Abstract
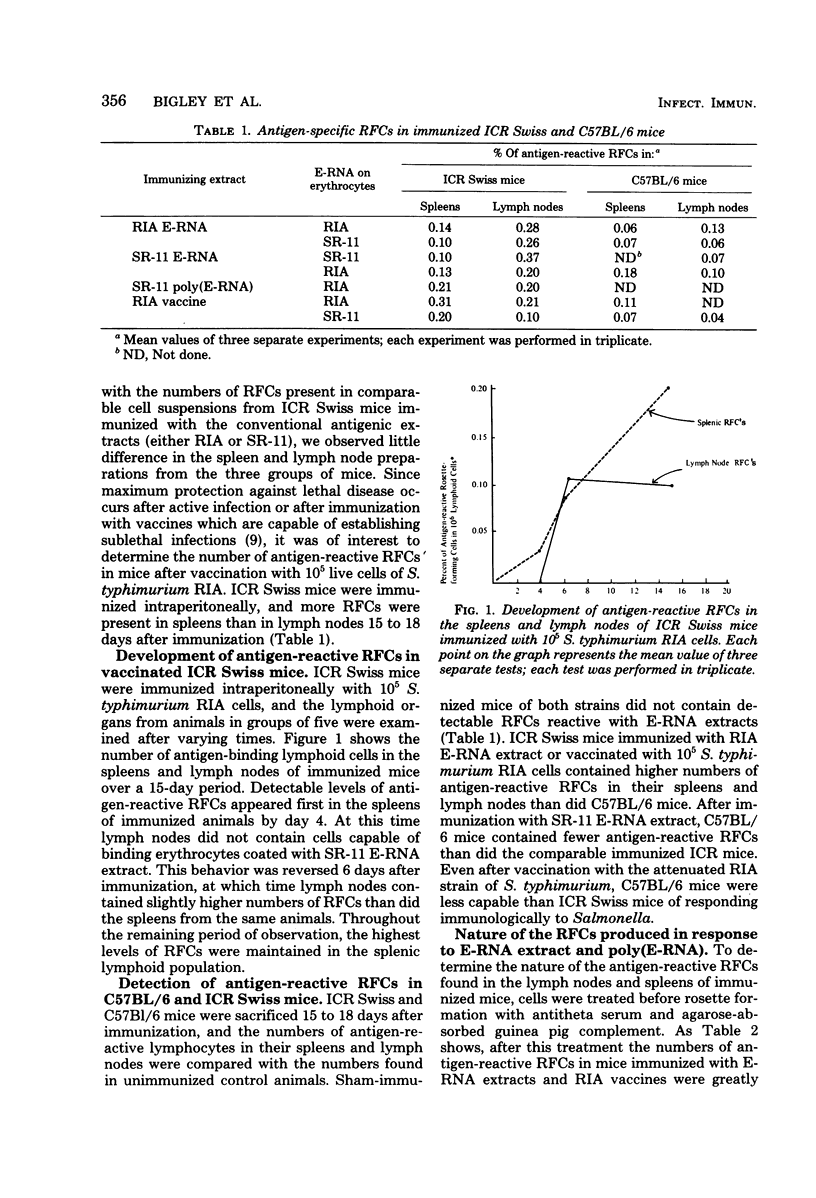
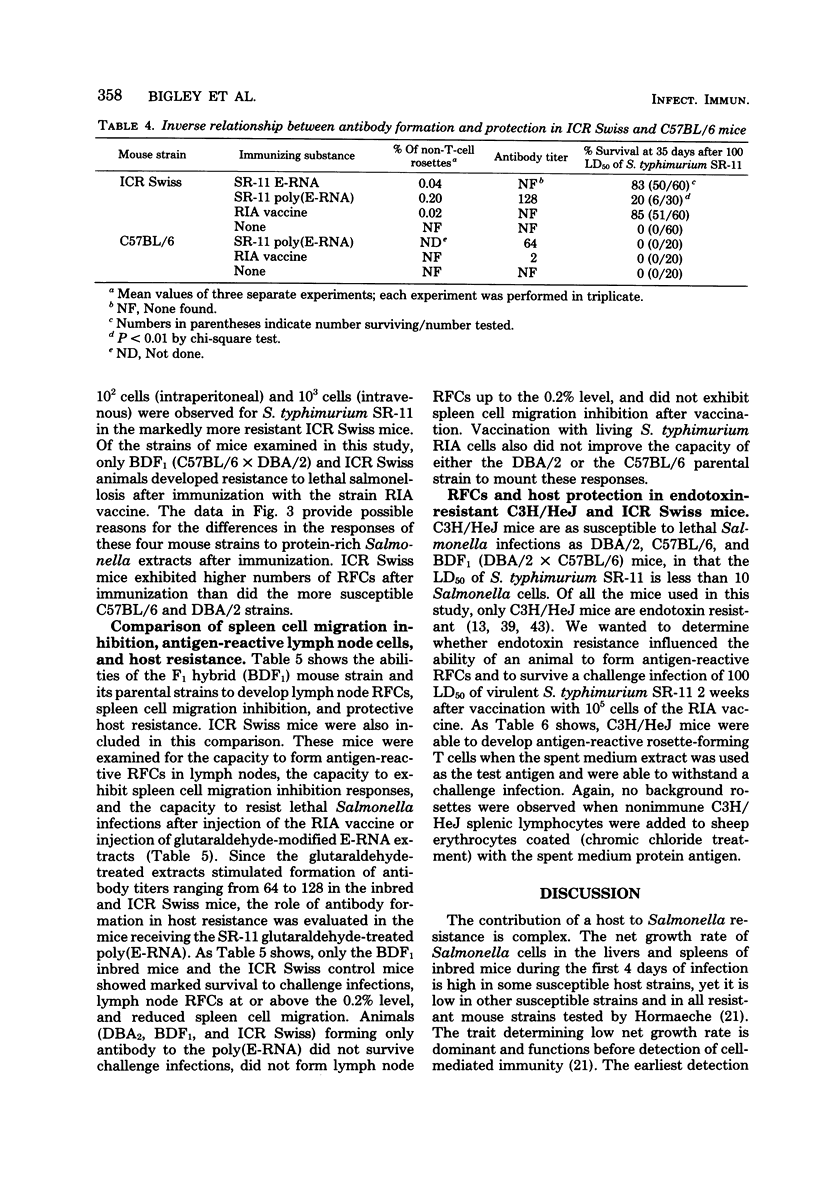
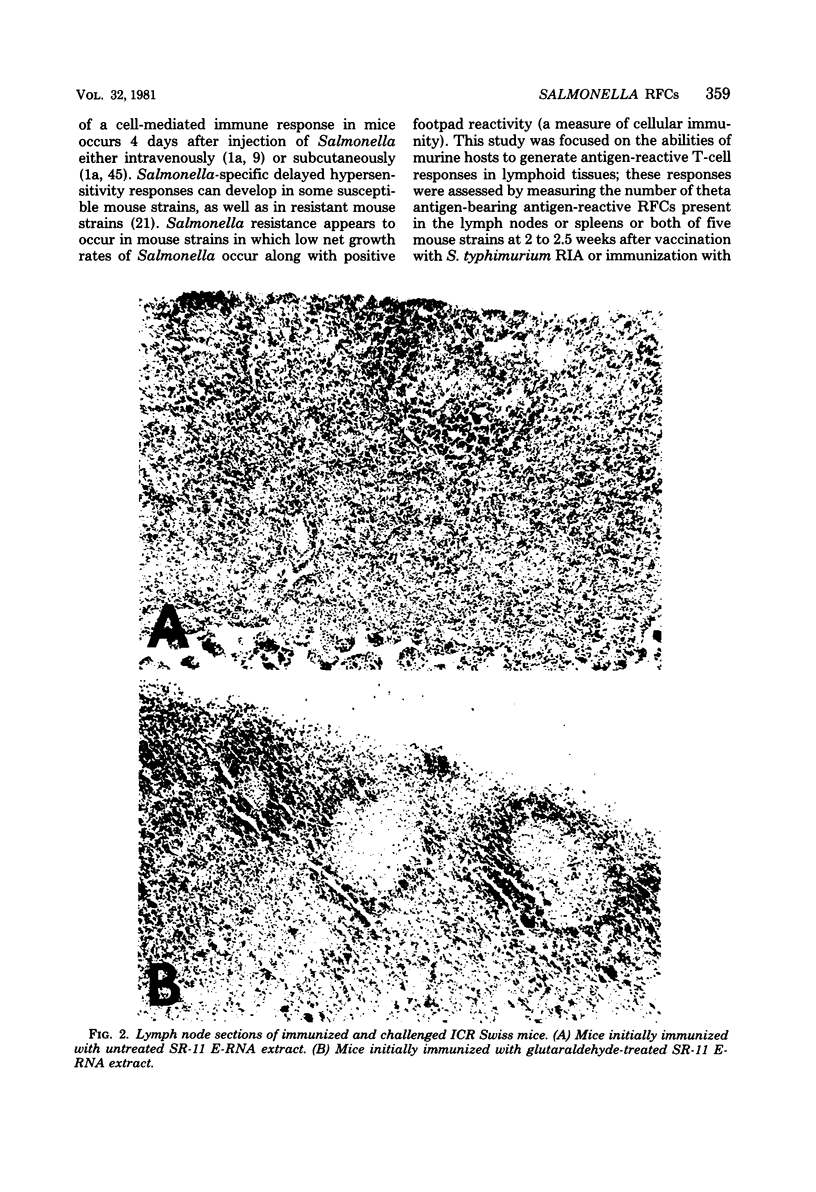
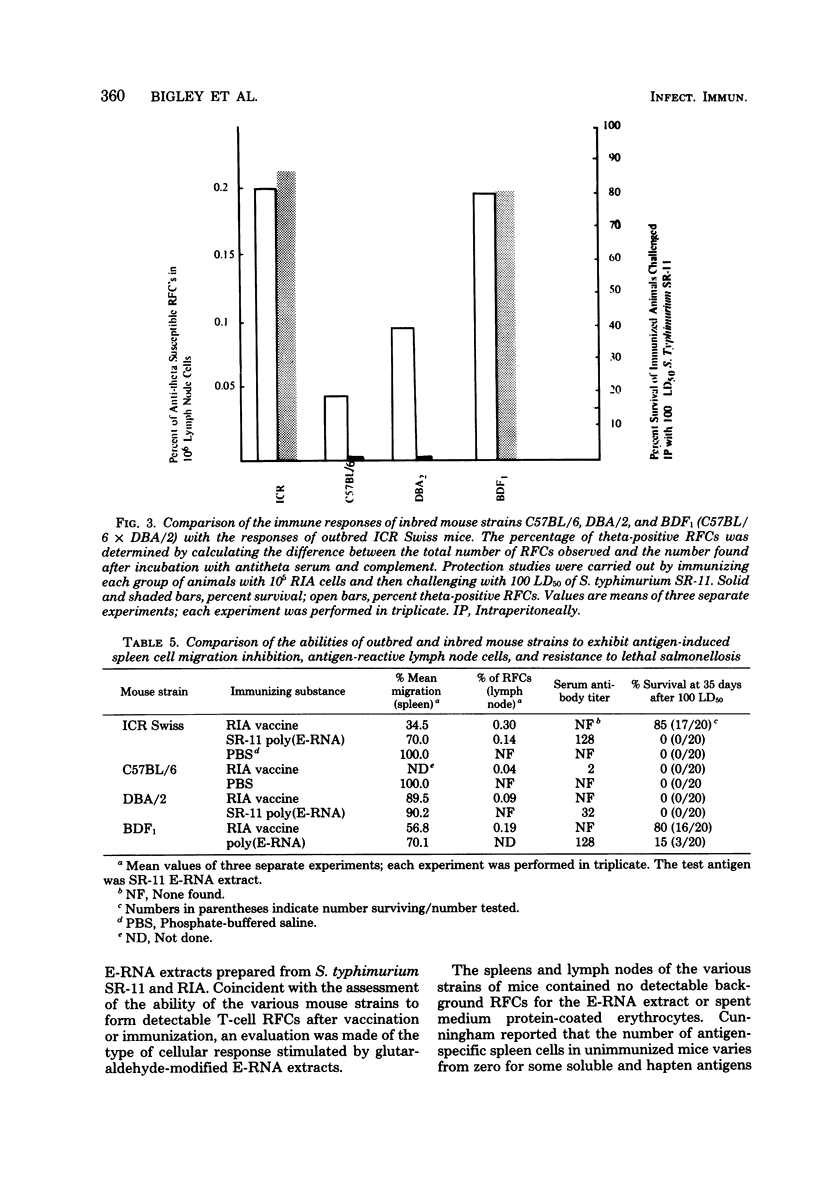
To assess the separate contributions of host T cells and the physical state of the antigen in the development of effective. Salmonella resistance, glutaraldehyde-treated and untreated protein- and ribonucleic acid-rich extracts (E-RNA extracts) of virulent Salmonella typhimurium SR-11 or attenuated S. typhimurium RIA were used to immunize Salmonella-resistant Salmonella-susceptible strains of mice for the purpose of determining whether antigen-specific T-cell or B-cell responses were formed and, if so, which responses predominated. The resistance imparted to each mouse strain after vaccination with S. typhimurium RIA was used as the standard for comparison. The inbred mouse strains C57BL/6 and DBA/2 and their F1 hybrid (strain BDF1), outbred ICR Swiss mice, and endotoxin-resistant C3H/HeJ mice were examined for the capacity to develop resistance to lethal Salmonella infections, as well as the ability to generate antigen-reactive T cells. Only the BDF1, C3H/HeJ, and ICR Swiss mice were able to develop resistance to challenge infections mediated by the virulent SR-11 strain of S. typhimurium after vaccination with the living, attenuated RIA strain of S. typhimurium or immunization with E-RNA extracts. We developed an assay to identify the antigen-reactive rosette-forming lymphocytes present in lymph nodes and spleens of immunized mice. Levels of 0.2% or higher of theta antigen-bearing, antigen-reactive rosette-forming cells were found in the lymph nodes or spleens or both of only