Abstract
A great deal is now known about the protein components of tight junctions and adherens junctions, as well as how these are assembled. Less is known about the molecular framework of gap junctions, but these also have membrane specializations and are subject to regulation of their assembly and turnover. Thus, it is reasonable to consider that these three types of junctions may share macromolecular commonalities. Indeed, the tight junction scaffolding protein zonula occluden-1 (ZO-1) is also present at adherens and gap junctions, including neuronal gap junctions. On the basis of these earlier observations, we more recently found that two additional proteins, AF6 and MUPP1, known to be associated with ZO-1 at tight and adherens junctions, are also components of neuronal gap junctions in rodent brain and directly interact with connexin36 (Cx36) that forms these junctions. Here, we show by immunofluorescence labeling that the cytoskeletal-associated protein cingulin, commonly found at tight junctions, is also localized at neuronal gap junctions throughout the central nervous system. In consideration of known functions related to ZO-1, AF6, MUPP1, and cingulin, our results provide a context in which to examine functional relationships between these proteins at Cx36- containing electrical synapses in brain—specifically, how they may contribute to regulation of transmission at these synapses, and how they may govern gap junction channel assembly and/or disassembly.
Keywords: AF6, Cingulin, Connexin36, Electrical synapse, Gap junctions, MUPP1, Neurons, PDZ domain
As evident in this special tribute issue of Journal of Membrane Biology to the long and distinguished career of Dr. Ross Johnson as a gap junctionologist, gap junctions remain at center stage regarding their relevance in health and disease. As well, these entities continue to entertain the imagination of researchers in the field and still seem to yield endless surprises concerning their myriad functions, their complex regulation, and, as is becoming evident more recently, their rich structural organization. One organ system in which studies of connexins and gap junctions have generated its share of such surprises over the past decade is the central nervous system (CNS). In brain, for example, the large repertoire of connexins expressed by glial cells is unparalleled by any other cell type in the body. In addition, huge advances in understanding the functional importance of widespread electrical synapses formed by gap junctions between neurons in the mammalian CNS (Bennett 1997; Bennett and Zukin 2004; Connors and Long 2004; Nagy et al. 2004) have left many wondering how, in the past, these functionally essential structures could have been overlooked, neglected, or even dismissed as a fundamental means of neurotransmission in higher vertebrates, along with chemical transmission. Three areas of our focus have been documentation of brain regions in which electrical synapses occur, identification of the structural components of neuronal gap junctions that form these synapses, and elucidation of how transmission at these synapses may be regulated.
Analogous to protein markers that are used for the identification of nerve terminals that contain specific chemical synaptic transmitters or their cognate postsynaptic receptors, there is a large body of evidence that has led to the acceptance of connexin36 (Cx36) as a reliable immunohistochemical marker for electrical synapses. Cx36 has a broad distribution pattern and is found in many electrically coupled networks in adult rodent brain (Connors and Long 2004; Hormuzdi et al. 2004; Sohl et al. 2004, 2005). Indeed, the typically plasma membrane-associated punctate immunoreactivity for Cx36 widely seen in brain corresponds at the ultrastructural level to sites of interneuronal gap junctions (Kamasawa et al. 2006; Rash et al. 2000, 2007a, b; Li et al. 2008a), and Cx36 is found at locations in brain where functional electrical synapses occur (Fuduka et al. 2006; Baude et al. 2007; Liu and Jones 2003; Muller et al. 2005). Specific examples of electrically coupled neuronal networks wherein Cx36 has been identified, among many others, include interneurons in the cerebral cortex (Bennett and Zukin 2004), principal neurons in the inferior olive (Llinas et al. 1974), the soma of proprioceptive neurons in the trigeminal mesencephalic nucleus (Curti et al. 2012a) and the long-projection noradrenergic neurons in the locus coeruleus (Rash et al. 2007b).
Like other cell–cell junctions, such as tight junctions and adherens junctions, gap junctions including those forming electrical synapses are emerging as multimolecular composites whose structure and regulation is governed in part by their associated proteins. In particular, protein sequence analysis has revealed that most members of the connexin family of gap junction proteins contain a PDZ (postsynaptic density-95/disks large/zonula occludens-1) ligand motif at their C-terminus. By virtue of this motif, many of these connexins have been demonstrated to interact with the PDZ domain-containing protein zonula occludens-1 (ZO-1). ZO-1 is a member of the membrane associated guanylate kinase (MAGUK) family of PDZ proteins, which are hallmarked by multiple PDZ domains, one SH3 domain and one GUK domain. ZO-1 was initially described in peripheral endothelial cells as a tight junction-associated protein. In the context of tight junctions, the PDZ domains of ZO-1 and zonula occludens-2 (ZO-2) have a pivotal role in the formation of claudin-based tight junction strands (Umeda et al. 2006). It is via their C-terminal motif that the claudins interact with the first PDZ domain of the zonula occludens (ZO) family of proteins and with the 10th PDZ domain of multi-PDZ domain protein-1 (MUPP1) (Jeansonne et al. 2003).
In brain, ZO-1 was originally reported to be localized almost exclusively at vascular endothelial cell tight junctions, ostensibly precluding its interaction with connexins found to be expressed in brain parenchyma. With reports of ZO-1 interactions with more and more connexins in peripheral tissues, it began to be inconceivable that none of the numerous connexins in brain had functional requirement for interaction with ZO-1. However, we noted that the extreme C-terminus YV residues in Cx36 are identical to those found in members of tight junction-associated proteins, including the claudins (Itoh et al. 1999). The presence of a potential C-terminus PDZ ligand motif in Cx36, together with reports of the mechanism whereby specific interactions occur between other connexins and ZO-1, prompted us to reevaluate the cellular expression and distribution of ZO-1 in brain. We found punctate immunohistochemical labeling of ZO-1 to be broadly distributed in neurons and glial cells in rodent CNS (Li et al. 2004a; Penes et al. 2005), providing the possibility of its interaction with glial connexins and with neuronal Cx36. We found ZO-1 in association with Cx36-containing gap junctions at virtually all electrical synapses examined and described a specific interaction of Cx36 with ZO-1, which required the C-terminal four amino acid PDZ domain ligand (SAYV) of Cx36 for interaction with the first of the three PDZ domains of ZO-1. This finding was later extended to include all members of the zonula occludens (ZO) family of proteins (ZO-1, ZO-2, ZO-3) (Li et al. 2004a, b, 2009; Ciolofan et al. 2006; Flores et al. 2008). The interaction of Cx36 and its fish ortholog Cx35 with ZO-1 is distinguished from that of other connexins previously examined in that while Cx36 and Cx35 interact with the first PDZ domain in ZO-1, other connexins interact with the second PDZ domain of ZO-1 (Derangeon et al. 2009).
