Abstract
Background: The purpose of this study is to asses the frequency of subclinical pregnancy loss (SPL) among women undergoing controlled ovarian hyperstimulation (COH) and in-vitro fertilization with ICSI.
Methods: The study was retrospectively conducted in a private IVF center. SPL was defined by a temporary rise in serum βhCG, along with the absence of signs of intra- and extra-uterine pregnancy by transvaginal ultrasonography. Overall 5273 COH and ICSI cycles with embryo transfer (ET) were segregated according to serum E2 levels percentiles (-24th, 25th, 74th, and 75th), women age and the type of spermatozoa for assisted fertilization (ejaculated and surgically retrieved). Those groups were assessed for SPL rates.
Results: Among the 3125 (59.25) conception cycles, 305 (9.7%) were diagnosed as SPL. There was no difference in SPL rate among E2 percentile groups. Women older than 35 years of age had significantly higher rate of SPL compared to younger women. There was also no difference in SPL rate among pregnancies in whom surgically retrieved spermatozoa used or ejaculated spermatozoa used for assisted fertilization.
Conclusion: Our results demonstrated that SPL rate was not influenced by the levels of E2 during COH or the origin of spermatozoa used for assisted fertilization. However, maternal age was found to be detrimental for SPL.
KEY WORDS: ART, biochemical pregnancy, estradiol, subclinical pregnancy loss
INTRODUCTION
Pregnancies achieved using ART are easier to follow than are those conceived spontaneously, offering a unique opportunity to observe early gestational life ultrasonographically. Early pregnancy loss significantly reduces the initial success of ART and decreases its efficiency, thus increasing the psychological burden on patients and therefore early prediction of outcome is important in pregnancies following assisted reproduction treatment (ART). Gestational sac is not reliably visible until a certain day after LH surge (1). Therefore, as a result of this inability of US identify very early pregnancy abnormalities, a significant amount of pregnancies detected by biochemical markers could not be demonstrated sonographically. Those pregnancies are named with various terminology such as biochemical, chemical, oocult, or subclinical pregnancy loss (SPL). In this regard, the blackbox of very early embryonic life in-vivo could only be assessed by serum markers.
In assisted reproductive therapy (ART), controlled ovarian hyperstimulation (COH) is used to recruit a large cohort of oocytes and in this regard, the serum concentration of estradiol (E2) is elevated, exposing endometrium and implanted concepti to supraphysiological levels of E2. Assessment of the relationship between E2 level and IVF outcome has been a focus of interest for many years. Pregnancy loss can be the result of abnormal concepti or decreased uterine receptivity (2). Adverse effects of supraphysiological E2 may include alterations in both endometrial receptivity and oocyte/embryo quality (3–5). Although elevated E2 levels were thought to alter the endometrial environment and lead to reduced implantation of embryos, other studies have not confirmed this effect (6–8) and the impact of supraphysiological E2 levels on endometrial receptivity remained controversial. Moreover, it has been proposed that, elevated levels of E2 may increase the miscarriage rates in women undergoing IVF (9). Differently said, supraphysiological levels of hormonal milleu in endometrium can be detrimental to very early survival of implanted conceptii.
In addition to hormonal milleu origin of spermatozoa utilized for assisted fertilization was thought to be important for early gestational life. It has been suggested that offspring from ICSI carry an increased rate of chromosomal aberrations (10). Those abnormalities, however, seem to be related to the underlying parental risk of abnormality and not to the ICSI procedure itself may eventually have impact on the fate of implanted conceptii.
Although some studies have assessed miscarriage rates in in-vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) pregnancies as well as the origin of spermatozoa utilized for assisted fertilization is considered in this regard (11–15), data on early pregnancy outcome of ART conceptions relative to E2 levels reached during COH is scarce. Moreover, majority of studies investigated clinical pregnancies and in this regard SPL in terms of major outcome has not been addressed. Therefore, this study intended to evaluate SPL in women undergoing controlled ovarian hyperstimulation and intracytoplasmic sperm injection and possible confounding variables such as E2 levels, maternal age, and type of spermatozoa used were investigated.
MATERIALS AND METHODS
The database of the German Hospital at Istanbul was retrospectively evaluated, and women undergoing assisted conception treatment cycles between January 1999 and June 2002 (5778 cycles) were recruited for this study. To standardize serum E2 levels at the time of HCG administation relative to the population, the cycles were segregated into percentiles and subsequently divided into three groups. Group I consisted of 810 cycles, defined as women with E2 levels up to 24th percentile (113–1349 pg/mL); Group II consisted of 3325 cycles, defined as women with E2 levels between the 25th and 74th percentiles (1350–3249 pg/mL); and Group III consisted of 1643 cycles, defined as women with E2 levels between the 75th and 100th percentiles (>3250 pg/mL).
