Abstract
Purpose: This study was performed to evaluate whether a lower dose (0.2 mg) of cetrorelix would prevent premature LH surge in patients undergoing controlled ovarian hyperstimulation. Methods: Controlled ovarian hyperstimulation was carried out in 45 patients, starting on menstrual cycle day 3 with recombinant FSH (r-FSH), and a cetrorelix of 0.2 mg was administered from day 5 evening of ovarian stimulation until the day before hCG injection. Results: There was a statistically significant decrease in serum LH level one day after the first cetrorelix injection and on the day of hCG administration. Serum LH concentrations were maintained constantly low during the follicular phase with no premature LH surge occurring in any of the patients. Clinical pregnancy was achieved for 18 women (40%), with one of these experiencing intrauterine fetal death before 12 week' gestation. Conclusion: This study demonstrates that a daily dose of cetrorelix 0.2 mg is able to prevent premature LH surge.
Keywords: Cetrorelix, GnRH antagonist, In vitro fertilization, LH surge
Introduction
One of the main causes of the relatively low efficacy of ovarian stimulation agents is the onset of premature luteinizing hormone (LH) surge. The ability of gonadotropin releasing hormone (GnRH) antagonists to induce a rapid, reversible suppression of gonadotropin release by competitive blockage of the GnRH receptors, preventing LH surges in in vitro fertilization (IVF) cycles has been previous reported [1–3]. The use of cetrorelix acetate, a type of GnRH antagonists, in multiple daily injection to prevent LH surge during controlled ovarian hyperstimulation, was proposed by Diedrich et al. [1]. In order to determine the minimal effective dose, Albano et al. have compared daily doses of 0.5, 0.25 and 0.1 mg of cetrorelix in women undergoing IVF. Dosages of both 0.5 and 0.25 mg were able to prevent LH surges while premature LH surges were observed in two out of seven patients at 0.1 mg [2]. Therefore, a minimum effective daily dose of 0.25 mg citrorelix was recommended for clinical use. An incidence of premature luteinization of 0.9% was observed in a phase III clinical trial [3].
In most controlled studies using GnRH antagonist, there is a trend to find slightly but statistically significant lower pregnancy rates as compared with GnRH agonist protocol [4]. This difference may be related to the deleterious effect of GnRH antagonist on the endometrium, resulting in a lower rate of embryo implantation [5]. Increasing GnRH antagonist dosage is associated with lower implantation rates and a reduction in ongoing pregnancies [6]. Thus, based on the concept of preventing the premature LH surge with less possible harmful effects on reproductive outcome, the minimum dose of GnRH antagonist should be determined through further investigation. A daily dose 0.2 mg cetrorelix (between 0.1 and 0.25 mg) was chosen for the present study and the efficacy of preventing premature LH surge during controlled ovarian stimulation was assessed.
Materials and methods
This non-randomized, observational study investigated the efficacy of 0.2 mg multiple-dose cetrorelix acetate (Cetrotide, Serono, Geneva, Switzerland) for prevention of premature LH surge in IVF cycles. After approval had been obtained from the Ethics Committee of Shin Kong Wu Ho-Su Memorial Hospital, 45 women aged 22–37 years (32.3±3.9, mean±SD) were enrolled. Mean body weight was 55.4±7.2 kg, and mean body mass index (BMI) was 21.8±2.7 kg/m2. Baseline FSH level was 6.76±1.64 mIU/ml. The causes of infertility were endometriosis (n = 8, 17.8%), male factor (n = 15, 33.3%), tubal factor (n = 12, n = 26.7%) and unexplained infertility (n = 10, 22.2%). The exclusion criteria included age >38 year, day 3 FSH level >15 mIU/ml, BMI >28 kg/m2, irregular menstrual cycle and more than three previous IVF attempts. All couples were required to sign informed consents.
