Abstract
The auditory cortex encodes information about sounds through the combined activity of large numbers of neurons, but the way this population activity is organized has not been clear. In this issue of Neuron, Bathellier et al. (2012) show that firing in the superficial layers of auditory cortex is organized into a small number of attractor-like neuronal assemblies, whose responses can predict an animal’s sound discrimination performance.
The brain processes sensory information through the combined activity of large numbers of neurons. Until fairly recently, it was only possible to record from neurons one a time. These recordings have revealed much about sensory coding and enabled scientists to hypothesize how larger neuronal populations might represent sensory stimuli. Now that techniques such as two-photon imaging and multichannel electrophysiology allow hundreds of neurons to be recorded simultaneously, one can directly see how moderately sized neuronal populations actually operate. The brain, of course, works the same way however many neurons an experimenter manages to record from, so any population recording must be consistent with what was earlier seen at the single neuron level. Nevertheless, the results of population recordings often contradict hypotheses that had been inferred from single neuron studies. In this issue of Neuron, Bathellier et al. (2012) provide an excellent example of this, in a study of population coding in the superficial layers of mouse auditory cortex.
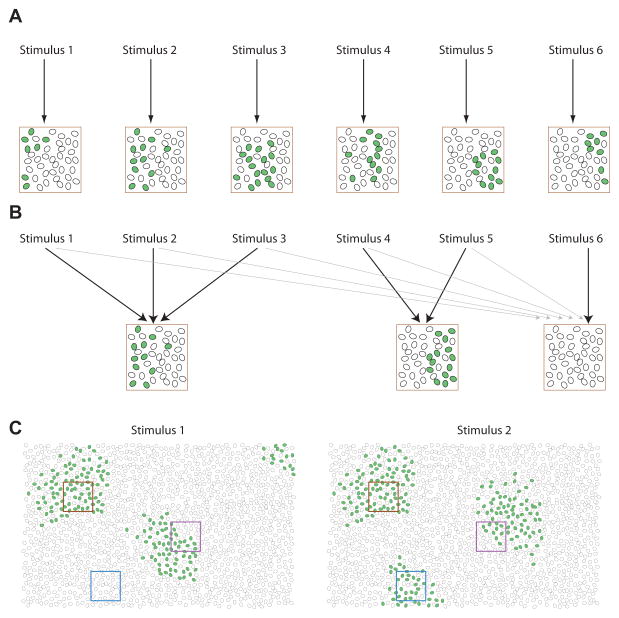
A picture of cortical population coding that one might have assumed based on single-cell studies is illustrated in Figure 1Afig1. Because neuronal tunings are diverse, and because neighboring neurons have more similar tuning than distant neurons, one might expect each stimulus to evoke a distinct spatial activity pattern, with more similar stimuli evoking more similar patterns. In this picture, no pair of stimuli would produce an exactly identical population response, so the firing pattern of even a relatively small set of neurons could in principle identify which of many stimuli was presented, limited only by the noise in neuronal responses. This is intuitively appealing, because it suggests the information coding capacity of the population is being used efficiently to represent a large number of potential stimuli.
Figure 1.
Spatial Organization of Neuronal Activity in Superficial Auditory Cortex
(A) The population coding scheme one might expect based on trial-averaged recordings of single neurons. Each stimulus evokes a different pattern of activity across the population, with physically or perceptually similar stimuli evoking more similar firing patterns. Each oval represents a neuron, with green shading indicating spiking in response to the stimulus.
(B) Illustration of Bathellier et al.’s results. The activity patterns produced in a local patch of superficial auditory cortex are restricted to a small number of discrete “response modes.” Each stimulus can evoke at most one mode, but each mode can be evoked identically by many stimuli. Some stimuli produce no response at all (stimulus 6), and any stimulus evokes its mode only probabilistically (black and gray arrows).
(C) Hypothesized global activity patterns underlying these results. Each stimulus evokes a set of discrete cell assemblies spread over the cortical surface, with the combination of active assemblies depending on the stimulus. Each square represents a different field of view for the two-photon microscope, with the three colors indicating different behaviors seen in individual recordings. Brown, only one response mode is found, which is evoked by both stimuli; blue, a single response mode evoked by only one stimulus; magenta, a field of view containing two discrete response modes.
Bathellier et al.’s experiments suggest a different picture (Figure 1B). Using two-photon calcium imaging, they recorded the activity of up to 100 neurons in the superficial layers of auditory cortex, while presenting a set of ?60 brief acoustic stimuli including tones and segments of complex sounds. In contrast to the picture suggested in Figure 1A, the number of patterns the population actually produced was very limited. Many stimuli produced no reliable response at all; but when a response was evoked, it typically consisted of the same subset of cells, forming a stereotyped spatial pattern termed a “response mode.” In most recordings, only one response mode was seen whatever the stimulus; in a smaller number of recordings two or three modes were seen, with each mode evoked by a distinct set of stimuli. When more than one mode was seen they were spatially segregated, with centers- of mass typically more than 50 μm apart (the true separation is probably larger since the modes could extend beyond the imaging window), although one neuron could participate in more than one response mode. The modes therefore appear to consist of partially overlapping assemblies of probably several hundred neurons, arranged in local clusters of size the order several hundred microns.