Women with history of recurrent pregnancy loss and as well as frozen thawed embryo transfer cycles were not considered in this study.
The ovulation induction protocol for ovarian stimulation began with pituitary desensitization by GnRH agonist (Lucrin; Abbott, France) in the mid-luteal phase of the preceding menstrual period. Administration of gonadotrophins (two to six ampolues, Metrodin® HP, 75 IU; Serono, Aubonne, Switzerland, or Humegon®, 75 IU; Organon, Oss, The Netherlands) was initiated when serum E2 concentration fell below 50 pg/mL. The starting regimen was fixed for the first 4 days, with the dose of gonadotrophins subsequently adjusted according to the individual ovarian response. When at least two follicles reached 18 mm in diameter, HCG (Pregnyl®; Organon) 5000–10,000 IU was administered i.m. Oocytes were retrieved 32–38 h after HCG injection. ICSI method was used for assisted fertilization in our center for all patients regardless of infertility etiology. Three days after oocyte retrieval, the embryos were transferred transcervically under ultrasound guidance. The luteal phase was supported by 50 mg/day progesterone in oil (G. Streuli and Co., Uznach, Switzerland) following oocyte retrieval and then by 100 mg/day following ET (16, 17).
Serum βhCG concentration was measured in all patients 12 days after embryo transfer. Pregnancy was defined as positive serum βhCG level (≥5 mIU/mL) on at least two occasions following ET. Clinical pregnancy was defined as the demonstration of an intrauterine gestational sac with yolk sac by transvaginal ultrasonogram (TVUS) (Aplio, Powervision and Corevision, Toshiba Corporation, Japan) following ET (17). All pregnancies underwent scanning by transvaginal ultrasonogram 3 weeks (18–22 days) after ET, and serial controls were performed until 10 weeks after ET. SPL was defined by a temporary rise in serum HCG, along with the absence of signs of intra- and extra-uterine pregnancy by transvaginal ultrasonography (2). Miscarriages following demonstration of any gestational sac on TVUS were not considered as SPL. Definition of ectopic pregnany relied exclusively on the diagnosis by TVUS or during laparoscopy and ectopic pregnancies (66 cases, 2.0%) were excluded from the inclusion criteria of pregnancy. SPL rate was calculated per(+)serum HCG.
Serum E2 was measured by competitive immunoassay using a commercial kit (Immulite Diagnostic Products Corporation, Los Angeles, CA), with intra- and inter-assay coefficients of variation of 6.3 and 6.4%, respectively. Serum βhCG was measured by competitive immunoassay using a commercial kit (Access, Beckman Coulter Inc., Fullerton, CA), with intra- and inter-assay coefficients of variation of 1.67 and 2.42%, respectively.
This study was approved by the institutional review board of the German Hospital at Istanbul. For group comparisons, statistical analysis used χ2 test, Fisher’s exact test, analysis of variance with Bonferoni post hoc test, and Kruskall–Wallis rank sum test with Dunn post hoc test, as applicable. In addition, logistic regression analysis was utilized to determine any relationship between peak E2 levels and SPL rates in assessed cycles. A p-value <0.05 was considered statistically significant.
RESULTS
Among the assessed 5778 cycles, 5273 (91.2%) commenced ET and 3125 (59.2%) revealed positive serum HCG following ET. SPL detected in 305 (9.7%) and the remaining 2820 (53.4% clinical pregnancy rate/ET) had evidences of sonographic visualization of implantation. During this period 11.9% of all clinical pregnancies (including singleton and multiples) miscarried prior to second trimester. SPL rate was significantly lower than first trimester miscarriage rate (p=0.007, OR: 1.2, 95%CI: 1.0–1.4).