After ultrasonographic exclusion of ovarian cyst greater than 12 mm, controlled ovarian hyperstimulation was started on cycle day 3 (6:00–8:00 p.m.) with recombinant-FSH (Gona-F, Serono, Geneva, Switzerland) 225 IU/day for 4 days with the dosage then adjusted according to the follicular response. Ultrasound monitoring was started on the morning of day 5 ovarian stimulation and then every 1–3 days as necessary. Cetrorelix (Cetrotide, Serono, Geneva, Switzerland) was injected subcutaneously once daily in doses of 0.2 mg, from the evening of day 5 ovarian stimulation until the day before hCG (Pregnyl, NY Organon, The Netherlands) administration. The 3 mg package of cetrorelix acetate was dissolved in 3 ml of injection-grade water to a final concentration of 1 mg/ml. HCG (10000 IU) was injected until at least two follicles had reached 18 mm with appropriate serum estradiol (E2) levels. Oocyte retrieval was performed 36 h after hCG administration and then IVF or intracytoplasmic sperm injection (ICSI) performed. Embryo transfer was conducted on day 2 or 3. The luteal phase was supported by 600 mg of vaginally administered micronized progesterone (Utrogestan, Piette International S.A., Brussel, Belgium). A premature LH surge is defined as LH level ≥10 mIU/ml with progesterone elevation ≥1.0 ng/ml.
Concentrations of LH, estradiol (E2) and progesterone (P4) were measured on the morning of day 5 of gonadotropin stimulation until the day of hCG administration. Serum LH levels were measured via immunometric assay using an Immulite® kit (Diagnostic Products Corporation, Los Angeles, CA, USA), the sensitivity for LH was 0.1 mIU/ml. The intra- and inter-assay coefficients of variation (CV) were 6.5 and 7.1%, respectively. Estradiol and progesterone were measured by competitive immunoassay using an Immulite kit with intra- and inter-assay CVs of 6.3 and 6.4% for estradiol and 6.3 and 5.8% for progesterone respectively. Sensitivity was 15 pg/ml for estradiol and 0.2 ng/ml for progesterone.
Values are expressed as the mean±standard deviation (SD). Paired t-test was used for the statistical analysis where appropriate. A P value < 0.05 was considered statistically significant. Analysis was performed using the SPSS statistical package Windows (Ver. 10.0; SPSS Inc., Chicago, IL, USA).
Results
All patients underwent oocyte retrieval and embryo transfer (n = 45). There was a statistically significant decrease in serum LH level one day after the first cetrorelix acetate injection and on the day of hCG administration (Table 1). It was noted that a daily injection of 0.2 mg cetrorelix, starting from day 5 evening of gonadotropin stimulation, effectively suppressed the LH surge till hCG administration in all patients (Fig. 1). Serum LH concentration were constantly low during the follicular phase. No patients experienced premature LH surge. Serum E2 steadily increased during cetrorelix injection (Fig. 2). Table 2 shows the data of the clinical results, including the period of cetrorelix injection, amount of gonadotropin used, number of oocytes retrieved, fertilization rate, and clinical and ongoing pregnancy rates. There were 18 clinical pregnancies (40%) with one mother experiencing intrauterine fetal death before 12 week' gestation.
Table 1.
Serum LH levels (mean±SD) on day 5 of gonadotropin stimulation (cetrorelix acetate started in the evening of day 5), day 6 of gonadotropin stimulation (one day after cetrorelix acetate injection), and the day of hCG injection
| LH | Values (mIU/ml) |
|---|---|
| Day 5 of gonadotropin stimulation | 2.6±1.6a.b |
| Day 6 of gonadotropin stimulation | 2.2±1.3a |
| Day of hCG | 2.0±1.6b |
a.bP < 0.05, paired t-test.
Fig. 1.
