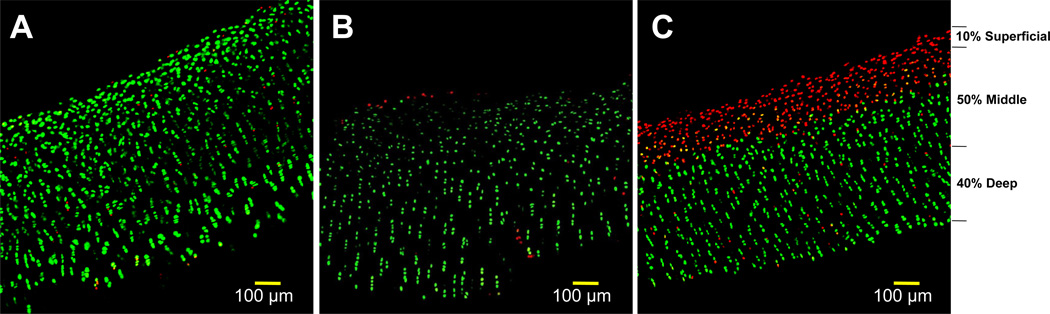
After 45 minutes in the staining solution in a 37°C incubator, the specimens were examined with a confocal laser microscope, and full thickness cartilage images were obtained (Figure 1). Live and dead cells were counted in a blind fashion using image analysis software (ImageJ, NIH, Bethesda MD) and percentage viability was calculated for the entire sample as well as in each cartilage zone: superficial (top 10%), middle (central 50%), and deep (lower 40%).30 Quantification of the chondrocyte live/dead densities was tabulated and reported upon following previously described techniques.17,41,42
Figure 1.
Representative confocal microscopy images of stored allograft specimens with live/dead staining (live cells fluoresce green and dead fluoresce red). (A) baseline image, (B) etanercept treated at 4-weeks, (C) typical storage at 4-weeks. Zones are indicated next to image C.
Glycosaminoglycan (GAG) Content
Tissue blocks were fixed in 10% neutral buffered formalin with 1% cetylpyridinium chloride (CPC) for 24 hours. CPC prevents loss of GAG from tissue during processing.40 The specimens were embedded in paraffin, sectioned onto slides and stained with Safranin O/fast green (an orthochromatic dye that selectively and semiquantitatively stains sulfated GAGs) to assess proteoglycan distribution.34
Quantification of GAG content was achieved using image analysis software (ImageJ, NIH).37 The image was desaturated and calibrated to a linear lookup table (LUT) with value ranging from 0 to 100 (0 being background and 100 being the highest intensity) of GAG stained in the entire population. Area measurements were made of the total cross-sectional cartilage area and the corresponding GAG-stained area. The proportion of GAG staining area to total area is recorded to assess overall percentage GAG area. The intensity of the stain was determined as the average LUT over the entire stained area. A composite GAG content score was determined by multiplying the stain intensity by the area percentage.
Histomorphologic Analysis
Sections were examined by transmitted light microscopy for qualitative and quantitative histological studies. All evaluations were performed blindly with qualified physicians and technical personnel. Qualitative assessment emphasized general indices of cartilage integrity or breakdown. Histological images were recorded with a digital camera attached to a light microscope. High-magnification (360×) images of the sections were used to assess the parameters of degeneration of the articular cartilage.22, 27, 28 At low magnification (40×) the surface was traced for histomorphometric parameters such as cartilage height and surface roughness.15 Idealized curves were established for the surface of the cartilage and subchondral plate. The area between these two curves was used to establish cartilage height, percentage cartilage loss, and surface roughness. Surface roughness was calculated as the root mean square of the surface profile.
Gene Expression by Reverse Transcriptase PCR
The cartilage specimens were pulverized in liquid nitrogen and total RNA was isolated using the acid-guanidinium-thiocyanate-phenol extraction procedure. Starting with 1 µg of total RNA, first - strand cDNA was synthesized using oligo (dT)15 primers. Based on published sequences, PCR primer sets specific to selected coding regions of TNF-α and GAPDH were constructed. Cycle studies were undertaken to determine the linear range of amplification for each gene. ImageJ image analysis software (version 1.61) was used to quantitatively scan RT-PCR profiles following agarose gel electrophoresis and ethidium bromide visualization. The ImageJ software measures relative mean density over a fixed gray scale range after correction for background. All values were normalized to GAPDH.