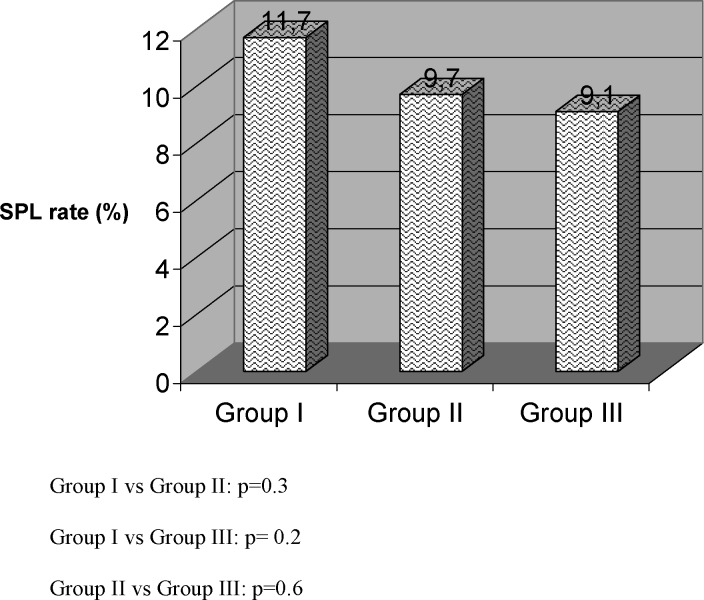
In Table I COH cycle characteristics according to E2 percentile groups (Groups I–III) were summarized. We found that age, mean peak E2 levels, and the number of oocytes retrieved all differed among the three groups. Clinical pregnancy rate was significantly different in all groups being the highest in Group III and lowest in Group I. SPL was detected in 35, 180, and 90 among Groups I, II, and IIII, respectively. However, SPL rate did not differ between groups (Fig. 1). In addition logistic regression analysis also did not reveal any correlation with E2 level and SPL. In addition to this, a subgroup analysis of Group III revealed 305 women had ≥5000 pg/mL of E2 with a SPL rate of 9.2% (16/173), which was not significantly different from other percentile E2 groups.
Table I.
A Breakdown of COH Characteristics by Groups According to E2 Percentile of Women Undergoing COH and ICSI–ET
| Group I (n=810) | Group II (n= 3325) | Group III (n=1643) | |
|---|---|---|---|
| Age (years) | 35.8±4.9a | 31.97±4.8b | 30.2±4.7c |
| Mean±SD range | 19–46 | 19–45 | 18–44 |
| Peak estradiol (pg/mL) | 866.6 ±320.7a | 2223.8±505.0b | 4264.3±766.4c |
| Mean±SD range | 113–1349 | (1351–3251) | (3252–9700) |
| Total oocytes retrieved | 5.3±3.0a | 13.9±5.9b | 20.8±6.7c |
| Mean±SD range | 1–13 | 3–45 | 4–61 |
| Number of cycles with ET | 723 | 3051 | 1499 |
| Number of(+)HCG | 298 | 1842 | 985 |
| Number of clinical pregnancies | 263 | 1662 | 895 |
| Implantation rate (%) | 20.9d | 29.2e | 29.7e |
| Clinical pregnancy rate/ET (%) | 36.3f | 54.4g | 59.7h |
Note. Values within rows following different superscript letters are significantly different: a vs. b, a vs. c, and b vs. c, p=0.001 (one way ANOVA with Bonferroni post multiple comparison test); d vs. e: p=0.0001, OR: 0.6, 95%CI: 0.5–0.7; f vs. g: p=0.0001, OR: 0.4, 95%CI: 0.4–0.5; f vs. h: p= 0.0001, OR: 0.4, 95%CI: 0.3–0.4; g vs. h: p=0.0009, OR: 0.8, 95%CI: 0.7–0.9.
Fig. 1.
Subclinical pregnancy loss rates in groups of women by E2 percentile.
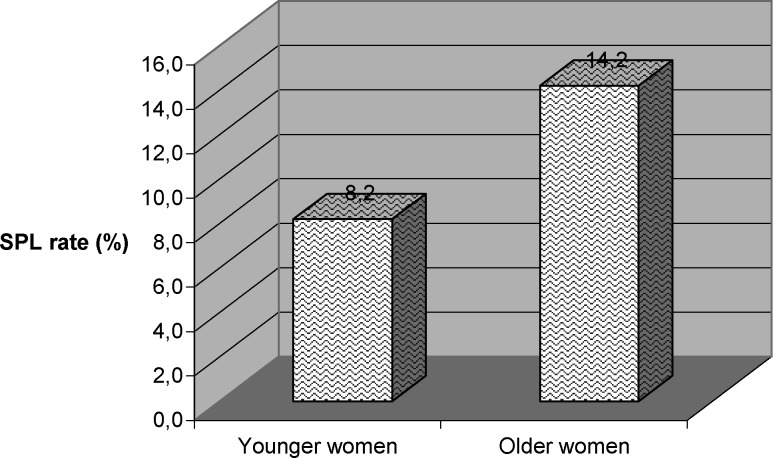
Out of 2332 women, aged less than 34 years and with positive HCG, 192 experienced SPL, whereas out of 793 women, aged more than 35 years and with positive HCG, 113 experienced SPL. Women aged 35 years or more had significantly higher rates of SPL compared to younger women (p=0.0001, OR: 0.5, 95%CI: 0.4–0.7) (Fig. 2)
Fig. 2.