the BDF1, C3H/HeJ, and ICR Swiss mice (i.e., in the “Salmonella responder” strains). Mouse strains C57BL/6 and DBA/2, which failed to develop resistance to lethal infections after immunization with the S. typhimurium RIA vaccine or with the E-RNA extracts, lacked effective numbers of antitheta antigen-sensitive rosette-forming cells. Modification of the effective E-RNA extracts by polymerization with glutaraldehyde resulted in a marked diminution in their abilities to induce resistance to salmonellosis in the two responder mouse strains tested (BDF1 and ICR Swiss), even though detectable levels of antibody were induced.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Bigley N. J., Smith R. A., Warren P., Minahan W. T., Kreps D. P. Antigenic modification: its relation to protective host resistance in murine salmonellosis. Infect Immun. 1981 Mar;31(3):1273–1276. doi: 10.1128/iai.31.3.1273-1276.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Mackaness G. B., Collins F. M. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme D. H. Resistance to salmonella infections in inbred mouse strains. Bull N Y Acad Med. 1970 Jul;46(7):499–508. [PMC free article] [PubMed] [Google Scholar]
- Carter P. B., Collins F. M. Growth of typhoid and paratyphoid bacilli in intravenously infected mice. Infect Immun. 1974 Oct;10(4):816–822. doi: 10.1128/iai.10.4.816-822.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Effect of specific immune mouse serum on the growth of Salmonella enteritidis in mice preimmunized with living or ethyl alcohol-killed vaccines. J Bacteriol. 1969 Feb;97(2):676–683. doi: 10.1128/jb.97.2.676-683.