Statistical Analysis
All data were analyzed using two-way analysis of variance with Bonferroni/Dunn correction. All data are expressed as mean ± standard deviation.
RESULTS
Chondrocyte Viability
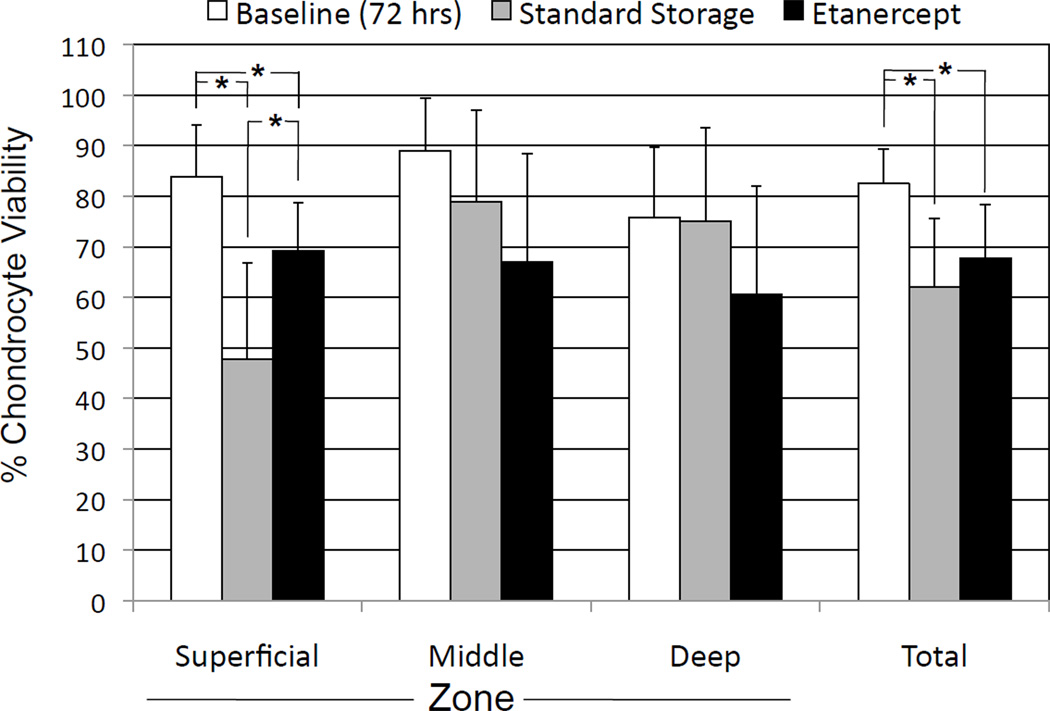
At 28 days, Etanercept was found to significantly maintain the cell viability in the superficial zone compared with the standard storage group. The superficial zone viability in the Etanercept group was 69.3% ± 9.4% compared with 47.8% ± 19.1% in the standard storage group. This difference was significant with P = 0.0125. The effect of etanercept on cell viability was not significant (P > 0.05) on total cell viability or cell viability in the middle and deep cartilage zones (P = 0.1899 and 0.1711, respectively). There was also no significant effect on the total cell count (P = 0.4437). It should be noted that there was a significant decline (P < 0.05) in total chondrocyte viability after storage in both the etanercept group and standard storage group when compared with baseline (Figure 2).
Figure 2.
Chondrocyte viability at baseline (72 hours) and 4 weeks of storage in allograft storage media with and without etanercept. *P < 0.05
Glycosaminoglycan Content
The glycosaminoglycan (GAG) component of the cartilage matrix, as measured by safranin O staining, did not change significantly after 28 days of storage. There were no significant differences in the stained percentage of tissue (etanercept = 73 ± 20% and control = 70 ± 25%, P = 0.79) or intensity of stain (etanercept = 53 ± 14% and control = 47 ± 13%, P = 0.39) between the groups as well as in the composite relative GAG scores (etanercept = 41 ± 18% and control = 36 ± 20%, P = 0.62). These results are illustrated in Figure 3.
Figure 3.
No differences were seen between glycosaminoglycan composite scores (safranin O stain intensity and percentage of total cartilage area) with and without etanercept.