Subclinical pregnancy loss rates in groups of women by age.
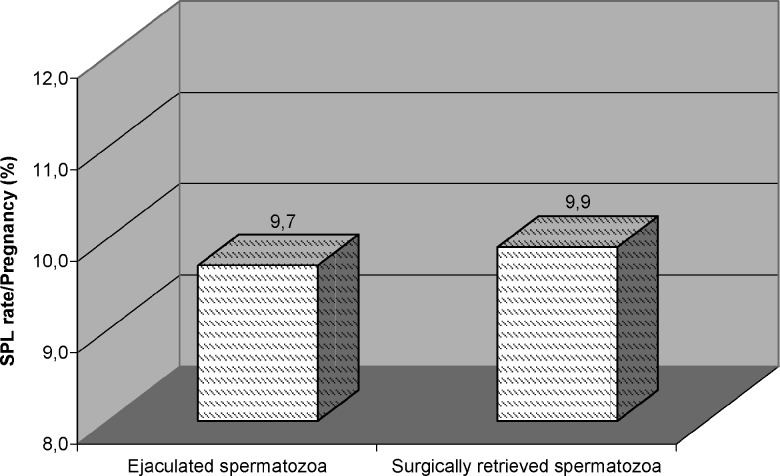
Surgically retrieved spermatozoa used in 373 pregnancies and 37 (9.9) of them had SPL. When SPL rate among this group was compared to SPL rate in ejaculated spermatozoa used pregnancies no difference was detected (Fig. 3).
Fig. 3.
Subclinical pregnancy loss rates according to origin of spermatozoa used for assisted fertilization.
DISCUSSION
Since the first report of a pregnancy loss detected by the measuremnt of βhCG (18), it has become clear that a large number of conceptions fail before the woman becomes aware that she might have been pregnant. These early pregnancy losses, sometimes called occult pregnancies (19), have been defined as pregnancies. It has become clear that from the moment of fertilization, there is a continuous reduction or selection of conception products showing chromosome abnormalities and approximately 30% of conceptus can be lost following implantation and prior to missed period (20). The introduction of sensitive assays for serum (hCG and the possibility provided by IVF to observe the events from ovulation to on-going pregnancy has enabled the previously elusive black box of early pregnancy to be explored. The most commonly employed marker of pregnancy is (hCG and a rise in serum (hCG on two consecutive occasions from day 11 after ET indicated pregnancy. In addition, in vitro studies have shown that HCG is produced by trophoblastic cells of unhatched blastocyst and may be detected from 7 days onwards fertilization (21,22).
The current study solely addressed SPL rates in a group of women undergoing assisted conception treatment with IVF or ICSI. Clinical miscarriage rates among pregnancies conceived by ICSI have been reported elsewhere. However, this is the first report with a large series demonstrating the survival rate of implanted embryos following ET until a period in which sonographic visualization is available. Our results confirmed that almost 90% of women with positive serum HCG would demonstrate gestational sac under sonogram. In other words, approximately 80% of conceptions diagnosed by measuring serum HCG following 12 days after ET would be lost by the end of the first trimester.
In the current study, the definition of clinical pregnancy was based on the demonstration of gestational sac by TVUS. Gestational sacs can be visualized by TVUS 25.5 days after the last menstrual period, whereas cardiac activity cannot be visualized until 44.5 days (1). Therefore, we were able to examine the life span of implanted embryos at the earliest stage possible permitting visualization by sonograpghy. Thus, it was proposed that a single determination of the βhCG level 2 weeks after ET, combined with TVUS 2–3 weeks later was a reliable follow-up in ART pregnancies (23). On the other hand, in numerous reports, clinical pregnancy was defined as the demonstration of fetal cardiac activity in which gestational sacs with yolk sacs without hearbeats were ignored although they have been recognized clinically. In other words, 8.5% of clinically identified gestations by sonography were considered somehow as preclinical pregnancy loss because they did not show viability later (24,25). However, our series exclusively consisted of subclinical pregnancies without any evidence of sonographic findings.