Using the YV motif of Cx36 as a guide for potential Cx36 interactors, together with considerations of structural similarities between gap junctions and other specialized tight and adhesion cell-cell junctions, we searched for additional interacting partners of Cx36. Recently, we demonstrated AF6 and MUPP1 colocalization with Cx36 in many brain areas. Coimmunoprecipitation and pull-down approaches revealed association of Cx36 with AF6 and MUPP1, which required the C-terminus PDZ ligand of Cx36 for interaction with the single PDZ domain of AF6 and with the 10th PDZ domain of MUPP1. As proof of principle for identifying shared proteins at tight, adherens and gap junctions, we examined Cx36-containing electrical synapses for the presence of cingulin. Cingulin is a cytoplasmic protein that is localized to tight junctions via its specific binding to ZO-1 and the actin cytoskeleton, and is emerging as an important participant in the regulation of RhoA signaling at tight junctions.
Materials and Methods
The primary antibodies used here were obtained from Life Technologies (Grand Island, NY, USA) and included mouse monoclonal anti-Cx36 (Cat. No. 36-4600), rabbit polyclonal anti-cingulin (Cat. No. 36-4401), and rabbit polyclonal anti-MUPP1 (Cat. No. 42-2700). A total of six adult C57BL/6 mice were used in this study and these were handled according to approved protocols by the Central Animal Care Committee of University of Manitoba, with minimization of the numbers of animals used.
Immunohistochemistry was conducted using protocols we have previously described (Li et al. 2012). Animals were deeply anesthetized by intraperitoneal injection of equithesin (3 mL/kg) and then transcardially perfused with 3 mL cold sodium phosphate buffer, pH 7.4 containing 0.9 % NaCl, 100 mm sodium nitrite and 1 U/mL heparin, followed by 40 mL of ice-cold sodium phosphate buffer, pH 7.6, containing 1 % freshly depolymerized paraformaldehyde and 0.2 % picric acid. The brains were removed and transferred to an ice-cold cryoprotectant solution consisting of 10 % sucrose in 25 mm sodium phosphate buffer, pH 7.4 and stored at 4 °C in this solution for 24–72 h.
Brain sections were cut on a cryostat at 10 µm thickness, slide mounted and then incubated for 20 min in 50 mm Tris-HCl, pH 7.4, containing 1.5 % sodium chloride (TBS) and 0.3 % Triton X-100 (TBSTr). For immunofluorescence labeling, brain sections were incubated with primary antibodies in TBSTr supplemented with 5 % normal goat serum for 18 h at 4 °C. Double immunofluorescence was conducted by incubation with anti-Cx36 antibody and simultaneously with a rabbit polyclonal antibody against either MUPP1 or cingulin. All primary antibodies were used at a concentration of 2–3 µg/mL. After incubation with primary antibodies, sections were washed for 1 h in TBSTr, and incubated for 1.5 h at room temperature simultaneously with Cy3-conjugated goat antimouse IgG diluted 1:300 (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and Alexa Flour 488-conjugated goat anti-rabbit IgG diluted 1:1,000 (Molecular Probes, Eugene, Oregon). After secondary antibody incubation, sections were washed in TBSTr for 20 min, then in 50 mm Tris-HCl buffer, pH 7.4 for 30 min, covered with antifade medium and coverslipped. Standard control procedures included omission of one of the primary antibodies with inclusion of each of the secondary antibodies to ensure the absence of inappropriate cross-reactions between primary and secondary antibodies or between different combinations of secondary antibodies. Conventional and confocal immunofluorescence images were acquired on a Zeiss Axioskop2 fluorescence microscope using Axiovision 3.0 software (Carl Zeiss Canada, Toronto, Ontario, Canada) and on an Olympus Fluoview IX70 confocal microscope using Olympus Fluoview software (Olympus Canada, Inc., Markham, ON, Canada). Images were assembled using Adobe Photoshop CS (Adobe Systems, San Jose, CA, USA) and CorelDraw Graphics Suite X5 (Corel Corporation, Ottawa, ON, Canada).
Results
Immunofluorescence Localization of MUPP1 and Cx36
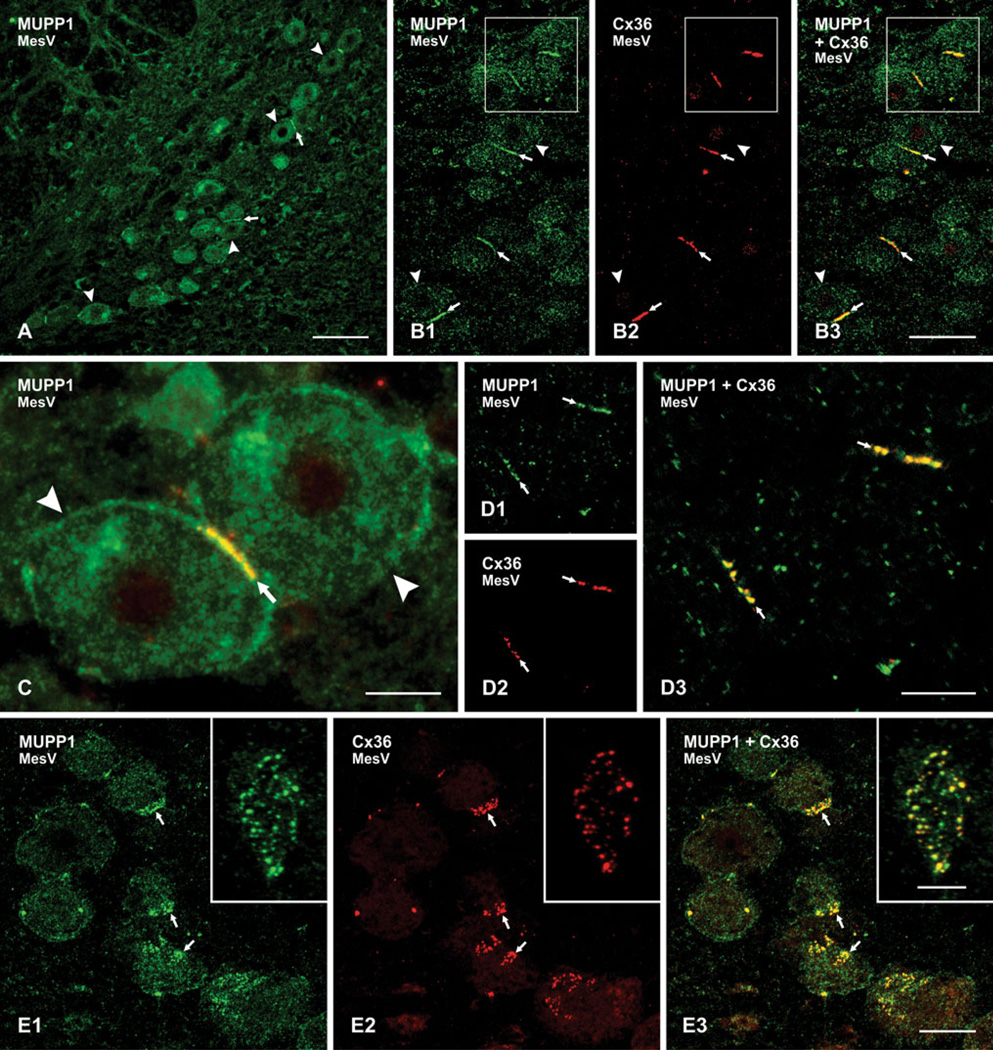
We have previously reported that MUPP1 is broadly distributed in various cell types of the CNS (Li et al. 2008b, 2012). On the basis of our observations, it is almost certain that this thirteen PDZ domain-containing protein will be found at a variety of neural cellular structures at which it serves a scaffolding function. In particular, we have documented its association with gap junctions formed by oligodendrocytes (Li et al. 2008b) and those formed by Cx36 between neurons in some regions of rodent brain (Li et al. 2012). Here, we examined relationships between MUPP1 and Cx36 in another region of brain, namely the mesencephalic trigeminal (MesV) nucleus in the brainstem of mouse. This nucleus was among the first in which electrical synapses in mammalian brain were identified (Hinrichsen and Larramendi 1970; Hinrichsen 1970; Baker and Llinas 1971). Recently, we reported on the localization of Cx36 between MesV neurons and the characteristics of the electrical coupling that Cx36-containing gap junctions mediate between these neurons (Curti et al. 2012a, b). In addition to being gap junctionally coupled, MesV neurons are further unusual because they are primary sensory neurons that convey proprioceptive signals from jawclosing muscles and periodontal ligaments, but have cell bodies located in the CNS rather than in sensory ganglia. We have noted that the absence of dendrites and the large size of MesV somata, together with the heavy concentration of Cx36 associated with their plasma membranes, make these cells ideal for the analyses of neuronal gap junction structure and function. Further, neuronal gap junctions in the MesV are also unique among electrically coupled neurons because the gap junctions between these cells occur exclusively at somato–somatic locations, allowing easy examination and analysis of the cell types between which gap junctions are found, which is not typically the case for other neuronal gap junctions in the CNS (Curti et al. 2012a, b).