Histomorphometry
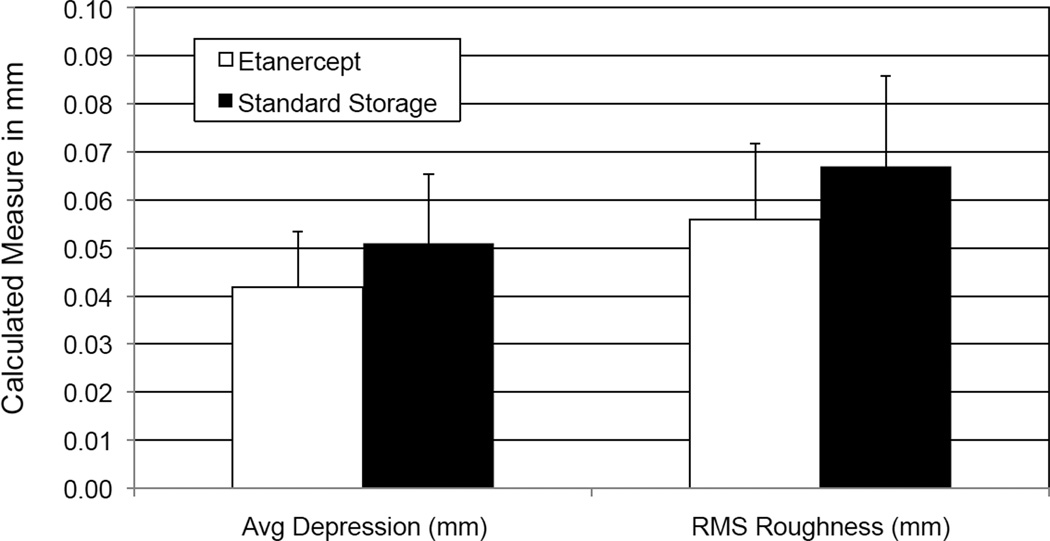
Histomorphometry did not demonstrate any changes in height of cartilage or surface roughness of the articular surface with and without addition of etanercept at 4°C. The standard storage group had an average depression of 0.042 ± 0.027 mm compared with 0.051 ± 0.03 mm for the etanercept group. The surface roughness was 0.067 ± 0.027 mm in the standard storage group compared with 0.056 ± 0.03 mm for the etanercept group (Figure 4).
Figure 4.
Histomorphometric parameters of average depression and root-mean square (RMS) roughness (both measured relative to idealized surface contour).
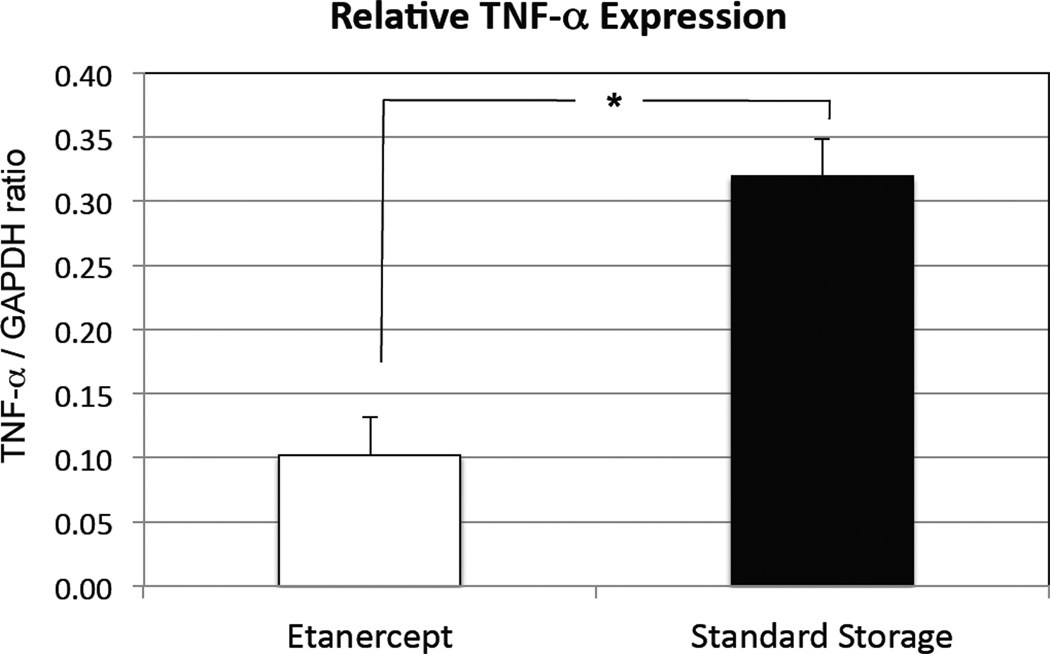
Semiquantitative PCR
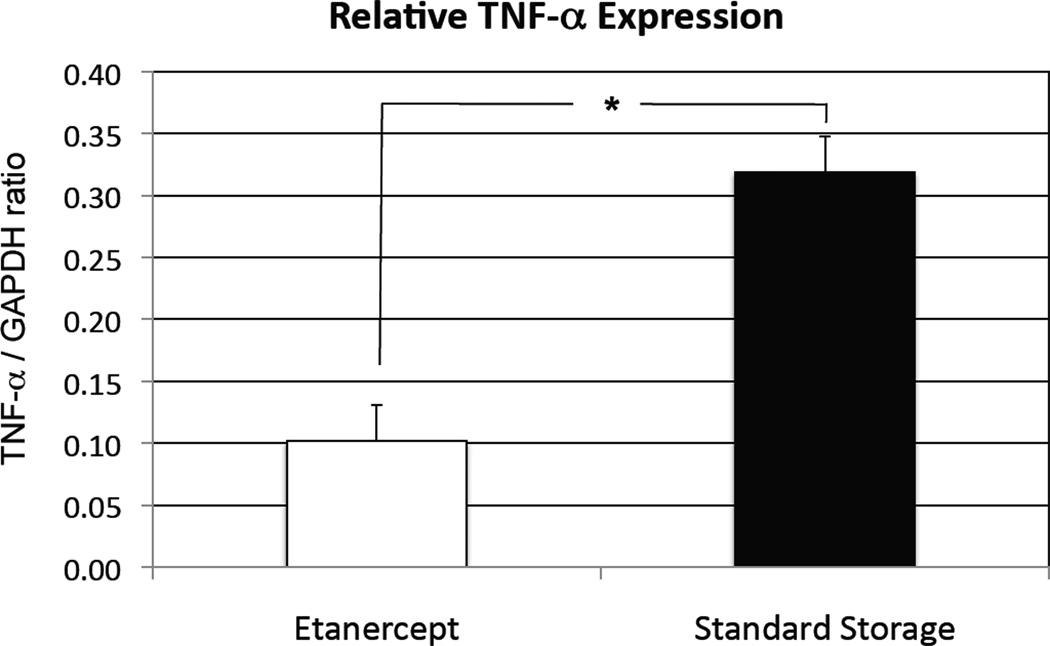
Semiquantitative PCR showed decreased TNF-α expression at 28 days with addition of Etanercept in the storage media at 4°C (TNF/GAPDH ratio of 0.102 ± 0.029) as compared with standard storage (0.319 ± 0.168) at 4°C. This result was significant at P < 0.001 (Figure 5).
Figure 5.
Semiquantitative polymerase chain reaction (PCR) at 4 weeks showing inhibition of tumor necrosis factor-alpha TNF-α. *P < 0.001
DISCUSSION
The clinical use of fresh osteochondral allografts for joint resurfacing of chondral and osteochondral defects now extends approximately three decades.25 In centers with extensive experience with this procedure, the paradigm was to rapidly transplant harvested tissue to preserve chondrocyte viability and other matrix parameters. Currently, however, commercial tissue banks are storing fresh osteochondral allografts for clinical use and providing the tissue usually no sooner than 2 weeks and sometimes as late as 40 days after harvest from the donor.
The work of our laboratories has shown that storage of fresh, human osteochondral allografts in culture media supplemented with fetal bovine serum provides enhanced preservation of chondrocyte viability and metabolic activity when compared with storage in lactated Ringer's solution.1, 2, 32, 41, 42 Based on these works, tissue banks have changed their storage protocols, and now fresh osteochondral allografts are placed directly into this enhanced culture media. This has helped to prolong the chondrocyte viability of stored tissue beyond that stored in lactated Ringer’s solution.
Despite improvements in storage media, chondrocyte cell death remains. Grafts lose significant numbers of cells to apoptosis during storage, especially in the superficial zone. This is particularly important as previous work has shown superficial zone chondrocytes strongly affect the function of joint lubrication with the secretion of superficial zone protein (SZP).35 As the grafts are expected to function as mature hyaline cartilage, the intact superficial zone functions to provide lubrication for articulation. The loss of superficial zone cells has been shown to compromise this functionality.
