Abstract
Laminins are the major constituents of blood vessel basement membranes (BMs). Each laminin is a trimer consisting of three assembled polypeptide chains, α, β and γ. More than 15 laminin isoforms are known to date and the expression of specific isoforms may change in certain pathological conditions. Here we show that during progression of glial tumors laminin-9 (α4β2γ1) is switched to laminin-8 (α4β1γ1), which is dramatically increased in glial brain tumors. Laminin-8 overproduction by glial tumor cells facilitates spread of glioma. Brain tumors with laminin-8 overexpression recur faster after standard treatment and patients have shorter survival time. Laminin-8 may be thus used as a predictor of tumor recurrence, patient survival and as a potential molecular target for glioma therapy.
Keywords: Laminin-8, Laminin-9, Basement Membrane, Extracellular Matrix, Angiogenesis, Human, Cancer, Tumor, Neoplasm, Glioma, Glioblastoma Multiforme, Recurrence, Survival, Invasion, Morpholino antisense, Review
2. INTRODUCTION
The majority of gioblastoma multiforme (GBM) tumors are highly invasive and rapidly develop recurrences at the primary site. Tumor prognoses and responses to therapy, however, can vary greatly even with the same histological diagnosis (1). It is generally recognized that the improvement of prognosis, prediction of response to treatment, and development of novel effective therapeutic approaches for glial tumors may largely depend upon the introduction into clinical practice of novel specific markers involved in the development of different gliomas and their subsequent recurrences. Attempts have been made to establish and characterize a number of glioma markers, but such studies have not altered existing therapeutic approaches, treatment success rates or disease outcome prediction (1, 2). Researchers then sought to identify novel glioma markers using powerful gene array technology (3–6).
3. LAMININ ISOFORM CHANGES DURING GLIOMA PROGRESSION
A number of genes and proteins have been identified with altered expression in glial tumors. Most recently, some of the markers have been tested for diagnostic and prognostic purposes. These markers include epidermal growth factor receptor (EGFR), tenascin-C, bcl-2 family of antiapoptotic proteins, survivin, Rho proteins, p53, and vascular endothelial growth factor (VEGF) and its receptors (7–12). Results of these tests have so far been controversial. Some reports suggest that specific proteins (Rho, VEGF, EGFR) could be used to discriminate between low-grade and high-grade gliomas. Increased expression of some proteins (EGFR, tenascin-C, survivin) correlated with shorter patient survival (13–15). At the same time, some of these markers, e.g., VEGF, are upregulated in a variety of tumors and are not glioma-specific. Other markers (bcl-2 family, EGFR) did not significantly correlate with survival when large groups of patients were studied (15–17). Therefore, before being considered as diagnostic and/or prognostic tools, these and other candidate markers have to be thoroughly analyzed in well-controlled studies involving significant numbers of patients and tumors of different grades. Unfortunately, the vast majority of existing markers of tumor invasion and progression have not yet been put through a rigorous pre-clinical and clinical testing.
3.1. Laminin-8 as a new glioma biomarker
In an earlier study (4) we found two genes that were consistently upregulated in all high-grade and low-grade gliomas and in tissues adjacent to GBMs, the most aggressive gliomas. One of these genes coding for EGFR was already known to be overexpressed in gliomas. The other one was coding for the α4 chain of laminin, which was not known to be overexpressed in any type of tumor. Laminin α4 chain is a constituent of three known laminins, laminin 8, laminin-9 and laminin-14 (18). Laminins are the major components of basement membranes that lie beneath the endothelial surface layer of blood vessels. Each laminin consists of three chains, α, β, and γ (4,18).
We found that during progression of human gliomas, the expression of capillary basement membrane (BM) laminins containing α4 chain switched from the predominant laminin-9 (α4β2γ1) to laminin-8 (α4β1γ1) (4, Figure 1). Laminin-8 and laminin-9 have similar structure but different β chains. Laminin-8 and its receptors, integrins α3β1 and α6β1, appear to be important to the functioning of endothelial cell BMs, which play a role in the maintenance of the blood-brain barrier (19,20). Recently, the association of laminin α4 chain with angiogenesis has been demonstrated in vivo and in vitro (21). Some cultured glioma cell lines can also produce α4 and β1-containing laminins. Laminin-8 is thought to play a role in cell migration during development, wound healing, and angiogenesis (19,22,23).