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Salmonellosis in orally infected specific pathogen-free C57B1 mice. Infect Immun. 1972 Feb;5(2):191–198. doi: 10.1128/iai.5.2.191-198.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Vaccines and cell-mediated immunity. Bacteriol Rev. 1974 Dec;38(4):371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon J., Hunter R. Selective induction of delayed hypersensitivity by a lipid conjugated protein antigen which is localized in thymus dependent lymphoid tissue. J Immunol. 1973 Jan;110(1):183–190. [PubMed] [Google Scholar]
- Cunningham A. J. The generation of antibody diversity: its dependence on antigenic stimulation. Contemp Top Mol Immunol. 1974;3:1–26. doi: 10.1007/978-1-4684-2838-4_1. [DOI] [PubMed] [Google Scholar]
- Dorf M. E., Benacerraf B. Complementation of H-2-linked Ir genes in the mouse. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3671–3675. doi: 10.1073/pnas.72.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein T. K., Angerman C. R. Immunity to experimental Salmonella infection: studies on the protective capacity and immunogenicity of lipopolysaccharide, acetone-killed cells, and ribosome-rich extracts of Salmonella typhimurium in C3H/HeJ and CD-1 mice. J Immunol. 1978 Sep;121(3):1010–1014. [PubMed] [Google Scholar]
- Elliott B. E., Haskill J. S., Axelrad M. A. Thymus-derived rosettes are not "helper" cells. J Exp Med. 1973 Nov 1;138(5):1133–1143. doi: 10.1084/jem.138.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEORGE M., VAUGHAN J. H. In vitro cell migration as a model for delayed hypersensitivity. Proc Soc Exp Biol Med. 1962 Nov;111:514–521. doi: 10.3181/00379727-111-27841. [DOI] [PubMed] [Google Scholar]