The distribution of immunofluorescence labeling for MUPP1 in the MesV nucleus is shown at low magnification in Fig. 1a, where large MesV somata display a moderate density of intracellular labeling for MUPP1 compared with lower densities in surrounding regions. Pairs and triplets of MesV somata are often seen in apposition to each other, and MUPP1 is invariably seen to be concentrated along the entire length of these appositions (Fig. 1a), shown at higher magnification in Fig. 1b1. Unlike labeling for MUPP1, immunoreactivity for Cx36 is restricted to areas of MesV somatic appositions, with little detectable intracellular labeling (Fig. 1b2). Specificity of Cx36 labeling has been validated using Cx36 knockout mice, as we have previously described (Curti et al. 2012a). Overlay of doublelabeled sections in Fig. 1b reveals a high degree of MUPP1 colocalization with Cx36 at MesV somatic appositions (Fig. 1b3), as shown at higher magnification in image overlay where red/green overlap appears as yellow (Fig. 1c).
Fig. 1.
Immunofluorescence labeling of MUPP1 and Cx36 in the MesV of adult mouse brain. a Low magnification of the MesV immunolabeled for MUPP1 showing the distribution of large neuronal somata in the nucleus (arrowheads), some of which are closely apposed to each other (arrows). b Double immunofluorescence showing MesV neurons (arrowheads) and dense concentration of labeling for MUPP1 (b1) and Cx36 (b2) at MesV somata appositions (arrows). c Magnification of two MesV somata (arrowheads), with overlay showing colocalization of immunolabeling for MUPP1 (green) and Cx36 (red) at the somatic apposition (arrow). d Laser scanning confocal double immunofluorescence of the same field (d1–d3), with labeling of MUPP1 and Cx36 at MesV somatic appositions viewed on edge, showing punctate appearance of immunolabeling (arrows). e The same field (e1–e3) showing confocal double immunofluorescence labeling of MUPP1 and Cx36 at MesV appositions viewed en face, shown at higher magnification in inset. Clusters of MUPP1-positive (e1, arrows) and Cx36-positive (e2, arrows) puncta are seen at soma surfaces, with many of these puncta displaying MUPP1/Cx36 colocalization, seen as yellow in overlay (E3, arrows). Scale bars = a, 100 µm; b, 50 µm; c, d, 10 µm; e, 20 µm; inset in e, 5 µm
Double immunofluorescence labeling for MUPP1 and Cx36 at MesV somatic contacts were subject to more detailed analysis by laser scanning confocal microscopy. Sections through MesV somata often yield on edge views of their somal appositions (i.e., views perpendicular to their plane of contact). In rare cases, these contacts are viewed en face when cells are sectioned tangential to their surface (i.e., parallel to their plane of contact). In edge views, confocal imaging revealed appositions to contain a series of linearly arranged MUPP1-positive (Fig. 1d1) and Cx36- positive puncta (Fig. 1d2), with substantial colocalization (Fig. 1d3). In en face views, the appositions were seen to consist of clusters of individual puncta, with various distances of separation between them (Fig. 1e, with inset showing higher magnification of a cluster). Confocal through focus indicated that the clusters of puncta were located at the cell surface, and image rotation in the y–z axis (not shown) indicated that most MUPP1-immunopositive puncta were also positive for Cx36.