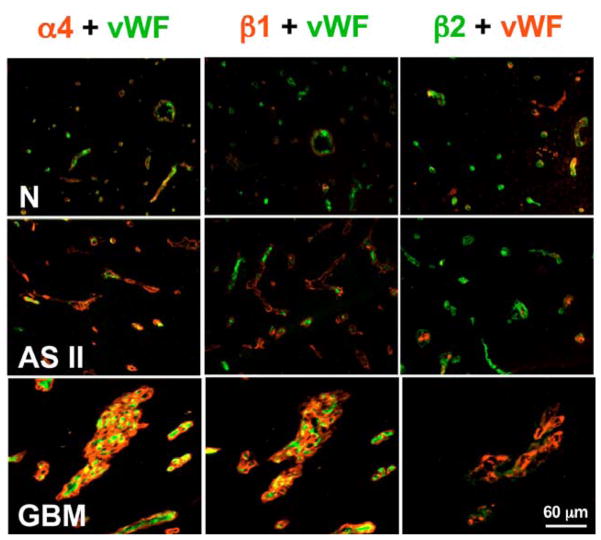
Figure 1.
Double immunofluorescent staining of brain tissues for laminin-8 (α4β1γ1) and laminin-9 (α4β2γ1) chains, and for von Willebrand factor (vWF) specific for endothelial cells. N, Normal brain, where microvessels are positive for vWF, and α4 and β1 laminin chains are barely visible. At the same time, β2 laminin chain is prominent in vessel walls positive for vWF. This pattern is compatible with small amounts of laminin-9. AS II, Astrocytoma grade II with stronger staining for laminin α4 chain in brain microvessels. Expression of laminin β1 chain is higher than in normal brain and β2 is still strong. This pattern is compatible with predominance of laminin-9.GBM, glioblastoma multiforme with very bright staining of α4 and β1 laminin chains but very weak β2 chain in brain vessels. This pattern is compatible with predominance of laminin-8. Reproduced with permission from Cancer 101, 604–612 (2004)
3.2. Development of in vitro system to block laminin-8
To examine the involvement of laminin-8 in glioma invasion, a reliable in vitro system was needed where it was possible to quantify invasion rates and to optimize the dosage of antisense laminin oligonucleotides. We used a cell culture system to meet these important needs. To better mimic the in vivo situation in glial tumors where the major cell types are glial (astrocytes) and endothelial cells (21), we needed to combine glioma cells with brain endothelium in a co-culture (24, Figure 2). In such a situation, endothelial cells can develop capillary-like structures, and this process is faster when endothelial cells are cultured with tumor astrocytes than with normal embryonic brain astrocytes (25). We hypothesized that in glioma-endothelium co-cultures there would be more laminin-8 produced, and that this laminin might increase glioma invasion in a Matrigel assay. Research into these issues could facilitate GBM diagnosis and prognosis, and eventually increase survival of brain cancer patients.
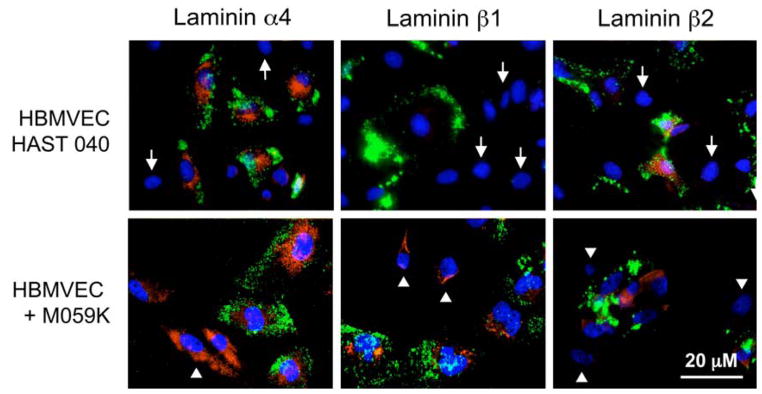
Figure 2.
Laminin α4, β1, and β2 chain staining of co-cultures. Live co-cultures were exposed to Ac-LDL (green color, to reveal endothelial cells) and then fixed and simultaneously stained for select laminin chains (red color) and nuclei (DAPI, blue color). In endothelial-normal astrocyte co-cultures (HBMVEC+HAST040) α4 and β2 chains are expressed in Ac-LDL-positive endothelial cells only but not in Ac-LDL-negative astrocytes (arrows). β1 chain is largely absent. In endothelial-glioma co-cultures (HBMVEC+M059K), α4 chain is expressed by both cell types and β2 chain, only by endothelial cells. Importantly, β1 chain is now expressed not only by Ac-LDL-negative glioma cells (arrowheads) but also by Ac-LDL-positive endothelial cells. Reproduced with permission from: Mol Cancer Ther 2, 985–994 (2003)
To probe the role of laminin-8 in glioma invasion, the use of antisense oligonucleotides to block its expression was attempted. The potential of antisense is widely recognized but it remained unfulfilled since, until recently, the available oligonucleotides suffered from poor specificity, instability, and undesirable non-antisense effects (26, 27). These problems have been largely solved by the new generation of antisense oligonucleotides that offer the promise of safe and effective therapeutics for various diseases including cancer (28,29). New-generation antisense oligonucleotides are being used in studies to find effective medications and treatments for many disorders, including viruses and cancers. Antisense technology is being refined not only for drug validation and diagnostic purposes but also for the development of future treatments for patients.