- Giuliano V. J., Jasin H. E., Hurd E. R., Ziff M. Enumeration of B-lymphocytes in human peripheral blood by a rosette method for the detection of surface-bound immunoglobulin. J Immunol. 1974 Apr;112(4):1494–1499. [PubMed] [Google Scholar]
- Gold E. R., Fudenberg H. H. Chromic chloride: a coupling reagent for passive hemagglutination reactions. J Immunol. 1967 Nov;99(5):859–866. [PubMed] [Google Scholar]
- Hormaeche C. E. Natural resistance to Salmonella typhimurium in different inbred mouse strains. Immunology. 1979 Jun;37(2):311–318. [PMC free article] [PubMed] [Google Scholar]
- Katz D. H. Adaptive differentiation of murine lymphocytes. III. T and B lymphocytes display reciprocal preference for one another to develop optimal interacting partner cell sets. J Immunol. 1979 May;122(5):1937–1942. [PubMed] [Google Scholar]
- Katz D. H., Dorf M. E., Benacerraf B. Control of t-lymphocyte and B-lymphocyte activation by two complementing Ir-GLphi immune response genes. J Exp Med. 1976 Apr 1;143(4):906–918. doi: 10.1084/jem.143.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis J. M., Bigley N. J. Cytophilic macroglobulin reactive with bacterial protein in mice immunized with ribonucleic acid-protein fractions of virulent Salmonella typhimurium. Infect Immun. 1972 Sep;6(3):390–397. doi: 10.1128/iai.6.3.390-397.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Hahn H. H., Mackaness G. B. The mediator of cellular immunity. V. Development of cellular resistance to infection in thymectomized irradiated rats. Cell Immunol. 1973 Feb;6(2):186–199. doi: 10.1016/0008-8749(73)90021-x. [DOI] [PubMed] [Google Scholar]