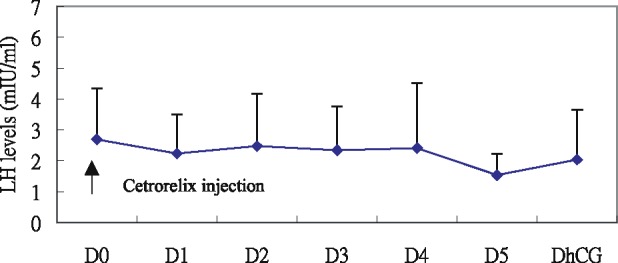
Mean serum LH (mIU/ml) concentrations during controlled ovarian hyperstimulation with gonadotropin in association with 0.2 mg cetrorelix aceate. Values are presented relative to the first day of cetrorelix acetate administration. D = day
Fig. 2.

Serum E2 values relative to the day of cetrorelix hCG injection (D0). D = day
Table 2.
Cycle outcome
| Variables | Values |
|---|---|
| Total amount of r-FSH (units) | 1980.6±302 |
| No. of patients undergoing oocyte retrieval | 45 |
| No. of patients undergoing embryo transfer | 45 |
| Days with cetrorelix acetate | 5.2±1.2 |
| Days of stimulation | 9.1±1.2 |
| No. of oocyte retrieval | 13.1±6.3 |
| Fertilization rate, % of oocytes fertilized | 73.4±8.7 |
| Clinical pregnancies | 18 (40.0%) |
| Ongoing pregnancies | 17 (37.8%) |
Discussion
Recently studies have shown that GnRH antagonists prevent LH rise during ovarian stimulation for IVF [1, 2], with clinical efficacy confirmed in a large multicenter phase III clinical trial [3]. However, decreases in pregnancy rate have been reported in GnRH antagonist-stimulated cycles in comparison to GnRH agonist long protocols [4]. Further, it has been suggested that an adverse effect of GnRH antagonists might occur on either oocyte quality, embryo development or the endometrium [5]. It has been demonstrated that implantation and fertilization rates are inversely associated with the GnRH antagonist dose used [6, 7], indicating that the higher dose of GnRH antagonist results in poor embryo and oocyte quality. In vitro, the murine preimplantation embryonic development can be blocked completely by increasing the concentration of the GnRH antagonist [8], suggesting that the implantaton is influenced by the dosage of GnRH antagonist. However, conflicting reports suggest that the GnRH antagonist has no direct negative effect on the quality of the oocytes and embryos [9, 10]. Comparisons of cryopreservation of pronucleate zygotes after cycles of GnRH antagonist and GnRH agonist long protocol reveal that cetrorelix acetate has no detrimental effect on the outcome of subsequent freeze-thaw cycles [9]. Kol et al. [10] also reported that the ongoing pregnancy rate in cryopreservation cycles appeared to be good and unrelated to the dose of GnRH antagonist.
The pregnancy rate in IVF is not only influenced by the quality of oocytes and embryos but also by the endometrial environment when the embryos are transferred. The human endometrial GnRH receptors have recently been identified [11] and a direct effect on the endometrium with relatively high doses of GnRH antagonist cannot be excluded. However, Engel et al. [12] suggest that neither granulose cell capacity nor endometrial receptivity seem to be affected by cetrorelix.
Although the effect of GnRH antagonists on the oocytes, embryos and endometrium remained controversial, the lower dose of GnRH antagonists to inhibit LH surge would do no harm to the outcome of pregnancies in IVF cycles. The present study demonstrated that a daily dose of 0.2 mg cetrorelix acetate was effective to suppress LH concentrations and prevention of premature LH surge. To the best of our knowledge, this is the lowest dose used successfully.
Daily antagonist administration was initially performed according to a fixed scheme, starting after 5 days evening or 6 days morning of ovarian stimulation with exogenous gonadotropins. Prior to that period, LH rise is thought to be unlikely. Because cetrorelix injection is not necessary on the day of hCG injection in this study, the total duration of cetrorelix treatment in this study is similar to that used in previous trial, which cetrorelix injection commenced on day 6 (5.2 vs. 5.7 days) [13]. In the present study, the lower dose of cetrorelix acetate (0.2 mg) starting on day 5 evening of stimulation before the day of hCG also could reduce total antagonist dosage. On the other hand, flexible multiple dose antagonist regimen, initiated according to the follicular size, could result in less amount of gonadotropin and reduced GnRH antagonist dosage [14]. Whether this lower dosage of cetrorelix acetate could be utilized in a flexible multiple dose protocol to further decrease the antagonist dosage may warrant further study.