The most promising types of antisense oligonucleotides are Morpholino and peptide nucleic acid (PNA; they have nucleobases attached to a neutral “peptide-like” backbone) oligonucleotides (26, 28). Our new study used short strands of chemically modified genetic material (Morpholino™ antisense oligonucleotides) to block the messenger RNA (mRNA). They work well in the presence or absence of serum, are totally resistant to nucleases, and remain intact in culture medium and in cells indefinitely. Morpholino oligonucleotides have a high affinity for RNA and efficiently invade even quite stable secondary structures in mRNAs. They have the highest sequence specificity of all antisense types over a very broad concentration range and appear to be free of non-antisense effects (28,29). They have high activity in a cell-free translation system and can block target protein production in cultured cells (26). Morpholino are also effective in vivo (30). Given these properties, Morpholino oligonucleotides have been chosen here to inhibit the expression of laminin-8 chains in culture. Special experiments (cell viability assay (32)) have shown that Morpholino antisense treatment did not affect the viability of any cell line used.
Matrigel invasion assay was developed for quantitative measurement of the invasiveness of tumor cells through a BM matrix. Most tested cells characterized as invasive and metastatic in vivo are able in vitro to invade Matrigel, which is a BM-like material from the mouse Engelbreth-Holm-Swarm tumor (33,34).
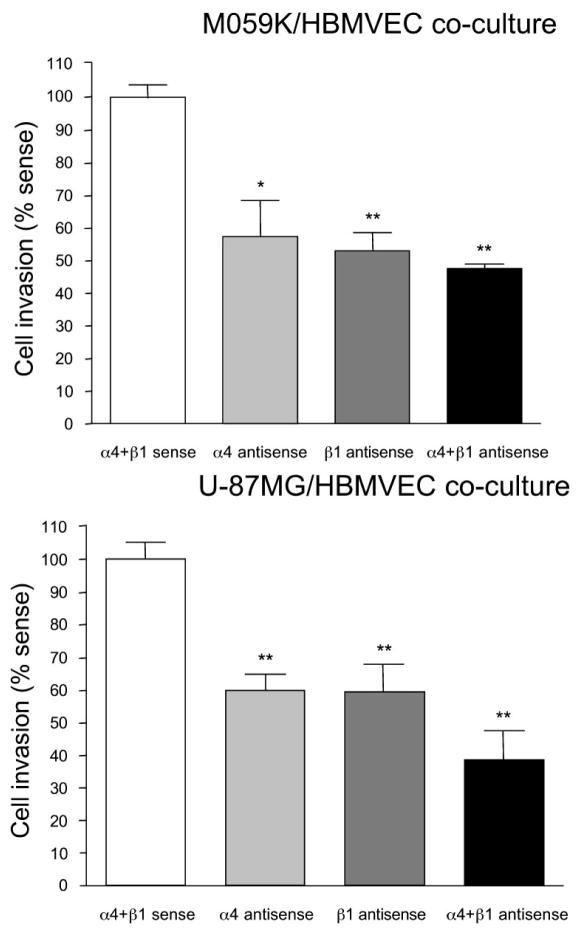
When co-cultures of human U-87MG or M059K glioma cells with normal human brain endothelial cells (HBMVEC) were treated by antisense, the inhibition of invasiveness on Matrigel was 62% for U-87MG+HBMVEC and 53% for M059K+HBMVEC compared to control cells treated with corresponding sense oligonucleotides. In our experiments, α4 and β1 laminin chain expression was inhibited more efficiently with a lower concentration of antisense oligonucleotides (0.25 + 0.25 mM) than with a higher concentration (0.5 + 0.5 mM), although no apparent toxicity was noticed at either concentration (Figure 3).
Figure 3.