The activation of a response mode was a discrete event. In recordings where multiple modes were observed, Bathellier et al. (2012) presented weighted superimpositions of two sounds, each driving one mode. The resulting firing pattern did not smoothly interpolate between the two response modes, but suddenly switched from one mode to the other, for a particular value of the weighting. This suggests a “winner-take-all” form of competition between response modes.
Although this picture is different to what many scientists may have assumed about population codes, it is not inconsistent with previous studies. When the same data was analyzed with single-neuron methods, standard results such as V-shape tuning curves were seen. The fact that these tuning curves show a continuous variation of firing rate with tone frequency might seem to contradict the all-or-none activation of response modes. However, because different stimuli can have different efficacies of evoking the mode, single cells can have continuously varying trial-averaged rates. The existence of discrete modes also makes the surprising prediction that neurons belong to the same mode should have very similar tuning. This is also consistent with previous observations: both silicon probe and optical recordings have shown that neighboring neurons in superficial auditory cortex can have strongly correlated activity, but that the probability of seeing these high correlations falls rapidly with interneuronal distance (Sakata and Harris, 2009; Rothschild et al., 2010).
For technical reasons, with two-photon microscopy one can only image the superficial layers of cortex. And there are reasons to believe that the organization revealed by this study is in fact specific to the superficial layers. As mentioned above, an organization of discrete local assemblies is consistent with previous reports of sparse activity in superficial cortex, and the existence of strong correlations between local but not distal pairs (Sakata and Harris, 2009; Rothschild et al., 2010). However, these phenomena are not observed in deep layers of cortex, where activity is less sparse, and correlations weaker and less dependent on distance. This suggests that deep layer activity has a different organization, with smooth variation across the cortical surface rather than discrete localized modes (Sakata and Harris, 2009). These differences in activity patterns might in turn arise from differences in connectivity and physiology between cortical layers. For example, lateral excitatory connections in the superficial layers fall off rapidly within a distance of a few hundred microns (Oswald and Reyes, 2008), while deep-layer connectivity extends much further (Schubert et al., 2007).
The fact that only a handful of response modes could be evoked within the 200 μm area scanned by the two-photon microscope does not mean that the entire auditory cortex has such a limited repertoire. Indeed, in different recordings—including multiple fields of view in a single mouse—the sets of stimuli activating the response modes were different. This suggests that any one stimulus evokes many response modes spread over the superficial auditory cortex, with the precise combination of modes activated depending on the stimulus (Figure 1C). Thus, the picture that emerges from this study is quite similar to our original assumption about population coding (Figure 1A) but with the fundamental coding unit being not a single neuron, but an assembly that inexorably fires together. In support of this idea, Bathellier et al. (2012) found that when they pooled together all their recordings, the population activity patterns produced could not only accurately decode a large number of stimuli, but predict the behavioral discriminations made by mice.
One of the reasons that this picture is counterintuitive is that it seems inefficient. If a hundred neurons can only fire together or not at all, why not just replace them with a single neuron? Bathellier et al. (2012) showed that predicting the stimulus from population activity could be done just as well after replacing the firing rates of all their neurons with a few numbers summarizing the activation of each mode. Why would the brain waste valuable resources using a hundred neurons to encode a single number? Although this question cannot be answered at present, this type of organization has some remarkable similarities with some long-hypothesized theories of cortical function, which we now describe.
Cell Assemblies and Attractor Dynamics in Cortical Circuits
One of the most influential theories for cortical function is the “cell assembly hypothesis,” first proposed over half a century ago (Hebb, 1949; see Harris, 2005, for a more recent review). A cell assembly was hypothesized to be a group of neurons that are reciprocally connected by excitatory synapses, so that once a sufficient subset of the assembly fires, the whole assembly will be activated through mutual excitation. In Hebb’s original formulation, assemblies were sculpted by experience-dependent plasticity, with frequently coactivated neurons wired together through what is now called Hebb’s rule. The benefit of this scheme is that when an animal later experiences a stimulus that is similar but not identical to the stimulus that created the assembly (such as a visual image that is partly occluded), the whole assembly will be reactivated, allowing the animal to respond as it would to the original stimulus.