Because previous studies on GnRH antagonist daily administration were performed mostly on Caucasians, it cannot be rule out that our results could be due to racial differences. Hwang et al. [15] demonstrated that a single dose of 2.5 mg cetrorelix was effective for prevention of LH surge in IVF cycles for Taiwanese women using clomid in combination with gonadotropin while this study also showed that daily injection of 0.2 mg cetrorelix is enough to prevent LH surge in Asian women. The ganirelix dosage has to be adjusted according to the patient’s body weight in controlled ovarian stimulation [16]. For cetrorelix-controlled IVF cycles, no body weight adjustment is necessary [17]. Therefore, the lower dosage of cetrorelix acetate may also be effective to prevent LH surge in Caucasians.
In conclusion, our results show that premature LH surge can be prevented effectively with a daily cetrorelix acetate dose of 0.2 mg over 5 days (total 1.0 mg). Further, the treatment is cost effective because a package of 3 mg cetrorelix acetate can be used for two to three IVF cycles. In addition, this lower dose of cetrorelix acetate may reduce the potentially deleterious effect on IVF pregnancy rate. However, large prospective randomized controlled studies are necessary to compare this protocol with the traditional daily dose of 0.25 mg cetrorelix with regards to character of controlled ovarian stimulation and pregnancy outcome.
References
- 1.Diedrich K, Diedrich C, Santos E, Zoll C, al-Hasani S, Reissmann T, Krebs D, Klingmuller D. Suppression of the endogenous luteinizing hormone surge by the gonadotrophin-releasing hormone antagonist Cetrorelix during ovarian stimulation. Hum Reprod. 1994;9:9–791. doi: 10.1093/oxfordjournals.humrep.a138597. [DOI] [PubMed] [Google Scholar]
- 2.Albano C, Smitz J, Camus M, Riethmuller-Winzen H, Van Steirteghem A, Devroey P. Comparison of different doses of gonadotropin-releasing hormone antagonist Cetrorelix during controlled ovarian hyperstimulation. Fertil Steril. 1997;67:67–922. doi: 10.1016/S0015-0282(97)81407-0. [DOI] [PubMed] [Google Scholar]
- 3.Felberbaum RE, Albano C, Ludwig M, Riethmuller-Winzen H, Grigat M, Devroey P, Diedrich K. Ovarian stimulation for assisted reproduction with HMG and concomitant midcycle administration of the GnRH antagonist cetrorelix according to the multiple dose protocol: a prospective uncontrolled phase III study. Hum Reprod. 2000;15:15–1020. doi: 10.1093/humrep/15.5.1015. [DOI] [PubMed] [Google Scholar]
- 4.Al-Inany H, Aboulghar M. GnRH antagonist in assisted reproduction: a Cochrane review. Hum Reprod. 2002;17:17–885. doi: 10.1093/humrep/17.4.874. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez ER. Embryo implantation and GnRH antagonists: embryo implantation: the Rubicon for GnRH antagonists. Hum Reprod. 2000;15:15–1216. doi: 10.1093/humrep/15.6.1211. [DOI] [PubMed] [Google Scholar]
- 6.Ganirelix Dose-Finding Study Group A double-blind, randomized, dose-finding study to assess the efficacy of the GnRH antagonist Ganirelix (Org 37462) to prevent premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation with recombinant follicle stimulating hormone. Hum Reprod. 1998;13:13–3031. [PubMed] [Google Scholar]