Measurement of invasion in co-cultures after antisense treatment. The Matrigel invasion assay was carried out as described in Materials and Methods. Note significant decrease in the fraction of cells that invaded through Matrigel in antisense-treated cultures. A more pronounced effect is seen with a combination of antisense oligonucleotides. Similar results were obtained with M059K and U-87MG glioma cell lines. *, p<0.04; **, p<0.001 by ANOVA. Invasion in sense-treated cultures was taken as 100%. Reproduced with permission from: Mol Cancer Ther 2, 985–994 (2003)
The use of antisense technology in vivo may offer an effective future tumor treatment because of its efficiency, specificity and ease of delivery to tumor cells (28, 29). This technology is being continuously developed and refined not only for the drug validation and diagnostic purposes but also for the development of future treatments. The present data emphasize the feasibility of antisense approach using laminin-8 as a target for treatment of brain gliomas. Reduction of tumor invasion by antisense to laminin-8 may slow the growth and spread of aggressive two GBMs cell lines, U-87MG and M059K (Figure 4). In combination with other treatment methods or with blocking of other targets as well (EGFR or matrix metalloproteinases, MMPs) it may prolong disease-free periods and increase survival of glioma patients. Future developments of laminin-8 blocking for therapeutic purposes may also include the use of specific monoclonal antibodies and/or small interfering RNA (siRNA) that is an emerging promising approach for gene silencing.
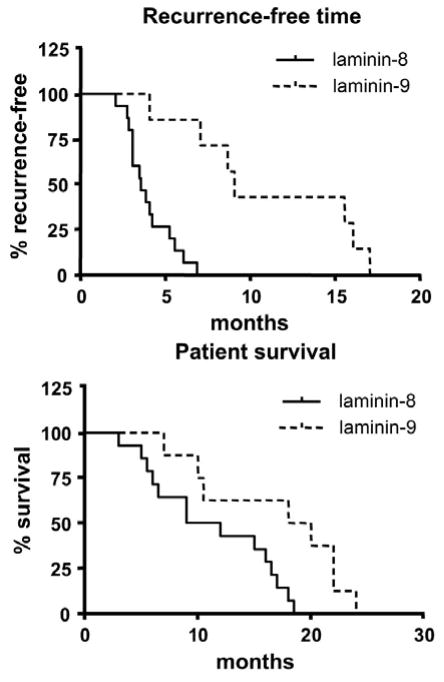
Figure 4.
Laminin-8 associates with decreased time of recurrent tumor development and decreased survival of GBM patients compared to laminin-9. Statistical analysis was done using Kaplan-Meier test. p=0.0002 for tumor recurrence, and p<0.015 for patient survival. Reproduced with permission from Cancer 101, 604–612 (2004)
It remains to be established how laminin-8 promotes glioma invasiveness. One possible mechanism may be stimulation of cell migration. Laminin-8 rather weakly supported cell adhesion and spreading compared to laminin-5 or laminin 10/11 (20,37). At the same time, laminin-8 stimulated cell migration better than several other laminin isoforms (20). Increased expression of laminin-8 in both glioma cells and glioma-adjacent capillary endothelial cells (4, 20, this report) may reduce glial cell adhesion and enhance migration, which is necessary for local tumor invasiveness. A recent gene microarray analysis of 85 gliomas identified the top 44 genes that distinguish the major gene clusters of gliomas. Gene expression of laminin β1 chain (part of laminin-8) was one of them out of ten thousand genes (38).
3.3. Laminin-8 is a predictor of glioma outcome. Clinical data
We have recently examined a significantly larger number of brain tumors (from 60 patients) including glial tumors of different grades in an attempt to correlate laminin-8 expression with recurrence development and patient survival (39). Immunohistochemistry and Western blotting were used to detect laminin isoforms of interest.
For 23 GBM patients laminin-8 expression was compared with tumor recurrence and patient survival time. For one patient, who was twice operated, the death record was not found. Clinically, all 17 GBM patients with high laminin-8 levels developed tumor recurrences at a mean of 3.92 months after surgery, whereas six patients with high laminin-9 expression developed recurrences at a mean of 11.43 months after surgery. This difference was highly significant (p=0.0002, Figure 4). The new data with expanded number of patients fully confirmed our previous study on 9 GBM patients (4). The mean survival time for 16 GBM patients whose tumors expressed laminin-8 was 11.2 months, whereas for eight GBM patients whose tumors expressed laminin-9 the mean survival time was 16.7 months (p<0.015, Figure 4).