Later computational work made this idea precise by constructing formal models of recurrent network dynamics (e.g., Gardner-Medwin, 1976; Hopfield, 1982). In these models, the stored assembly patterns are “attractors”—stable activity patterns to which network activity evolves. The response modes described by Bathellier et al. (2012) are similar to attractors in their all-or-none nature, their discrete spatial patterns, and the fact that locally, only one mode can be activated at a time. Nevertheless, there are differences between the organization reported by Bathellier et al. (2012) and the simplest attractor models. First, the assemblies of superficial auditory cortex are spatially localized, unlike the disordered patterns typically stored in a Hopfield net; second, it is presumably possible for several spatially separated cortical assemblies to be active simultaneously (as illustrated in Figure 1C); and third, the number of assemblies expressed (1 assembly for at least 100 neurons) is much lower than the predicted capacity of most autoassociative networks (Tsodyks and Feigelman, 1988).
Some of these discrepancies are rectified in a class of models known as “bump attractor” networks, in which localized recurrent excitation and lateral inhibition cause firing in localized groups of neurons (Amari, 1977). These models are often proposed as a mechanism to memorize continuous variables such as an animal’s location in space (McNaughton et al., 2006). Interestingly however, getting such networks to exhibit a truly continuous spectrum of attractors, rather discrete attractors in fixed positions, requires exquisite fine tuning of synaptic connections (Renart et al., 2003). The organization found by Bathellier et al. (2012)—which contained a small number of discrete, spatially separated modes—could thus be a more natural behavior for locally recurrent circuits that are not fine tuned.
Like all surprising results, this study raises many questions. First, how general is this organization of neuronal activity? Evidence for attractor dynamics in other networks has been inconsistent (e.g., Wills et al., 2005; Leutgeb et al., 2005). Is a similar organization of discrete modes seen in other cortical regions, other cortical layers, and other brain structures? Second, would a similar pattern be seen in actively behaving animals, as well as the anesthetized and awake passive mice studied here? Third, what causes particular groups of cells to form an assembly? In Hebb’s original theory, the composition of cell assemblies was determined by experience. But Bathellier et al. (2012) could predict the one mouse’s classification choices from the mode organization observed in different animals, suggesting that auditory cortical assemblies arise either from an innate process, or at least from commonalities in the sensory experience of these mice. Finally, why should the cortex work like this, using hundreds of neurons do convey a single number? Although this may seem inefficient from the perspective of information coding, the brain is not just there to represent external stimuli, but to act on them. Is cortical attractor dynamics in fact a fundamental mechanism of decision making? Characterizing cortical dynamics in behaving animals, and how it changes with learning, may well answer these questions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amari S. Biol Cybern. 1977;27:77–87. doi: 10.1007/BF00337259. [DOI] [PubMed] [Google Scholar]
- Bathellier B, Ushakova L, Rumpel S. Neuron. 2012;76 doi: 10.1016/j.neuron.2012.07.008. this issue, *bxs. [DOI] [PubMed] [Google Scholar]
- Gardner-Medwin AR. Proc R Soc Lond B Biol Sci. 1976;194:375–402. doi: 10.1098/rspb.1976.0084. [DOI] [PubMed] [Google Scholar]
- Harris KD. Nat Rev Neurosci. 2005;6:399–407. doi: 10.1038/nrn1669. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior. New York: Wiley; 1949. [Google Scholar]
- Hopfield JJ. Proc Natl Acad Sci USA. 1982;79:2554–2558. doi: 10.1073/pnas.79.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Treves A, Meyer R, Barnes CA, McNaughton BL, Moser MB, Moser EI. Neuron. 2005;48:345–358. doi: 10.1016/j.neuron.2005.09.007. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Nat Rev Neurosci. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- Oswald AM, Reyes AD. J Neurophysiol. 2008;99:2998–3008. doi: 10.1152/jn.01160.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renart A, Song P, Wang XJ. Neuron. 2003;38:473–485. doi: 10.1016/s0896-6273(03)00255-1. [DOI] [PubMed] [Google Scholar]
- Rothschild G, Nelken I, Mizrahi A. Nat Neurosci. 2010;13:353–360. doi: 10.1038/nn.2484. [DOI] [PubMed] [Google Scholar]
- Sakata S, Harris KD. Neuron. 2009;64:404–418. doi: 10.1016/j.neuron.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D, Kötter R, Staiger JF. Brain Struct Funct. 2007;212:107–119. doi: 10.1007/s00429-007-0147-z. [DOI] [PubMed] [Google Scholar]
- Tsodyks MV, Feigelman MV. Europhys Lett. 1988;6:101–105. [Google Scholar]
- Wills TJ, Lever C, Cacucci F, Burgess N, O’Keefe J. Science. 2005;308:873–876. doi: 10.1126/science.1108905. [DOI] [PMC free article] [PubMed] [Google Scholar]