This study has demonstrated that the superficial region possessed the highest cell density when etanercept was added compared with standard storage at 4°C. Moreover, the superficial zone seemed to be the most sensitive to storage in the allograft storage media and also showed the best preservation in the group stored with the addition of etanercept.
The addition of etanercept, which is commercially available, significantly improves chondrocyte viability in the superficial zone when added to the current storage paradigm. This is clinically relevant as maintaining the viability of the superficial zone will benefit outcomes by decreasing chondrocyte death in the articulating layer of hyaline cartilage. With preservation of the superficial zone, the mechanical integrity of the cartilage will be stabilized. The loss of the superficial zone chondrocytes increases the rate of degeneration with the corresponding loss of cartilage matrix proteins. Additionally, maintaining the cellular viability for increased periods of time increases the window for a suitable recipient to be found before the graft is too degraded for successful implantation.
While we did see a significant difference in the cellular viability between the groups, there are weaknesses to this study that will need to be addressed in further research. First, the goat model, while convenient and a good animal model for osteoarthritis is not identical to human cartilage tissue. Goats were chosen for the availability of tissue and to develop operative and allograft harvesting techniques for the in vivo study. The use of a goat knee model best approximated the clinical application of this procedure without the need for human tissue.
Etanercept was given at only one dose and changing the dosage level could also affect the cellular viability. In previous research on etanercept, there does not appear to be a toxic level in either animal or human testing.11 The dosage level applied was based on the concentration of drug in the joint fluid of a typical patient taking etanercept for clinical reasons. The serum concentration of etanercept in humans is 2.4 ± 1 ug/mL.11 Toxicology studies in monkeys11 were performed at up to 30 times the human dose without any evidence of dose-limiting toxicity.
The stored grafts were in dowel or plug form, rather than as hemicondyles, as is the typical human graft. While this does diminish the amount of bone on the backside of the graft, it should not change the exposure of the superficial zone to the media, which was our primary concern.
Despite the limitations in the present study, etanercept has the potential to be of great clinical use in allograft storage. It is readily available, non-deleterious to cartilage and other tissue11 and in our study significantly prolongs chondrocyte viability in the superficial layer. Clinically, there is an elevated risk of local reaction or even infection with treatment by etanercept and it is not indicated for patients with sepsis.11 Allergic reactions associated with administration of etanercept during clinical trials have been reported in < 2% of patients.11 This is not necessarily pertinent for ex vivo use. However, the transplanted volume of media (if any) should be minimized so as not to elicit a systemic effect.
Further work to assess the preservation of stored human osteochondral allografts would be beneficial and clinically significant.
ACKNOWLEDGMENT
This work was supported by NIH Grant AR055637 and the Joint Restoration Foundation (Centennial, CO).
Footnotes
DISCLOSURE: The primary author has no relationships to disclose.
REFERENCES
- 1.Allen RT, Robertson CM, Pennock AT, et al. Analysis of stored osteochondral allografts at the time of surgical implantation. Am J Sports Med. 2005 Oct;33(10):1479–1484. doi: 10.1177/0363546505275010. [DOI] [PubMed] [Google Scholar]
- 2.Ball ST, Amiel D, Williams SK, et al. The effects of storage on fresh human osteochondral allografts. Clin Orthop Relat Res. 2004 Jan;(418):246–252. doi: 10.1097/00003086-200401000-00043. [DOI] [PubMed] [Google Scholar]