Enhanced expression of laminin-8 in high-grade compared with low-grade gliomas suggests its involvement in tumor progression. Overexpression of laminin-8 in tumor-adjacent tissues may facilitate the spread of microinvasive glioma foci not removed by surgery or available therapy and lead to recurrence. This may explain why laminin-8 may be predictive of glioma recurrence. Given these patterns of laminin-8 (and to a lesser extent, laminin-9) expression, we hypothesize that laminin-8 plays a role in glioma progression and tumor recurrence, and that inhibiting its expression may inhibit glioma invasion and recurrence. In summary, the majority of high-grade gliomas had increased levels of laminin-8 (α4β1γ1) in blood vessel walls, whereas in low-grade tumors laminin-9 (α4β2γ1) predominated. GBMs that predominantly expressed laminin-8 had a shorter time to tumor recurrence and patient survival than GBMs that predominantly expressed laminin-9. Laminin-8 may play a role in the process of invasiveness in human gliomas as well as in their progression. It thus appears to be a promising target for new therapeutic approaches possibly with combination with other tumor markers or standard chemo- and radiation therapy.
A new glioma-endothelial co-culture model suitable for studying laminin-8 expression and its inhibition in vitro by antisense oligonucleotides was developed to examine the role of laminin-8 in cell migration and invasion. Morpholino antisense oligonucleotides proved to be efficient inhibitors of laminin-8 expression in co-cultures. These antisense oligonucleotides also significantly inhibited invasion of two different glioma cell lines in vitro. The results suggest that laminin-8 may play an important role in glioma invasion. Morpholino oligonucleotides may provide an efficient method to block laminin-8 expression for future therapeutic purposes.
4. PERSPECTIVE
Laminins are the major constituents of blood vessel BMs. Gradual increase of laminin-8 expression with a switch from laminin-9 in low-grade glial tumors (grade I-II) to moderate in grade III and significantly high expression in 76% of GBMs (grade IV) may be associated with neovascularization and, thus, contribute to tumor aggressiveness. Overall, laminin-8 overexpressed in GBMs, together with factors promoting tumor growth (e.g., EGFR), might be an important prognostic factor for predicting GBM time to recurrence and patient survival time. As invasion-promoting factor it may be potentially used as a target for glioma therapy.
In combination with several new well-characterized proteins associated with glioma progression, such as tenascin-C, MMP-2 and MMP-9 (4, 38, 40–44), laminin-8 may be an important tool for potential diagnosis or treatment of gliomas. Previously, only laminin-5 was shown to play a role in melanoma invasion (45). Our present data suggest that vascular laminin-8 also plays a significant role in glioma cell invasiveness. Since matrix-degrading proteinases are also important for glioma invasion (46), future research should explore whether proteolysis of laminin is required for glioma invasion. Our recent data suggest that laminin-8, which may facilitate tumor invasion, contributes to tumor regrowth after therapy. Laminin-8 may be used as a predictor of tumor recurrence, patient survival and as a potential molecular target for glioma therapy. Pre-clinical study of laminin-8 inhibition is in progress to develop treatment regimens for glial tumor prevention.
Our recent study on breast cancer (47) also showed that the expression of laminin β2 chain (a constituent of laminin-9) was mostly seen in vascular BMs of normal breast and carcinomas in situ but not in invasive carcinomas or metastases. In contrast, laminin β1 chain (a constituent of laminin-8) appeared in vessel walls of carcinomas and their metastases. A similar change from laminin-11 (α5β2γ1) to laminin-10 (α5β1γ1) was observed in vascular BMs during breast tumor progression. Therefore, a switch from β2-containing to β1-containing laminins may not be confined to brain glioma but appears to be a more general phenomenon associated with tumor development and progression. In this respect, blocking the expression of laminin β1 chain (possibly, together with α4 and/or α5 chains) could have a potential importance for the development of future antiangiogenic therapies for cancer.
The original work described in this review was conducted under appropriate protocols approved by Cedars-Sinai Medical Center Institutional Review Board.