- Medina S., Vas S. I., Robson H. G. Effect of nonspecific stimulation on the defense mechanisms of inbred mice. J Immunol. 1975 Jun;114(6):1720–1725. [PubMed] [Google Scholar]
- Misfeldt M. L., Johnson W. Identification of protective cell surface proteins in ribosomal fractions from Salmonella typhimurium. Infect Immun. 1979 Jun;24(3):808–816. doi: 10.1128/iai.24.3.808-816.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misfeldt M. L., Johnson W. Variability of protection in inbred mice induced by a ribosomal vaccine prepared from Salmonella typhimurium. Infect Immun. 1976 Sep;14(3):652–659. doi: 10.1128/iai.14.3.652-659.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser I., Hohmann A., Schmidt G., Rowley D. Salmonellosis in mice: studies on oral immunization with live avirulent vaccines. Med Microbiol Immunol. 1980;168(2):119–128. doi: 10.1007/BF02121760. [DOI] [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant J., Glynn A. A., Wilson B. M. Protective effects of a supernatant factor from Salmonella typhimurium on Salmonella typhimurium infection of inbred mice. Infect Immun. 1978 Oct;22(1):125–131. doi: 10.1128/iai.22.1.125-131.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWLEY D., TURNER K. J., JENKIN C. R. THE BASIS FOR IMMUNITY TO MOUSE TYPHOID. 3. CELL-BOUND ANTIBODY. Aust J Exp Biol Med Sci. 1964 Apr;42:237–248. doi: 10.1038/icb.1964.25. [DOI] [PubMed] [Google Scholar]
- Rampy P. A., Jutila J. W. Influence of lipopolysaccharide on graft versus host reactivity of lipopolysaccharide-unresponsive C3H/HeJ mice. Infect Immun. 1979 Oct;26(1):137–142. doi: 10.1128/iai.26.1.137-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson H. G., Vas S. I. Resistance of inbred mice to Salmonella typhimurium. J Infect Dis. 1972 Oct;126(4):378–386. doi: 10.1093/infdis/126.4.378. [DOI] [PubMed] [Google Scholar]