This study differs from the previous reports basically in two aspects. First, in many of studies, E2 cutoff levels in COH cycles were chosen arbitrarily (26). Cutoff levels, dependent on absolute E2 concentration in a single center, however, expressed as either pg/mL or pmol/L, cannot be applied universally. Objective analysis requires that E2 concentrations be sorted into percentiles and categorized by groups and calibrated in each center (27). Therefore, we sorted patients by E2 into three groups. It should also be noted that the 25th to the 75th window includes women with ±1SD of the distribution, representing the normality of the population.
It should be taken in consideration that extra- and intra-follicular influences such as the elevated E2 are able to disturb maturation leading to aneuploidy and oocyte meiosis is very sensitive to exogenous factors which could lead to abnormal zygotes (28). It has been shown that exaggerated levels of E2 have a negative impact on only oocyte quality (7,28,29). Moreover, elevated E2 levels have been shown to convert endometrium into a hostile environment for embryo implantation (30), with a histological appearance of prematurely advancing endometrium (31). Conceivably, both endometrial and oocyte factors may contribute to an increased rate of early pregnancy loss. On the other hand, the findings of this study suggest that postimplantation period of embryos at least 2 weeks apart appear not to be effected by the levels of serum E2. Therefore, endometrium exposed to supraphysiological levels of E2 cannot deleteriously impact the survival of implanted conceptii.
Second, previous studies reported their results originating from patients conceived by various techniques of assisted conception (ICSI, IVF, or IUI). Inclusion of only ICSI cycles made our assessed group homogeneous in one part and comparisons in this regard became more reliable.
Taking into consideration of those differences, several authors reported more frequent SPL rates in their series. In this regard, Poikkeus et al. reported 20% of SPL among 774 pregnancies conceived by IVF or ICSI (32). Legro et al. reported of 23.3% SPL among 77 women conceived by IVF (33). Sugantha et al. found 22.4% SPL among 397 pregnancies conceived by IUI or IVF or ICSI (34). However, those authors included visualized gestational sacs without hearbeats in their series of SPL. On the other hand, similar to our SPL definition, Coulam et al. evaluated 1675 fresh IVF ET cycles and detected 17% of SPL rate in their series, whereas Levy et al. reported 14.8% SPL rate in 750 IVF cycles (2–35).
Two types of SPL have been identifed on the basis of the pattern of βhCG expression (36,37). In the first type, βhCG levels decline abruptly over 2 days and this type suggests endometrial factors preventing completion of implantation. In the second type, a reduced rate of βhcg rise occurs and this type indicates abnormal embryonic development after implantation. Conceivably possible mechanisms leading to SPL are related with either endometrial factors or embryonic factors.
An increase in maternal age effect on aneuploidy which is known to be present in spontaneous abortions and liveborns has also been demonstrated in morphologically and developmentally normal preimplantation embryos (38). Therefore, oocytes of older women are more prone to nondisjunction caused by meiotic errors at the gamete level. Our results also demonstrated that older women had significantly higher rate of SPL compared to younger women.
Although it has been observed that surgically retrieved spermatozoa had a higher incidence of chromosomal abnormalities (39), no correlation has been demonstrated with increased SPL and usage of non-ejaculated spermatozoa for assisted fertilization so far. We have recently reported that miscarriage rates were not effected from the origin of spermatozoa used for ICSI (40). However, Poikkeus et al. claimed that SPL is lesser among fertile couples suffering from male factor infertility (32). Contrary to this, the current study revealed that SPL does not increase by usage of surgically retrieved spermatozoa.
Luteal phase deficiency in ART cycles can result from the use of GnRH agonist or poor progesterone production after granulosa cell removal during oocyte retrieval; therefore, luteal phase support is routinely recommended. Progesterone in oil or micronized progesterone can be supplemented during luteal phases of IVF cycles. Several clinical trials favored intramuscular progesterone in oil administration rather than vaginal application of micronized progesterone in terms of high SPL rates (41–43). From this point of view, homogeneity in the administration of progesterone for luetal phase support in our series rendered our comparisons reliable.
As a conclusion, our results demonstrated that at least 10% of probability of SPL after the detection of positive serum βhcg result following ICSI and this rate was not influenced by the level of serum E2 reached during COH or the origin of spermatozoa used for assisted fertilization. Maternal age as in all miscarriages is the significant variable increasing the SPL rate.