References
- 1.Shapiro WR, Shapiro JR. Biology and treatment of malignant glioma. Oncology. 1998;12:233–240. [PubMed] [Google Scholar]
- 2.Gonzales MF. Classification and pathogenesis of brain tumors. In: Kaye AH, Laws ER Jr, editors. Brain tumors. Churchill Livingstone; Edinburgh: 1997. [Google Scholar]
- 3.Ljubimova JY, Khazenzon NM, Chen Z, Neyman YI, Turner L, Riedinger MS, Black KL. Gene array analysis of differentially expressed genes in human glial tumors. Int J Oncol. 2001;18:287–295. doi: 10.3892/ijo.18.2.287. [DOI] [PubMed] [Google Scholar]
- 4.Ljubimova JY, Lakhter AJ, Loksh A, Yong WH, Riedinger MS, Miner JH, Sorokin ML, Ljubimov AV, Black KL. Overexpression of α4 chain-containing laminins in human glial tumors identified by gene microarray analysis. Cancer Res. 2001;61:5601–5610. [PubMed] [Google Scholar]
- 5.Sehgal A. Molecular changes during the genesis of human gliomas. Semin Surg Oncol. 1998;14:3–12. doi: 10.1002/(sici)1098-2388(199801/02)14:1<3::aid-ssu2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 6.Lal A, Lash AE, Altschul SF, Velculescu V, Zhang L, McLendon RE, Marra MA, Prange C, Morin PJ, Polyak K, Papadopoulos N, Vogelstein B, Kinzler KW, Strausberg RL, Riggins GJ. A public database for gene expression in human cancers. Cancer Res. 1999;59:5403–5407. [PubMed] [Google Scholar]
- 7.Qin H, Sun Y, Benveniste EN. The transcription factors Sp1, Sp3, and AP-2 are required for constitutive matrix metalloproteinase-2 gene expression in astroglioma cells. J Biol Chem. 1999;274:29130–29137. doi: 10.1074/jbc.274.41.29130. [DOI] [PubMed] [Google Scholar]
- 8.Huncharek M, Kupelnick B. Epidermal growth factor receptor gene amplification as a prognostic marker in glioblastoma multiforme: results of a meta-analysis. Oncol Res. 2000;12:107–112. doi: 10.3727/096504001108747576. [DOI] [PubMed] [Google Scholar]
- 9.Ueki K, Nishikawa R, Nakazato Y, Hirose T, Hirato J, Funada N, Fujimaki T, Hojo S, Kubo O, Ide T, Usui M, Ochiai C, Ito S, Takahashi H, Mukasa A, Asai A, Kirino T. Correlation of histology and molecular genetic analysis of 1p, 19q, 10q, TP53, EGFR, CDK4, and CDKN2A in 91 astrocytic and oligodendroglial tumors. Clin Cancer Res. 2002;8:196–201. [PubMed] [Google Scholar]
- 10.Muracciole X, Romain S, Dufour H, Palmari J, Chinot O, Ouafik L, Grisoli F, Branger DF, Martin PM. PAI-1 and EGFR expression in adult glioma tumors: toward a molecular prognostic classification. Int J Radiat Oncol Biol Phys. 2002;52:592–598. doi: 10.1016/s0360-3016(01)02699-2. [DOI] [PubMed] [Google Scholar]
- 11.Chakravarti A, Noll E, Black PM, Finkelstein DF, Finkelstein DM, Dyson NJ, Loeffler JS. Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J Clin Oncol. 2002;20:1063–1068. doi: 10.1200/JCO.2002.20.4.1063. [DOI] [PubMed] [Google Scholar]
- 12.Forget MA, Desrosiers RR, Del M, Moumdjian R, Shedid D, Berthelet F, Beliveau R. The expression of rho proteins decreases with human brain tumor progression: potential tumor markers. Clin Exp Metastasis. 2002;19:9–15. doi: 10.1023/a:1013884426692. [DOI] [PubMed] [Google Scholar]
- 13.Kulla A, Liigant A, Piirsoo A, Rippin G, Asser T. Tenascin expression patterns and cells of monocyte lineage: relationship in human gliomas. Mod Pathol. 2000;13:56–67. doi: 10.1038/modpathol.3880010. [DOI] [PubMed] [Google Scholar]
- 14.Herold-Mende C, Mueller MM, Bonsanto MM, Schmitt HP, Kunze S, Steiner HH. Clinical impact and functional aspects of tenascin-C expression during glioma progression. Int J Cancer. 2002;98:362–369. doi: 10.1002/ijc.10233. [DOI] [PubMed] [Google Scholar]
- 15.Yao Y, Kubota T, Sato K, Kitai R, Takeuchi H, Arishima H. Prognostic value of vascular endothelial growth factor and its receptors Flt-1 and Flk-1 in astrocytic tumours. Acta Neurochir (Wien) 2001;143:159–166. doi: 10.1007/s007010170122. [DOI] [PubMed] [Google Scholar]