Immunofluorescence Localization of Cingulin and Cx36
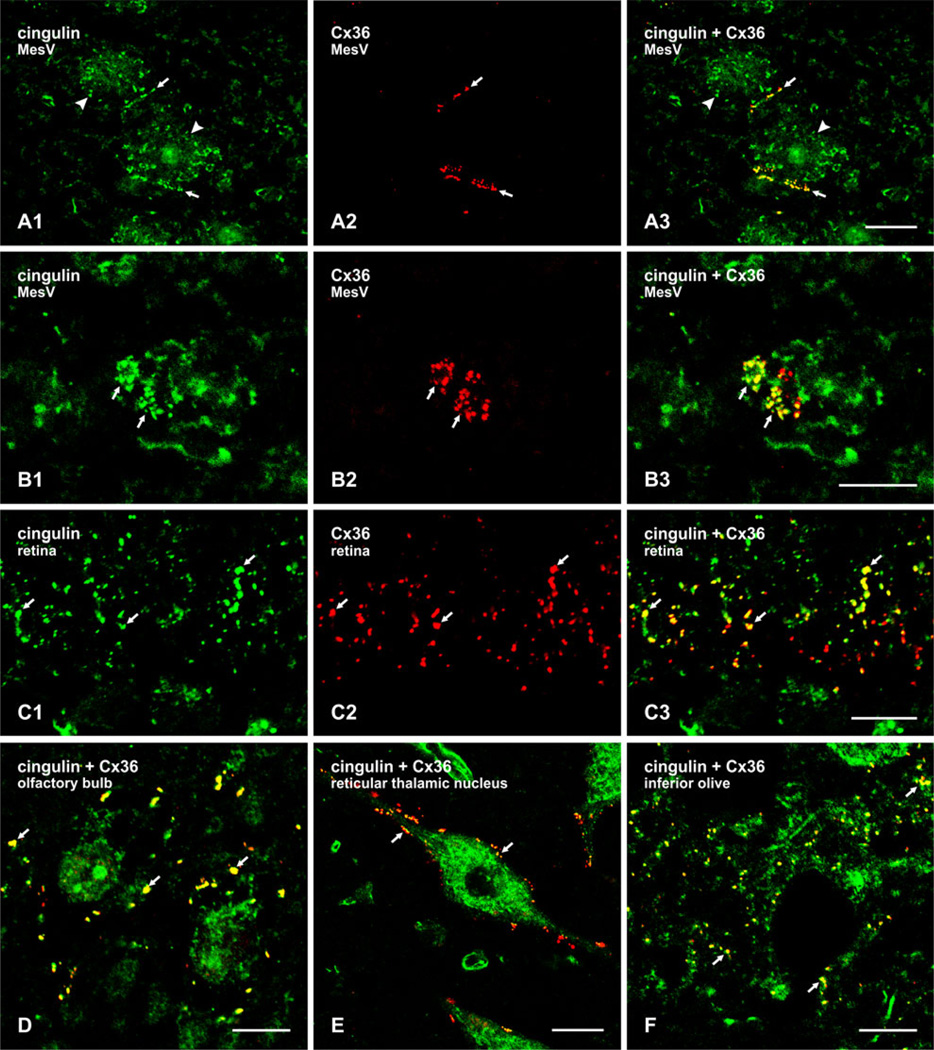
On the basis of structural similarities between tight junctions, adherens junctions and gap junctions, each located at plasma membrane appositions with proteinacious substructure, close relationships of the former two junctions with cytoskeletal elements, and the co-occurrence of at least three proteins at these junctions (ZO-1, MUPP1 and AF6), it might be predicted that gap junctions may also harbor cytoskeletal-related proteins. The first of these we have examined in numerous brain structures is cingulin. In the MesV nucleus, labeling for cingulin at cell-cell contacts had an appearance very similar to that of labeling for MUPP1 and Cx36, at both appositions viewed on edge (Fig. 2a) or en face (Fig. 2b). Punctate labeling for cingulin at appositions was robust (Fig. 2a1, b1), and Cx36-puncta (Fig. 2a2, b2) were nearly always colocalized with cingulin- immunopositive puncta (Fig. 2a3, b3). MesV somata contained additional cingulin immunoreactivity that was localized intracellularly and not associated with Cx36 (Fig. 2a1, b1). Further examples of cingulin colocalization with Cx36 are shown in the inner plexiform layer in sections of retina (Fig. 2c), and by image overlay only (cingulin, green; Cx36, red; green/red overlap, yellow) in the mitral cell layer of the olfactory bulb (Fig. 2d), the reticular thalamic nucleus (Fig. 2e) and the inferior olive (Fig. 2f). In these four brain regions, cingulin/Cx36 colocalization was not complete as there appeared to be small subpopulations of Cx36-positive puncta in each region that were devoid of labeling for cingulin, suggesting that cingulin/Cx36 association may be a dynamic process. Similar results concerning colocalization of these two proteins were obtained in numerous other brain regions, including cerebral cortex, hippocampus, striatum, midbrain and various areas of brainstem (not shown).
Fig. 2.
Laser scanning confocal double immunofluorescence labeling of cingulin and Cx36 in various regions of adult mouse brain. a The same field (a1–a3) showing neuronal somata (arrowheads) in the MesV, with punctate labeling of cingulin (a1, arrows) and Cx36 (a2, arrows) at appositions between somata, and colocalization of these puncta, as seen by red/green overlap in overlay (a3, arrows). b En face view of MesV neuronal somatic apposition, showing clusters of cingulin-immunopositive (b1, arrows) and Cx36-positive puncta (b2, arrows), with colocalization of the majority of these puncta. c The same field (c1–c3) of a vertical section of retina, showing the inner plexiform layer. Labeling for both cingulin (c1, arrows) and Cx36 (c2, arrows) is punctate, and the majority of Cx36-immunopositive puncta is also immunopositive for cingulin, as seen by yellow in overlay (a3, arrows). d–f Double immunofluorescence punctate labeling of cingulin (green) and Cx36 (red), shown only by image overlay in the olfactory bulb at the level of the mitral cell layer (d), the reticular thalamic nucleus (e), and the inferior olivary nucleus (f). Cingulin/Cx36 colocalization seen as yellow puncta (arrows) are evident in each of the brain areas. Scale bars = a, e, 20 µm; b, c, d, f, 10 µm
Discussion
Although ultrastructural confirmation of the localization of AF6,MUPP1, and cingulin to neuronal gap junctions has yet to be conducted, their immunohistochemical colocalization with Cx36 is strongly indicative of their association with electrical synapses. Electrical synapses are found to occur in various neuronal subtypes and appear to have diverse functions and are likely subject to regulation by diverse mechanisms. We previously reported heterogeneous immunolabeling for AF6 and MUPP1 at electrical synapses, which appeared not only across different brain regions, but was also evident within individual brain nuclei. This heterogeneity may reflect differential expression or requirement for these proteins in various electrically coupled neuronal subtypes, or may reflect the dynamic functional and/or structural states of neuronal gap junctions. Cingulin appeared to be more consistently associated with neuronal gap junctions, although the proportion of these junctions containing cingulin remains to be determined by quantitative immunofluorescence approaches.
Like gap junctions between other cell types, it is known that those forming electrical synapses in brain are highly regulated. For example, modulation of gap junctional coupling between neurons is proposed to underlie rapid shifts in neuronal network connectivity (Schmitz et al. 2001). Further, neuronal gap junctions exhibit rapid alteration in coupling state in response to neuromediators (Landisman and Connors 2005; Hatton 1997), and have the same high turnover rate (half-life, 1–5 h) as gap junctions in other cell types (Flores et al. 2012). Various neurotransmitters contained in widespread CNS systems that have a broad influence on cognition are known to alter transmission at electrical synapses. These include dopamine (Cepeda et al. 1989; Hampson et al. 1992; He et al. 2000; Onn and Grace 1994, 1995, 1999; Onn et al. 2000; Rorig et al. 1995), serotonin (Rorig and Sutor 1996), histamine (Hatton and Yang 2001; He et al. 2000; Yang and Hatton 2002), acetylcholine (Perez Velazquez et al. 1997) and noradrenaline (Hopkins and Johnston 1988). Some of these transmitters are acted upon by drugs used to treat neurological disorders (e.g., Parkinson’s disease, schizophrenia, depression) and by drugs of addiction (e.g., cocaine, amphetamine). In view of these points, therapeutic and addictive drugs may exert their actions in part by modifying the activities of transmitters that regulate signal transmission at electrical synapses. For example, the antipsychotic drug haloperidol and withdrawal from chronic amphetamine increased neuronal gap junctional coupling in the basal ganglia (Onn and Grace 1994, 1995; Onn et al. 2000), and amphetamine and cocaine in animal models of addiction cause altered expression of Cx36 (McCracken et al. 2005a, b).