REFERENCES
- 1.Steinkampf MP, Guzick DS, Hammond KR, Blackwell RE. Identification of early pregnancy landmarks by transvaginal sonography: Analysis by logistic regression. Fertil Steril. 1997;68:168–170. doi: 10.1016/S0015-0282(97)81496-3. [DOI] [PubMed] [Google Scholar]
- 2.Coulam CB, Chapman C, Rinehart JS. What is a preclinical pregnancy loss. J Assist Reprod Genet. 1998;15:184–187. doi: 10.1023/A:1023044217810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon C, Cano F, Valbuena D, Remohi J, Pellicer A. Clinical evidence for a detrimental effect on uterine receptivity of high serum oestradiol concentrations in high and normal responder patients. Hum Reprod. 1995;10:2432–2437. doi: 10.1093/oxfordjournals.humrep.a136313. [DOI] [PubMed] [Google Scholar]
- 4.Yang JH, Chen HF, Lien YR, Chen SU, Ho HN, Yang YS. Elevated E2:oocyte ratio in women undergoing IVF and tubal ET. Correlation with a decrease in the implantation rate. J Reprod Med. 2001;46:434–438. [PubMed] [Google Scholar]
- 5.Ng EH, Lau EY, Yeung WS, Ho PC. Oocyte and embryo quality in patients with excessive ovarian response during in vitro fertilization treatment. J Assist Reprod Genet. 2003;20:186–191. doi: 10.1023/A:1023670010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharara FI, Lim J, McClamrock HD. Endometrial pattern on the day of oocyte retrieval is more predictive of implantation success than the pattern or thickness on the day of hCG administration. J Assist Reprod Genet. 1999;16:523–528. doi: 10.1023/A:1020545120256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng EH, Yeung WS, Yee Lan E, So WW, Ho PC. High serum oestradiol concentrations in fresh IVF cycles do not impair implantation and pregnancy rates in subsequent frozen-thawed embryo transfercycles. Hum Reprod. 2000;15:250–255. doi: 10.1093/humrep/15.2.250. [DOI] [PubMed] [Google Scholar]
- 8.Chen CH, Zhang X, Barnes R, Confino E, Milad M, Puscheck E, Kazer RR. Relationship between peak serum estradiol levels and treatment outcome in in vitro fertilization cycles after embryo transfer on day 3 or day 5. Fertil Steril. 2003;80:75–79. doi: 10.1016/S0015-0282(03)00504-1. [DOI] [PubMed] [Google Scholar]
- 9.Mahutte NG, Duleba AJ, Taylor HS, Arici A, Jones E, Sakkas A. Elevated estradiol levels are associated with increased miscarriage rates in women undergoing IVF/ICSI. Fertil Steril. 2002;78(Suppl 1):S253. doi: 10.1016/S0015-0282(02)04059-1. [DOI] [Google Scholar]
- 10.Bonduelle M, Van Assche E, Joris H, Keymolen K, Devroey P, Van Steirteghem A, Liebaers I. Prenatal testing in ICSI pregnancies: Incidence of chromosomal anomalies in 1586 karyotypes and relation to sperm parameters. Hum Reprod. 2002;17:2600–2614. doi: 10.1093/humrep/17.10.2600. [DOI] [PubMed] [Google Scholar]
- 11.Coulam CB, Opsahl MS, Sherins RJ, Thorsell LP, Dorfmann A, Krysa L, Fugger E, Schulman JD. Comparisons of pregnancy loss patterns after intracytoplasmic sperm injection and other assisted reproductive technologies. Fertil Steril. 1996;65:1157–1162. [PubMed] [Google Scholar]
- 12.Govaerts I, Devreker F, Koenig I, Place I, Van den Bergh M, Englert Y. Comparison of pregnancy outcome after intracytoplasmic sperm injection and in vitro fertilization. Hum Reprod. 1998;13:1514–1518. doi: 10.1093/humrep/13.6.1514. [DOI] [PubMed] [Google Scholar]