- 7.Felberbaum R, Reissmann T, Kupker W, Al-Hasani S, Bauer O, Schill T, Zoll C, Diedrich C, Diedrich K. Hormone profiles under ovarian stimulation with human menopausal gonadotropin (hMG) and concomitant administration of the gonadotropin releasing hormone (GnRH)-antagonist Cetrorelix at different dosages. J Assist Reprod Genet. 1996;13:13–222. doi: 10.1007/BF02065939. [DOI] [PubMed] [Google Scholar]
- 8.Raga F, Casan EM, Kruessel J, Wen Y, Bonilla-Musoles F, Polan ML. The role of gonadotropin-releasing hormone in murine preimplantation embryonic development. Endocrinology. 1999;140:140–3712. doi: 10.1210/en.140.8.3705. [DOI] [PubMed] [Google Scholar]
- 9.Seelig AS, Al-Hasani S, Katalinic A, Schopper B, Sturm R, Diedrich K, Ludwig M. Comparison of cryopreservation outcome with gonadotropin-releasing hormone agonists or antagonists in the collecting cycle. Fertil Steril. 2002;77:77–475. doi: 10.1016/S0015-0282(01)03008-4. [DOI] [PubMed] [Google Scholar]
- 10.Kol S, Lightman A, Hillensjo T, Devroey P, Fauser B, Tarlatzis B, Mannaerts B, Itskovitz-Eldor J. High doses of gonadotrophin-releasing hormone antagonist in in-vitro fertilization cycles do not adversely affect the outcome of subsequent freeze-thaw cycles. Hum Reprod. 1999;14:14–2244. doi: 10.1093/humrep/14.9.2242. [DOI] [PubMed] [Google Scholar]
- 11.Dong KW, Marcelin K, Hsu MI, Chiang CM, Hoffman G, Roberts JL. Expression of gonadotropin-releasing hormone (GnRH) gene in human uterine endometrial tissue. Mol Hum Reprod. 1998;4:4–898. doi: 10.1093/molehr/4.9.893. [DOI] [PubMed] [Google Scholar]
- 12.Engel JB, Riethmuller-Winzen H, Diedrich K. Extrapituitary effects of GnRH antagonists in assisted reproduction: a review. Reprod Biomed Online. 2005;10:10–234. doi: 10.1016/S1472-6483(10)60945-5. [DOI] [PubMed] [Google Scholar]
- 13.Albano C, Felberbaum RE, Smitz J, Riethmuller-Winzen H, Engel J, Diedrich K, Devroey P. Ovarian stimulation with HMG: results of a prospective randomized phase III European study comparing the luteinizing hormone-releasing hormone (LHRH)-antagonist cetrorelix and the LHRH-agonist buserelin. European Cetrorelix Study Group. Hum Reprod. 2000;15:15–531. doi: 10.1093/humrep/15.3.526. [DOI] [PubMed] [Google Scholar]
- 14.Al-Inany H, Aboulghar MA, Mansour RT, Serour GI. Optimizing GnRH antagonist administration: meta-analysis of fixed versus flexible protocol. Reprod Biomed online. 2005;10:10–570. doi: 10.1016/s1472-6483(10)61661-6. [DOI] [PubMed] [Google Scholar]
- 15.Hwang JL, Huang LW, Hsieh BC, Tsai YL, Huang SC, Chen CY, Hsieh ML, Chen PH, Lin YH. Ovarian stimulation by clomiphene citrate and hMG in combination with cetrorelix acetate for ICSI cycles. Hum Reprod. 2003;18:18–49. doi: 10.1093/humrep/deg021. [DOI] [PubMed] [Google Scholar]
- 16.Hardy RI, Tummon IS, Hosseinzadeh M. Luteinizing hormone (LH) surge on ganirelix (Antagon) is related to body mass index (BMI) Fertil Steril. 2001;73:73–179. [Google Scholar]
- 17.Engel JB, Ludwig M, Junge K, Howles CM, Diedrich K. No influence of body weight on pregnancy rate in patients treated with cetrorelix according to the single- and multiple-dose protocols. Reprod Biomed Online. 2003;6:6–487. doi: 10.1016/s1472-6483(10)62171-2. [DOI] [PubMed] [Google Scholar]