Just as understanding the biochemical machinery involved in chemical synaptic transmission has served as a basis for deciphering sites and mechanisms of drug action at chemical synapses, so too, knowledge of structural and regulatory components of electrical synapses is expected to provide insight into mechanisms for potential actions of drugs on cellular processes that regulate electrical synapses. Such knowledge is also essential for understanding how malfunction of these synapses may contribute to CNS disease. Despite the wealth of data indicating the dynamic nature of neuronal gap junctions, very little is known about the signaling pathways or the molecular mechanisms that underlie the regulation of electrical synaptic transmission. Indeed, it has been emphasized that a major barrier to progress in understanding electrical synapses in mammalian brain is the paucity of information on mechanisms of their regulation (Bennett and Zukin 2004; Connors and Long 2004; Hormuzdi et al. 2004). Identification of protein components of neuronal gap junctions represents a step toward eliminating that barrier, as discussed below.
Occurrence of ZO-1 at Gap Junctions
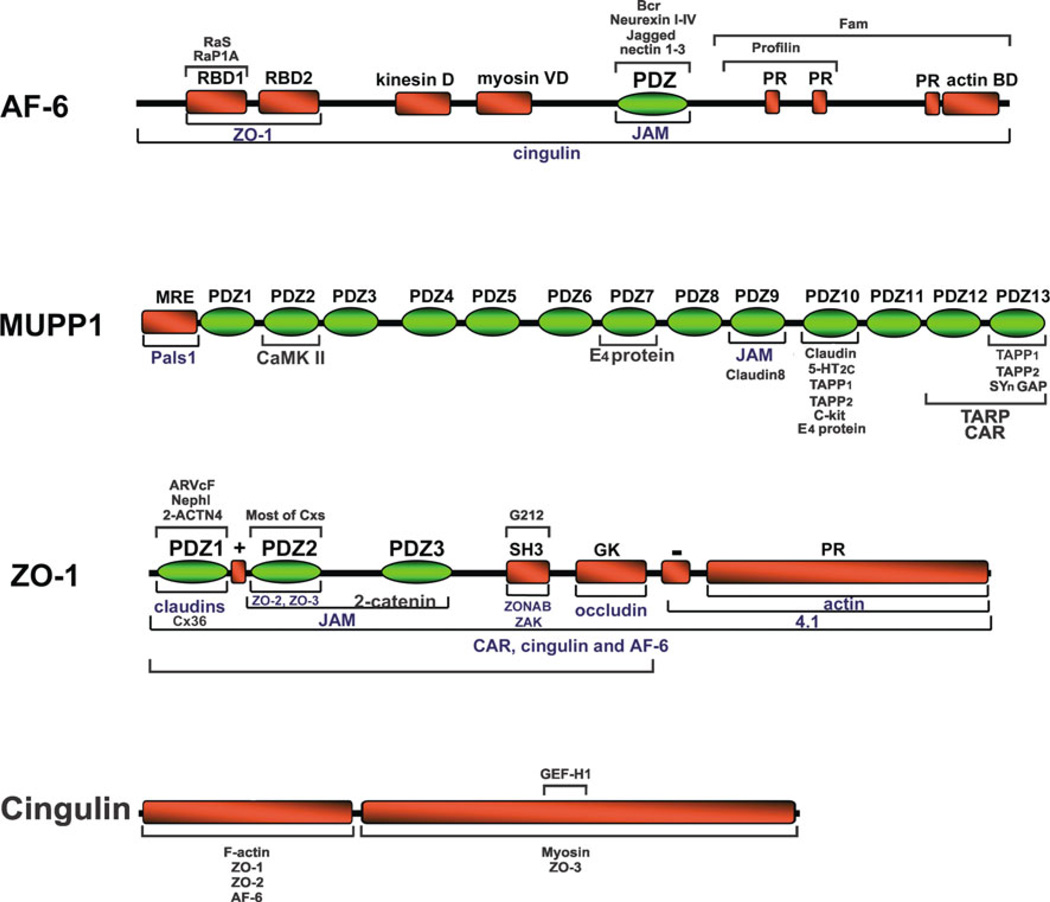
In its capacity as a multi-domain scaffolding protein (Fig. 3), ZO-1 interacts with transmembrane structural proteins of intercellular junctions, where it directs the assembly of other adaptor, structural and signaling proteins into functional membrane-associated complexes. ZO-1 is found at various classes of intercellular junctions and, at least in the case of gap junctions formed by connexin43, was found to be essential for gap junctional intercellular communication, gap junction accretion and disassembly (Akoyev and Takemoto 2007; Hunter et al. 2005; Laing et al. 2005). In HeLa cells expressing C-terminal-tagged connexin43 (Cx43) that lacked its capacity for binding of ZO-1, the usual internalization of Cx43 in response to inflammatory mediators did not occur, demonstrating that ZO-1 interactions with Cx43 is required for gap junction internalization (Baker et al. 2008). In lens epithelial cells, silencing of ZO-1 expression caused a stable interaction of protein kinase C-γ (PKC-γ) with Cx43-containing gap junctions, a complete loss of junctional dye transfer, and loss of the usual gap junction disassembly seen in response to TPA activation of PKC-γ, indicating a requirement for ZO-1 in disassembly of these junctions (Akoyev and Takemoto 2007). In lens and in vitro, unlike other connexins such as Cx43 that appear able to form gap junctions in the absence of direct interactions with ZO-1 (Hunter et al. 2005; Hunter and Gourdie 2008), the formation of functional gap junctions by Cx50 was recently shown to require the interaction of the C-terminus PDZ ligand of this connexin with ZO-1 (Chai et al. 2011). Although these studies directly address molecular processes that play a role in the essential function of ZO-1 at gap junctions, for the most part little is known at the molecular level regarding the exact mechanisms by which ZO-1 exerts its actions at these structures. Clues to mechanisms involved in ZO-1 actions on junction structure and signaling may be gleaned from studies of the proteins that ZO-1 is known to recruit in other systems.
Fig. 3.