- 13.Orvieto R, Ben-Rafael Z, Ashkenazi J, Yoeli R, Messing B, Perri T, Shalev Y, Bar-Hava I. Outcome of pregnancies derived from assisted reproductive technologies: IVF versus ICSI. J Assist Reprod Genet. 2000;17:385–387. doi: 10.1023/A:1009497809176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wennerholm UB, Bergh C, Hamberger L, Westlander G, Wikland M, Wood M. Obstetric outcome of pregnancies following ICSI, classified according to sperm origin and quality. Hum Reprod. 200;15:1189–1194. doi: 10.1093/humrep/15.5.1189. [DOI] [PubMed] [Google Scholar]
- 15.Schieve LA, Tatham L, Peterson HB, Toner J, Jeng G. Spontaneous abortion among pregnancies conceived using assisted reproductive technology in the United States. Obstet Gynecol. 2003;101:959–967. doi: 10.1016/S0029-7844(03)00121-2. [DOI] [PubMed] [Google Scholar]
- 16.Gibbons WE, Toner JP, Hamacher P, Kolm P. Experience with a novel vaginal progesterone preparation in a donor oocyte program. Fertil Steril. 1998;69:96–101. doi: 10.1016/S0015-0282(97)00457-3. [DOI] [PubMed] [Google Scholar]
- 17.Miles RA, Paulson RJ, Lobo RA, Press MF, Dahmoush L, Sauer MV. Pharmacokinetics and endometrial tissue levels of progesterone after administration by intramuscular and vaginal routes: A comparative study. Fertil Steril. 1994;62:485–490. doi: 10.1016/s0015-0282(16)56935-0. [DOI] [PubMed] [Google Scholar]
- 18.Morris NM, Udry JR. Daily immunologic pregnancy testing of initially nonpregnant women. Am J Obstet Gynecol. 1967;98:1148–1150. doi: 10.1016/0002-9378(67)90043-9. [DOI] [PubMed] [Google Scholar]
- 19.Walker EM, Lewis M, Cooper W, Marnie M, Howie PW. Occult biochemical pregnancy: Fact or fiction. Br J Obstet Gynaecol. 1988;95:659–663. doi: 10.1111/j.1471-0528.1988.tb06526.x. [DOI] [PubMed] [Google Scholar]
- 20.Chard T. Frequency of implantation and early pregnancy loss in natural cycles. Baillieres Clin Obstet Gynaecol. 1991;5:179–189. doi: 10.1016/S0950-3552(05)80077-X. [DOI] [PubMed] [Google Scholar]
- 21.Fishel SB, Edwards RG, Evans CJ. Human chorionic gonadotropin secreted by preimplantation embryos cultured in vitro. Science. 1984;24, 223:816–818. doi: 10.1126/science.6546453. [DOI] [PubMed] [Google Scholar]
- 22.Lopata A, Hay DL. The potential of early human embryos to form blastocysts, hatch from their zona and secrete HCG in culture. Hum Reprod. 1989;4(Suppl):87–94. doi: 10.1093/humrep/4.suppl_1.87. [DOI] [PubMed] [Google Scholar]
- 23.Fridstorm M, Garoff L, Sjoblom P, Hilensjo T. Human chorionic gonadotropin in early pregnancy after assisted reproduction. Acta Obstet Gynecol Scand. 1995;74:534–538. doi: 10.3109/00016349509024385. [DOI] [PubMed] [Google Scholar]
- 24.Ulug U, Jozwiak EA, Mesut A, Berksoy MM, Bahceci M. Survival rates during the first trimester of multiple gestations achieved by ICSI: A report of 1448 consecutive multiples. Hum Reprod. 2004;19:360–364. doi: 10.1093/humrep/deh090. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein SR. Sonography in early pregnancy failure. Clin Obstet Gynecol. 1994;37:681–692. doi: 10.1097/00003081-199409000-00020. [DOI] [PubMed] [Google Scholar]
- 26.Papageorgiou T, Guibert J, Goffinet F, Patrat C, Fulla Y, Janssens Y, Zorn JR. Percentile curves of serum estradiol levels during controlled ovarian stimulation in 905 cycles stimulated with recombinant FSH show that high estradiol is not detrimental to IVF outcome. Hum Reprod. 2002;17:2846–2850. doi: 10.1093/humrep/17.11.2846. [DOI] [PubMed] [Google Scholar]
- 27.Kosmas IP, Kolibianakis EM, Devroey P. Association of estradiol levels on the day of hCG administration and pregnancy achievement in IVF: A systematic review. Hum Reprod. 2004;19:2446–2453. doi: 10.1093/humrep/deh473. [DOI] [PubMed] [Google Scholar]
- 28.Plachot M. The human oocyte. Genetic aspect. Ann Genet. 1997;40:115–120. [PubMed] [Google Scholar]