- 3.Brehm W, Aklin B, Yamashita T, Rieser F, Trüb T, Jakob RP, Mainil-Varlet P. Repair of superficial osteochondral defects with an autologous scaffold-free cartilage construct in a caprine model: implantation method and short-term results. Osteoarthritis Cartilage. 2006 Dec;14(12):1214–1226. doi: 10.1016/j.joca.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Bugbee WD. Fresh osteochondral allografting. Operative Tech Sports Med. 2000;(8):158–162. [Google Scholar]
- 5.Bugbee WD. Alternatives to arthroplasty of the knee: Biologic resurfacing. Curr Opin Orthop. 2001;(12):1–7. [Google Scholar]
- 6.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001 Mar 22;344(12):907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 7.Chu CR, Convery FR, Akeson WH, et al. Articular cartilage transplantation. Clin Orthop. 1999;(360):159–168. [PubMed] [Google Scholar]
- 8.Chu CR, Coyle CH, Chu CT, Szczodry M, Seshadri V, Karpie JC, Cieslak KM, Pringle EK. In vivo effects of single intra-articular injection of 0.5% bupivacaine on articular cartilage. J Bone Joint Surg Am. 2010;92(3):599–608. doi: 10.2106/JBJS.I.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu CR, Szczodry M, Bruno S. Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev. 2010 Feb;16(1):105–15. doi: 10.1089/ten.teb.2009.0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czitrom AA, Keating S, Gross AE. The viability of articular cartilage in fresh osteochondral allografts. J Bone Joint Surg. 1990;(72A):574–581. [PubMed] [Google Scholar]
- 11.ENBREL® (etanercept) [package insert] Thousand Oaks, CA: Immunex Corporation; 2003. [Google Scholar]
- 12.Enneking WF, Campanacci DA. Retrieved human allografts: A clinicopathological study. J Bone Joint Surg. 2001;(83A):971–986. [PubMed] [Google Scholar]
- 13.Firestein GS, Kelley WN. Kelley's textbook of rheumatology. 8th ed. Philadelphia, PA: Saunders/Elsevier; 2009. [Google Scholar]
- 14.Gross AE, McKee NH, Pritzker KPH, et al. Reconstruction of skeletal deficits at the knee: A comprehensive osteochondral transplant program. Clin Orthop. 1983;(174):96–106. [PubMed] [Google Scholar]
- 15.Hacker SA, Healey RM, Yoshioka M, Coutts RD. A methodology for the quantitative assessment of articular cartilage histomorphometry. Osteoarthritis Cartilage. 1997;5(5):343–355. doi: 10.1016/s1063-4584(97)80038-6. [DOI] [PubMed] [Google Scholar]
- 16.Kim HT, Teng MS, Dang AC. Chondrocyte apoptosis: implications for osteochondral allograft transplantation. Clin Orthop Relat Res. 2008 Aug;466(8):1819–1825. doi: 10.1007/s11999-008-0304-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lane JG, Tontz WL, Jr, Ball ST, Massie JB, Chen AC, Bae WC, Amiel ME, Sah RL, Amiel D. A morphologic, biochemical, and biomechanical assessment of short-term effects of osteochondral autograft plug transfer in an animal model. Arthroscopy. 2001;17(8):856–863. doi: 10.1016/s0749-8063(01)90010-6. [DOI] [PubMed] [Google Scholar]
- 18.Lexer E. Joint transplantation. Clin Orthop. 1985;(197):4–10. [PubMed] [Google Scholar]
- 19.Lotz M, Hashimoto S, Kuhn K. Mechanisms of chondrocyte apoptosis. Osteoarthritis Cartilage. 1999 Jul;7(4):389–391. doi: 10.1053/joca.1998.0220. [DOI] [PubMed] [Google Scholar]
- 20.Malinin TI, Wagner JL, Pita JC, et al. Hypothermic storage and cryopreservation of cartilage. Clin Orthop. 1985;(197):15–26. [PubMed] [Google Scholar]
- 21.Mankin HJ, Doppelt S, Tomford WW. Clinical experience with allograft implantation: The first ten years. Clin Orthop. 1983;(174):69–86. [PubMed] [Google Scholar]
- 22.Mankin HJ, Dorfman H, Lippicllo L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteoarthritic human hip. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg. 1971;53-A:523–537. [PubMed] [Google Scholar]
- 23.Mankin HJ, Gebhardt MC, Tomford WW. The use of frozen cadaveric allograft in the management of patients with bone tumors of the extremities. Orthop Clin North Am. 1987;(18):275–289. [PubMed] [Google Scholar]
- 24.Marco F, Leon C, Lopez-Oliva F, et al. Intact articular cartilage preservation: In-vivo evaluation. Clin Orthop. 1992;(283):11–20. [PubMed] [Google Scholar]