Diagram of the domain structure of AF6, MUPP1, ZO-1, and cingulin. Bars above and below protein domain structures indicate known sites of interaction with other proteins. Where interaction has been documented but sites of interaction are not known, the bars span the entire length of the protein. (Modified from Gonzalez-Mariscal et al. 2003)
Molecular Organization of Tight and Adherens Junctions
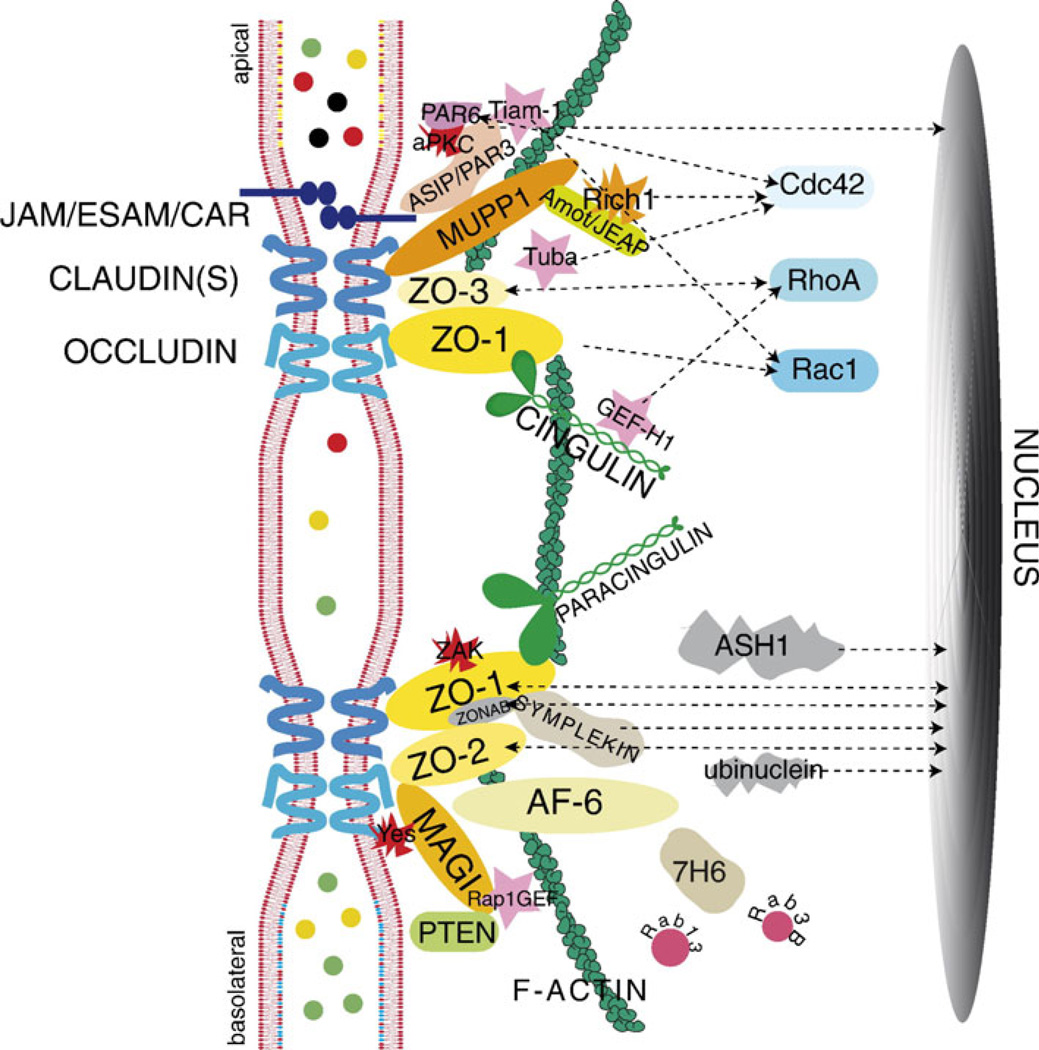
It is becoming clear that some of the proteins long known to be present at tight and adherens junctions also occur at gap junctions forming electrical synapses, suggesting that there may be other molecular commonalities between these three types of junctions. In considering future directions for deciphering the macromolecular organization of electrical synapses, it may be instructive to take brief account of the somewhat larger body of knowledge concerning the molecular components of tight junctions and adherens junctions. Tight junctions serve to control paracellular permeability across the intercellular space and to maintain cell polarity. They are composed of integral transmembrane proteins (Fig. 4) (for more detailed reviews, see Guillemot et al. 2008; Herve et al. 2011), including claudins, junctional adhesion molecules (JAMS) and the MARVEL family of proteins, of which the occludens are members. In addition, the cytoplasmic ‘‘submembrane plaque’’ of tight junctions contains multimolecular protein complexes that are responsible for anchoring the core proteins within the junction, regulating associations with the actin cytoskeleton (Fanning et al. 1998, 2002) and mediating signals from the plasma membrane to other cellular structures, including the nucleus and to adherens junctions. Many of the protein-protein interactions at tight junctions are mediated by binding of C-terminal PDZ ligands in some proteins to PDZ domains contained in other proteins. The three members of the ZO family each contain three PDZ domains that mediate a host of interactions. The ZO family members are considered the central scaffolding molecules of tight junctions, as indicated by failure of tight junction strand formation after targeted deletion of ZO-1 and depletion of ZO-2 in cultured epithelial cells (Umeda et al. 2006). In addition to its interaction with other proteins, ZO-1 can undergo self interaction forming a homodimer, which is thought to be mediated via its second PDZ domain (Utepbergenov et al. 2006). Other PDZ domain-containing proteins within the tight junction plaque include AF6 and MUPP1. In addition, there are several non-PDZ proteins in the tight junction cytoplasmic plaque, including cingulin and paracingulin, as well as a growing list of functionally diverse signaling proteins that include: regulators of membrane trafficking, protein kinases and regulators of small GTPases, such as guanine exchange factors (GEFs) and GTPase activating proteins (GAPs) (reviewed in Guillemot et al. 2008). Besides their roles in junction formation and stabilization, some of the tight junction-associated proteins are thought to serve as signaling molecules for regulation of such processes as cell differentiation, proliferation, and gene expression.
Fig. 4.