- 16.Strik H, Deininger M, Streffer J, Grote E, Wickboldt J, Dichgans J, Weller M, Meyermann R. BCL-2 family protein expression in initial and recurrent glioblastomas: modulation by radiochemotherapy. J Neurol Neurosurg Psychiatry. 2001;67:763–768. doi: 10.1136/jnnp.67.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou YH, Tan F, Hess KR, Yung WK. The expression of PAX6, PTEN, vascular endothelial growth factor, and epidermal growth factor receptor in gliomas: relationship to tumor grade and survival. Clin Cancer Res. 2003;9:3369–3375. [PubMed] [Google Scholar]
- 18.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- 19.Thyboll J, Kortesmaa J, Cao R, Soininen R, Wang L, Iivanainen A, Sorokin L, Risling M, Cao Y, Tryggvason K. Deletion of the laminin α4 chain leads to impaired microvessel maturation. Mol Cell Biol. 2002;22:1194–1202. doi: 10.1128/MCB.22.4.1194-1202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujiwara H, Kikkawa Y, Sanzen N, Sekiguchi K. Purification and characterization of human laminin-8. Laminin-8 stimulates cell adhesion and migration through α3β1 and α6β1 integrins. J Biol Chem. 2001;276:17550–17558. doi: 10.1074/jbc.M010155200. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez AM, Gonzales M, Herron GS, Nagavarapu U, Hopkinson SB, Tsuruta D, Jones JC. Complex interactions between the laminin α4 subunit and integrins regulate endothelial cell behavior in vitro and angiogenesis in vivo. Proc Natl Acad Sci USA. 2002;99:16075–16080. doi: 10.1073/pnas.252649399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, Copeland NG, Sanes JR. The laminin α chains: expression, developmental transitions, and chromosomal locations of α1–5, identification of heterotrimeric laminins 8–11, and cloning of a novel α3 isoform. J Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patarroyo M, Tryggvason K, Virtanen I. Laminin isoforms in tumor invasion, angiogenesis and metastasis. Semin Cancer Biol. 2002;12:197–207. doi: 10.1016/S1044-579X(02)00023-8. [DOI] [PubMed] [Google Scholar]
- 24.Boado RJ, Kazantsev A, Apostol BL, Thompson LM, Pardridge WM. Antisense-mediated down-regulation of the human huntingtin gene. J Pharmacol Exp Ther. 2000;295:239–243. [PubMed] [Google Scholar]
- 25.Minakawa T, Bready J, Berliner J, Fisher M, Cancilla PA. In vitro interaction of astrocytes and pericytes with capillary-like structures of brain microvessel endothelium. Lab Invest. 1991;65:32–40. [PubMed] [Google Scholar]
- 26.Nielsen PE. Peptide nucleic acid targeting of double-stranded DNA. Methods Enzymol. 2001;340:329–340. doi: 10.1016/s0076-6879(01)40429-0. [DOI] [PubMed] [Google Scholar]
- 27.Dias N, Stein CA. Antisense oligonucleotides: basic concepts and mechanisms. Mol Cancer Ther. 2002;1:347–355. [PubMed] [Google Scholar]
- 28.Summerton J, Weller D. Morpholino antisense oligomers: Design, preparation and properties. Antisense Nucleic Acid Drug Dev. 1997;7:187–195. doi: 10.1089/oli.1.1997.7.187. [DOI] [PubMed] [Google Scholar]
- 29.Lacerra G, Sierakowska H, Carestia C, Fucharoen S, Summerton J, Weller D, Kole R. Restoration of hemoglobin A synthesis in erythroid cells from peripheral blood of thalassemic patients. Proc Natl Acad Sci USA. 2000;97:9591–9596. doi: 10.1073/pnas.97.17.9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor MF, Paulauskis JD, Weller DD, Kobzik L. Comparison of efficacy of antisense oligomers directed toward TNF-α in helper T and macrophage cell lines. Cytokine. 1997;9:672–681. doi: 10.1006/cyto.1997.0212. [DOI] [PubMed] [Google Scholar]
- 31.Arora V, Knapp DC, Smith BL, Statdfield ML, Stein DA, Reddy MT, Weller DD, Iversen PL. c-Myc antisense limits rat liver regeneration and indicates role for c-myc in regulating cytochrome P-450 3A activity. J Pharmacol Exp Ther. 2000;292:921–928. [PubMed] [Google Scholar]
- 32.Khazenzon NM, Ljubimov AV, Lakhter AJ, Fujiwara H, Sekiguchi K, Sorokin LM, Petäjäniemi N, Virtanen I, Black KL, Ljubimova JY. Antisense inhibition of laminin-8 expression reduces invasion of human gliomas in vitro. Mol Cancer Ther. 2003;2:985–994. [PubMed] [Google Scholar]