- 29.Gelety TJ, Buyalos RP. The influence of supraphysiologic estradiol levels on human nidation. J Assist Reprod Genet. 1995;12:406–412. doi: 10.1007/BF02211139. [DOI] [PubMed] [Google Scholar]
- 30.Akagbosu F, Marcus S, Abusheikha N, Avery S, Brinsden P. Does ovarian hyperstimulation syndrome affect the quality of oocytes. Hum Reprod. 1998;13:2583–2584. doi: 10.1093/humrep/13.9.2583. [DOI] [PubMed] [Google Scholar]
- 31.Basir GS, O WS, Ng EH, Ho PC. Morphometric analysis of peri-implantation endometrium in patients having excessively high oestradiol concentrations after ovarian stimulation. Hum Reprod. 2001;16:435–440. doi: 10.1093/humrep/16.3.435. [DOI] [PubMed] [Google Scholar]
- 32.Poikkeus P, Hiilesmaa V, Tiitinen A. Serum HCG 12 days after embryo transfer in predicting pregnancy outcome. Hum Reprod. 2002;17:1901–1905. doi: 10.1093/humrep/17.7.1901. [DOI] [PubMed] [Google Scholar]
- 33.Legro RS, Paulson RJ, Lobo RA, Sauer MV. Association of early beta-human chorionic gonadotrophin values with pregnancy wastage and multiple implantation in a donor oocyte programme. Hum Reprod. 1995;10:3293–3296. [PubMed] [Google Scholar]
- 34.Sugantha SE, Webster S, Sundar E, Lenton EA. Predictive value of plasma human chorionic gonadotrophin following assisted conception treatment. Hum Reprod. 2000;15:469–473. doi: 10.1093/humrep/15.2.469. [DOI] [PubMed] [Google Scholar]
- 35.Levy T, Goldman JA, Dicker D, Ashkenazi J, Feldberg D. Very early pregnancy wastage in in vitro fertilization and embryo transfer (IVF-ET) J In Vitro Fertil Embryo Transf. 1991;8:250–253. doi: 10.1007/BF01139779. [DOI] [PubMed] [Google Scholar]
- 36.Lenton EA, Woodward AJ. The endocrinology of conception cycles and implantation in women. J Reprod Fertil Suppl. 1988;36:1–15. [PubMed] [Google Scholar]
- 37.Liu HC, Rosenwaks Z. Early pregnancy wastage in IVF patients. J In Vitro Fertil Embryo Transf. 1991;8:65–72. doi: 10.1007/BF01138657. [DOI] [PubMed] [Google Scholar]
- 38.Munne S, Alikani M, Tomkin G, Grifo J, Cohen J. Embryo morphology, developmental rates, and maternal age are correlated withchromosome abnormalities. Fertil Steril. 1995;64:382–391. [PubMed] [Google Scholar]
- 39.Palermo GD, Colombero LT, Hariprashad JJ, Schlegel PN, Rosenwaks Z. Cromosome analysis of epididymal and testicular sperm in azoospermic patients undergoing ICSI. Hum Reprod. 2002;17:570–575. doi: 10.1093/humrep/17.3.570. [DOI] [PubMed] [Google Scholar]
- 40.Bahceci M, Ulug U. Does underlying infertility aetiology impact on first trimester miscarriage rate following ICSI? A preliminary report from 1244 singleton gestations. Hum Reprod. 2005;20:717–721. doi: 10.1093/humrep/deh681. [DOI] [PubMed] [Google Scholar]
- 41.Damario MA, Goudas VT, Session DR, Hammitt DG, Dumesic DA. Crinone 8% vaginal progesterone gel results in lower embryonic implantation efficiency after in vitro fertilization – embryo transfer. Fertil Steril. 1999;72:830–836. doi: 10.1016/S0015-0282(99)00364-7. [DOI] [PubMed] [Google Scholar]
- 42.Propst AM, Hill JA, Ginsburg ES, Hurwitz S, Politch J, Yanushpolsky EH. A randomized study comparing Crinone 8% and intramuscular progesterone supplementation in in vitro fertilization – embryo transfer cycles. Fertil Steril. 2001;76:1144–1149. doi: 10.1016/S0015-0282(01)02872-2. [DOI] [PubMed] [Google Scholar]
- 43.Pritts EA, Atwood AK. Luteal phase support in infertility treatment: A meta-analysis of the randomized trials. Hum Reprod. 2002;17:2287–2299. doi: 10.1093/humrep/17.9.2287. [DOI] [PubMed] [Google Scholar]