Diagram of protein organization at tight junctions. The tight junction proteins JAM/ESAM/CAR, claudins, and occludin are shown crossing the membrane bilayers of 2 adjacent cells. These core proteins are shown interacting with various cytoplasmic proteins. Signaling proteins found at tight junctions include kinases (aPKC, ZAK, c-Yes), GEFs (Tiam1, GEF-H1, Tuba, Rap1GEF), and membrane traffic regulators (Rab3B and Rab13). Interactions of cytoplasmic tight junction proteins with signaling proteins (Cdc42, RhoA, and Rac1) are indicated by dotted arrows. Arrows to the nucleus indicate proteins having a dual nuclear/ junctional localization (PAR-3, PAR-6, ASH1, ZO-1, ZO-2, ZONAB, symplekin, ubinuclein). (Reprinted from Guillemot et al. 2008, with permission from Elsevier)
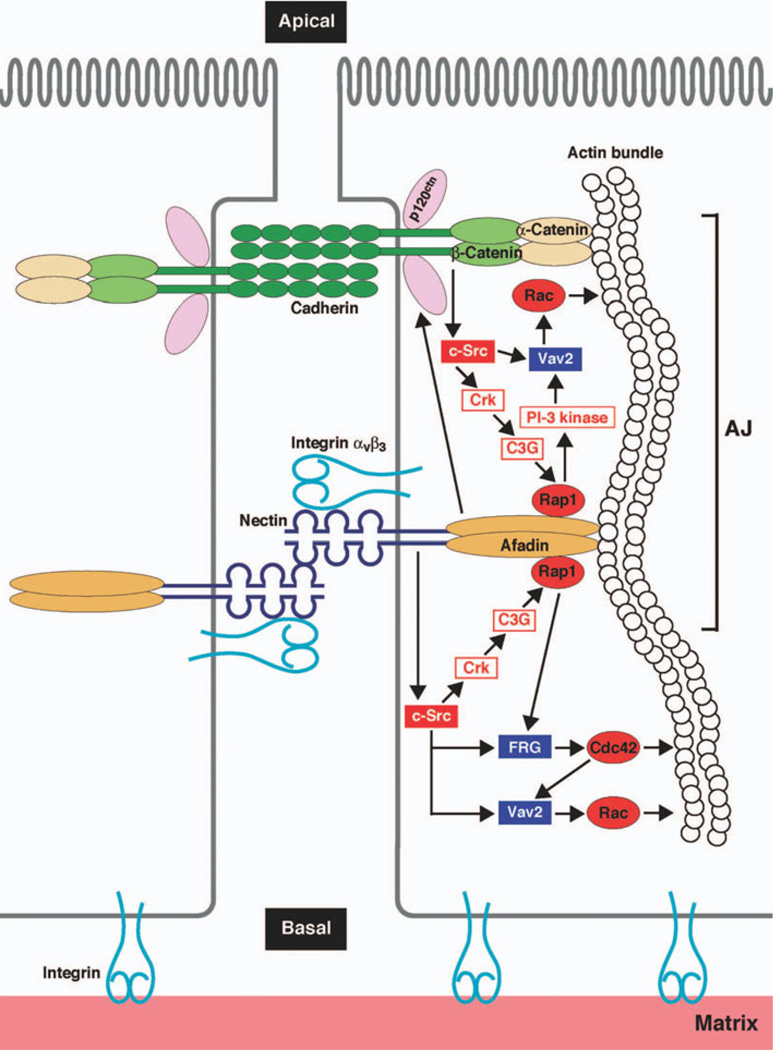
Adherens junctions (Fig. 5) (Ogita and Takai 2006) are also molecularly complex structures whose functions include the initiation and stabilization of intercellular adhesion, as well as regulation of intracellular signaling, the cytoskeleton, and gene expression (Niessen and Gottardi 2008). There are several subtypes of adherens junctions, including zonula adherens, which are found in highly polarized epithelial cells, where they encircle the cell in a belt-like fashion. Punctate versions of adherens junctions include puncta adherentia or nascent/primordial junctions, which are formed by interactions of cadherins, the nectin family of adhesion molecules and with the cytoplasmic catenin proteins. ZO-1 is among the PDZ domain proteins found at adherens junctions, where it interacts with various proteins, including alpha catenin and AF6 (aka afadin). These observations on tight and adherens junctions provide the molecular basis for considerations of regulatory and structural proteins as well as intracellular signaling pathways that contribute to modulation of transmission at electrical synapses composed of Cx36.
Fig. 6.
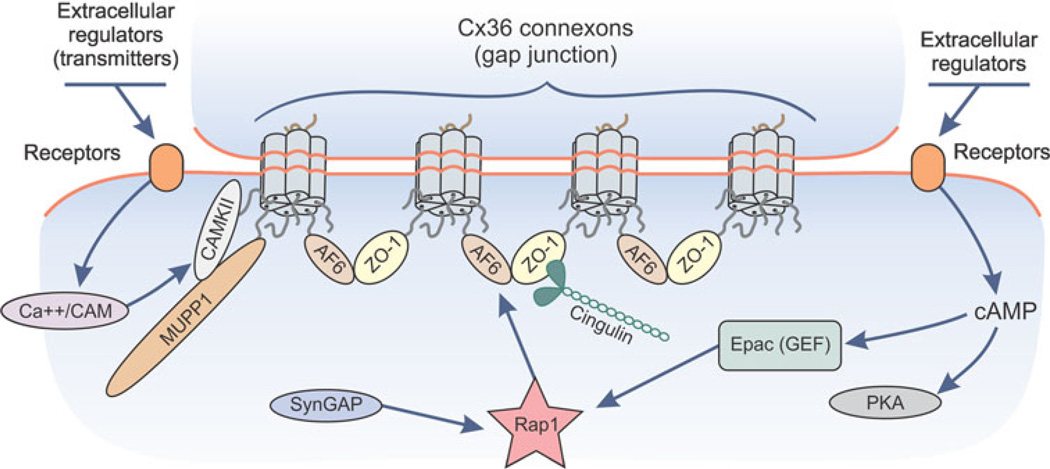
Hypothesized model for actions of the cAMP-Epac-Rap1 signaling pathway at neuronal gap junctions. The c-terminus PDZ domain ligand of Cx36 within different connexon hexamers are shown associating with ZO-1 and AF6. Association of ZO-1 and AF6 with each other is envisioned to result in clustering of connexons and maintenance of gap junction plaque integrity. Upon cAMP activation of the guanine nucleotide exchange factor (GEF) Epac, which acts on Rap1, Rap1 may be targeted to AF6, where it may either further stabilize junctions or disrupt ZO-1/AF6 association, resulting in untethering of a portion of connexons within a gap junction plaque and internalization of these connexons. SynGAP is shown because it is a GTPase activating protein (GAP) for Rap1 and is known to associate with MUPP1 at other subcellular locations, but as yet, neither Rap1 nor SynGAP has been examined for its presence at neuronal gap junctions
Scaffolding by MUPP1 at Electrical Synapses
Our previous finding of MUPP1 at neuronal gap junctions and demonstration of its direct interaction with the PDZ ligand of Cx36 added to the list of proteins that associate with PDZ domains of MUPP1 in several cell types and at various cell structures (Ullmer et al. 1998; Becamel et al. 2001; Ebnet et al. 2004; Krapivinsky et al. 2004; Balasubramanian et al. 2007; Guillaume et al. 2008). Presently, we extend that finding to its localization at electrical synapses between sensory neurons of the MesV nucleus in rodent brain, where it has been emphasized that experimental accessibility to the large MesV neuronal cell bodies is ideal for electrophysiological analysis of mechanisms controlling neurotransmission at gap junctions using in vitro preparations (Curti et al. 2012a, b).