- Rowley D. Phagocytosis and immunity. The central role of phagocytosis in immune reactions. Experientia. 1966 Jan 15;22(1):1–5. [PubMed] [Google Scholar]
- Rudbach J. A., Reed N. D. Immunological responses of mice to lipopolysaccharide: lack of secondary responsiveness by C3H/HeJ mice. Infect Immun. 1977 May;16(2):513–517. doi: 10.1128/iai.16.2.513-517.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A., Bigley N. J. Detection of delayed hypersensitivity in mice injected with ribonucleic acid-protein fractions of Salmonella typhimurium. Infect Immun. 1972 Sep;6(3):384–389. doi: 10.1128/iai.6.3.384-389.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A., Bigley N. J. Ribonucleic acid-protein fractions of virulent Salmonella typhimurium as protective immunogens. Infect Immun. 1972 Sep;6(3):377–383. doi: 10.1128/iai.6.3.377-383.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis G. A., Weinbaum F. I., Thorbecke G. J. Abscence of rosette-forming cells in delayed hypersensitive agammaglobulinemic chickens. J Immunol. 1973 Aug;111(2):457–463. [PubMed] [Google Scholar]
- Wilson J. D., Miller J. F. T and B rosette-forming cells. Eur J Immunol. 1971 Dec;1(6):501–503. doi: 10.1002/eji.1830010622. [DOI] [PubMed] [Google Scholar]
- Youdim S., Stuntman O., Good R. A. Thymus dependency of cells involved in transfer of delayed hypersensitivity to Listeria monocytogenes in mice. Cell Immunol. 1973 Sep;8(3):395–402. doi: 10.1016/0008-8749(73)90129-9. [DOI] [PubMed] [Google Scholar]