- 33.Albini A, Iwamoto Y, Aaronson SA, Kozlowski JM, McEwan RN. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47:3239–3245. [PubMed] [Google Scholar]
- 34.Kleinman HK, McGarvey ML, Hassell JR, Star VL, Gannon FB, Laurie GW, Martin GR. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 35.Jansen B, Wacheck V, Heere-Ress E, Schlagbauer-Wadl H, Hoeller C, Lucas T, Hoermann M, Hollenstein U, Wolff K, Pehamberger H. Chemosensitisation of malignant melanoma by BCL2 antisense therapy. Lancet. 2000;356:1728–1733. doi: 10.1016/S0140-6736(00)03207-4. [DOI] [PubMed] [Google Scholar]
- 36.Shi N, Boado RJ, Pardridge WM. Antisense imaging of gene expression in the brain in vivo. Proc Natl Acad Sci USA. 2000;97:14709–14714. doi: 10.1073/pnas.250332397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knott JC, Mahesparan R, Garcia-Cabrera I, Bolge Tysnes B, Edvardsen K, Ness GO, Mork S, Lund-Johansen M, Bjerkvig R. Stimulation of extracellular matrix components in the normal brain by invading glioma cells. Int J Cancer. 1998;75:864–872. doi: 10.1002/(sici)1097-0215(19980316)75:6<864::aid-ijc8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 38.Freije WA, Castro-Vargas FE, Fang Z, Horvath S, Cloughesy T, Liau LM, Mischel PS, Nelson SF. Gene expression profiling of gliomas strongly predicts survival. Cancer Res. 2004;64:6503–6510. doi: 10.1158/0008-5472.CAN-04-0452. [DOI] [PubMed] [Google Scholar]
- 39.Ljubimova JY, Fujita M, Khazenzon NM, Das A, Pikul BB, Newman D, Sekiguchi K, Sorokin LM, Sasaki T, Black KL. Association between laminin-8 and glial tumor grade, recurrence, and patient survival. Cancer. 2004;101:604–612. doi: 10.1002/cncr.20397. [DOI] [PubMed] [Google Scholar]
- 40.Herold-Mende C, Mueller MM, Bonsanto MM, Schmitt HP, Kunze S, Steiner HH. Clinical impact and functional aspects of tenascin-C expression during glioma progression. Int J Cancer. 2002;98:362–369. doi: 10.1002/ijc.10233. [DOI] [PubMed] [Google Scholar]
- 41.Zagzag D, Capo V. Angiogenesis in the central nervous system: a role for vascular endothelial growth factor/vascular permeability factor and tenascin-C. Common molecular effectors in cerebral neoplastic and non-neoplastic “angiogenic diseases”. Histol Histopathol. 2002;17:301–321. doi: 10.14670/HH-17.301. [DOI] [PubMed] [Google Scholar]
- 42.Qin H, Sun Y, Benveniste EN. The transcription factors Sp1, Sp3, and AP-2 are required for constitutive matrix metalloproteinase-2 gene expression in astroglioma cells. J Biol Chem. 1999;274:29130–29137. doi: 10.1074/jbc.274.41.29130. [DOI] [PubMed] [Google Scholar]
- 43.Kachra Z, Beaulieu E, Delbecchi L, Mousseau N, Berthelet F, Moumdjian R, Del Maestro R, Beliveau R. Expression of matrix metalloproteinases and their inhibitors in human brain tumors. Clin Exp Metastasis. 1999;17:555–566. doi: 10.1023/a:1006760632766. [DOI] [PubMed] [Google Scholar]
- 44.MacDonald TJ, DeClerck YA, Laug WE. Urokinase induces receptor mediated brain tumor cell migration and invasion. J Neurooncol. 1998;40:215–226. doi: 10.1023/a:1006150506789. [DOI] [PubMed] [Google Scholar]
- 45.Tsuj T, Kawada Y, Kai-Murozono M, Komatsu S, Han SA, Takeuchi K, Mizushima H, Miyazaki K, Irimura T. Regulation of melanoma cell migration and invasion by laminin-5 and α3β1 integrin (VLA-3) Clin Exp Metastasis. 2002;19:127–134. doi: 10.1023/a:1014573204062. [DOI] [PubMed] [Google Scholar]
- 46.Kondraganti S, Mohanam S, Chintala SK, Kin Y, Jasti SL, Nirmala C, Lakka SS, Adachi Y, Kyritsis AP, Ali-Osman F, Sawaya R, Fuller GN, Rao JS. Selective suppression of matrix metalloproteinase-9 in human glioblastoma cells by antisense gene transfer impairs glioblastoma cell invasion. Cancer Res. 2000;60:6851–6855. [PubMed] [Google Scholar]
- 47.Fujita M, Khazenzon NM, Bose S, Sekiguchi K, Sasaki T, Carter WG, Ljubimov AV, Black KL, Ljubimova JY. Overexpression of β1 chain-containing laminins in capillary basement membranes of human breast cancer and its metastases. Breast Cancer Res. 2005;7:R411–R421. doi: 10.1186/bcr1011. [DOI] [PMC free article] [PubMed] [Google Scholar]