- 25.Maury AC, Safir O, Heras FL, Pritzker KP, Gross AE. Twenty-five-year chondrocyte viability in fresh osteochondral allograft. A case report. J Bone Joint Surg Am. 2007 Jan;89(1):159–165. doi: 10.2106/JBJS.E.00815. [DOI] [PubMed] [Google Scholar]
- 26.McDermott AGP, Langer F, Pritzker KPH, et al. Fresh small-fragment osteochondral allografts: Long-term follow-up study on first 100 cases. Clin Orthop. 1985;(197):96–102. [PubMed] [Google Scholar]
- 27.McDevitt C, Gilbertson E, Muir H. An experimental model of osteoarthritis; early morphological and biochemical changes. J Bone Joint Surg Br. 1977 Feb;59(1):24–35. doi: 10.1302/0301-620X.59B1.576611. [DOI] [PubMed] [Google Scholar]
- 28.Meachim G, Emery IH. Quantitative aspects of patello-femoral cartilage fibrillation in Liverpool necropsies. Ann Rheum Dis. 1974 Jan;33(1):39–47. doi: 10.1136/ard.33.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niederauer GG, Slivka MA, Leatherbury NC, Korvick DL, Harroff HH, Ehler WC, Dunn CJ, Kieswetter K. Evaluation of multiphase implants for repair of focal osteochondral defects in goats. Biomaterials. 2000 Dec;21(24):2561–2574. doi: 10.1016/s0142-9612(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 30.Ostrander RV, Goomer RS, Tontz WL, et al. Donor Cell Fate in Tissue Engineering for Articular Cartilage Repair. Clin Orthop. 2001;(389):228–237. doi: 10.1097/00003086-200108000-00032. [DOI] [PubMed] [Google Scholar]
- 31.Pallante AL, Bae WC, Chen AC, Gortz S, Bugbee WD, Sah RL. Chondrocyte viability is higher after prolonged storage at 37 degrees C than at 4 degrees C for osteochondral grafts. Am J Sports Med. 2009 Nov;37(Suppl 1):24S–32S. doi: 10.1177/0363546509351496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pennock AT, Wagner F, Robertson CM, Harwood FL, Bugbee WD, Amiel D. Prolonged storage of osteochondral allografts: does the addition of fetal bovine serum improve chondrocyte viability? J Knee Surg. 2006 Oct;19(4):265–272. doi: 10.1055/s-0030-1248117. [DOI] [PubMed] [Google Scholar]
- 33.Robertson CM, Allen RT, Pennock AT, Bugbee WDDA. Upregulation of apoptotic and matrix-related gene expression during fresh osteochondral allograft storage. Clin Orthop Relat Res. 2006;(442):260–266. doi: 10.1097/01.blo.0000187058.42820.39. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg L. Chemical basis for the histological use of Safranin O in the study of articular cartilage. J Bone Joint Surg [Am] 1971;(53):69–82. [PubMed] [Google Scholar]
- 35.Schmidt TA, Schumacher BL, Klein TJ, Voegtline MS, Sah RL. Synthesis of proteoglycan 4 by chondrocyte subpopulations in cartilage explants, monolayer cultures, and resurfaced cartilage cultures. Arthritis Rheum. 2004 Sep;50(9):2849–2857. doi: 10.1002/art.20480. [DOI] [PubMed] [Google Scholar]
- 36.Scott DL, Kingsley GH. Tumor necrosis factor inhibitors for rheumatoid arthritis. N Engl J Med. 2006 Aug 17;355(7):704–712. doi: 10.1056/NEJMct055183. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu C, Coutts RD, Healey RM, Kubo T, Hirasawa Y, Amiel D. Method of histomorphometric assessment of glycosaminoglycans in articular cartilage. J Orthop Res. 1997 Sep;15(5):670–674. doi: 10.1002/jor.1100150507. [DOI] [PubMed] [Google Scholar]
- 38.Tomford WW, Duff GP, Mankin HJ. Experimental freeze-preservation of chondrocytes. Clin Orthop. 1985;(197):11–14. [PubMed] [Google Scholar]
- 39.Tomford WW, Springfield DS, Mankin HJ. Fresh and frozen articular cartilage allografts. Orthopedics. 1992;(15):1183–1188. doi: 10.3928/0147-7447-19921001-09. [DOI] [PubMed] [Google Scholar]
- 40.Troyer H, et al. Principles and Techniques of Histochemistry. Massachusetts: Little Brown & Co; 1980. [Google Scholar]
- 41.Williams SK, Amiel D, Ball ST, et al. Analysis of cartilage tissue on a cellular level in fresh osteochondral allograft retrievals. Am J Sports Med. 2007 Dec;35(12):2022–2032. doi: 10.1177/0363546507305017. [DOI] [PubMed] [Google Scholar]
- 42.Williams SK, Amiel D, Ball ST, et al. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003 Nov;85-A(11):2111–2120. doi: 10.2106/00004623-200311000-00008. [DOI] [PubMed] [Google Scholar]