Analogous to other systems where MUPP1 serves in an accessory scaffolding capacity for structural and signaling proteins, as indicated by its domain structure (Fig. 3),MUPP1 likely serves to anchor regulatory proteins at gap junctions composed of Cx36. In this context, reports of calcium-calmodulin kinase II (CaMKII) interaction with MUPP1 is of particular interest as various kinases, including protein kinase A (PKA), protein kinase G (PKG) and CaMKII, have an impact on phosphorylation status and coupling state of gap junctions composed of Cx36 or its fish ortholog Cx35 (Pereda et al. 1998; Ouyang et al. 2005; Kothmann et al. 2007; Moreno and Lau 2007). More recently, CaMKII was found to localize at neuronal gap junctions and to interact with specific peptide fragments ofCx36, culminating in phosphorylation of these fragments (Alev et al. 2008). Although phosphorylation of Cx36 mediated by CaMKII would require an effector/ ligand interaction, CaMKII is tethered to some of its sites of action in neurons by interacting with the PDZ2 domain of MUPP1 (Krapivinsky et al. 2004). Thus, it is possible that this same CaMKII/MUPP1 interaction occurs at gap junctions, where further interaction of Cx36 with the PDZ10 domain of MUPP1 poises CaMKII in a response-ready position for rapid signaling at electrical synapses.
Signaling and Scaffolding by Cingulin at Electrical Synapses
The current finding of cingulin colocalized with Cx36 adds yet another ZO-1 binding protein found at other types of cell-cell junctions to the roster of proteins at neuronal gap junctions in mouse brain. This provides further clues to the regulation of electrical synapses, although the molecular association of cingulin with Cx36 at neuronal gap junctions is not known and is currently under investigation by biochemical approaches. Cingulin was characterized as a component of the submembranous plaque of tight junctions, where it may be anchored as a parallel homodimer, as predicted by its secondary structure (Citi et al. 1989). It has three distinct structural domains; an N-terminal head domain, a central coiled-rod domain and a smaller globular tail (Cordenonsi et al. 1999). Interaction of cingulin with ZO-1 is not through a classical C-terminal PDZ ligand, which cingulin lacks, but rather through a conserved ZO-1 interaction motif (ZIM) located in the cingulin N-terminus ‘‘head’’ region (Cordenonsi et al. 1999; D’Atri et al. 2002). In addition to its association with ZO-1, cingulin interacts with other proteins (Fig. 3) at tight junctions, including alpha actin (D’Atri and Citi 2001). Association of actin with gap junctions appears to be important for connexin trafficking, and gap junction stability and channel permeability (Theiss and Meller 2002; Derangeon et al. 2008; Qu et al. 2009; Smyth et al. 2012). Besides its potential structural roles, cingulin participates in modification of gene expression via its direct binding to the guanine exchange factor GEF-H1, which is an activator of RhoA signaling. It appears that sequestration of GEF-H1 by cingulin at tight junctions contributes to inhibition of RhoA (Citi et al. 2009; Aijaz et al. 2005). In the context of gap junctions, altered RhoA activity in cardiac myocytes leads to rapid changes in gap junctional channel conductance via RhoA actions on the actin cytoskeleton (Derangeon et al. 2008). Although we have not yet determined if cingulin is present at gap junctions composed of other connexins, our finding of cingulin association with neuronal gap junctions raises the possibility that it may influence the stability and channel conductance of electrical synapses by a RhoA dependent mechanism similar to that seen in cardiac myocytes. It remains to be determined whether neuronal gap junctions also serve as platforms for cingulin-mediated signaling to other subcellular sites in neurons.
Electrical Synapses and cAMP/Epac/Rap1 Signaling
AF6 is targeted by the Epac/Rap1-dependant cAMP pathway that is independent of PKA. Although regulation of electrical synapses by cAMP/Epac signaling has not been directly explored, regulatory actions of cAMP on neuronal gap junction coupling have been described (Hampson et al. 1992; Hatton and Yang 2001; Rorig et al. 1995; Urschel et al. 2006; Xia and Mills 2004). Our finding of AF6 at gap junctions composed of Cx36 suggests that the Epac/Rap1 pathway may be operational at electrical synapses. AF6 is targeted by Rap1 after its activation by the cAMP effector Epac (de Rooij et al. 1998; Boettner et al. 2000, 2001, 2003; Caron 2003), a GEF that is distinct from the other major cAMP effector PKA. Epac-Rap1-AF6 signaling has been demonstrated in various cell types and contributes to the regulation of cell-cell contacts (e.g., tight junctions) through its influence on AF6/ZO-1 interaction (Yamamoto et al. 1997, 1999; Kooistra et al. 2006). To date there is one study concerning the impact of Epac on gap junctions, where PKA versus Epac activation had differential, but cooperative actions on cardiomyocyte gap junctions; Epac activation increased the recruitment of Cx43 to junctions, while PKA activation increased dye-coupling (Somekawa et al. 2005). To dissect the specific functions of these two separate pathways, cAMP analogs have been developed that activate either one or the other; 8-CPT-2Me-cAMP (8CPT) specifically activates Epac but not PKA (Enserink et al. 2002; Somekawa et al. 2005), whereas N6-benzoylcAMP specifically activates PKA but not Epac (Christensen et al. 2003; Holz et al. 2008; Lorenowicz et al. 2008).
In consideration of the foregoing, the presence of AF6 at electrical synapses suggests that its associated signaling moieties may contribute to the regulation of these synapses. Specifically, as outlined in Fig. 6, we propose that cAMP activation of Epac/Rap1 results in Rap1 targeting to AF6 and ZO-1 at neuronal gap junctions, where it may influence AF6/ZO-1 interaction and dynamically impact junction assembly/disassembly, channel conductance, remodeling and/or turnover. ZO-1 is found at virtually all Cx36-containing electrical synapses that we have examined, suggesting that some of these synapses simultaneously harbor ZO-1, AF6, MUPP1 and cingulin. As yet, we are aware of no reports of direct interactions between AF6 and MUPP1 at any cellular structures. But, as indicated in the summary of the domain structures and interactions of these three proteins in Fig. 3, ZO-1 has the capacity to simultaneously interact with AF6 and Cx36, but whether it does so at neuronal gap junctions remains to be determined. It is conceivable that ZO-1, AF6 and MUPP1 simultaneously interact with Cx36 at individual gap junctions, or each of these proteins associate with the constituent connexin molecules within an individual connexon. Additionally, connexins within distinct subregions of a neuronal gap junction plaque may selectively associate with one or more of these proteins. The latter may be of particular relevance to the potentially unique functional roles of ZO-1, AF6, MUPP1 and cingulin at electrical synapses, impacting on the reported segregation of assembly and disassembly of gap junctions that occurs at the periphery and centers, respectively, of the plaque (Segretain and Falk 2004).
The alternatively spliced AF6 isoform lacking the F-actin binding domain influences the interaction of this isoform with the cytoskeleton and may influence the effector functions of AF6 (Lorger and Moelling 2006). We have shown that both of these isoforms coimmunoprecipitate with Cx36 (Li et al. 2012). It remains to be determined if the relative proportion of the full-length and truncated isoforms targeted to gap junctions is dependent on dynamic changes in the requirements of electrical synapses for interactions with cytoskeletal elements to maintain stability or promote turnover.
Fig. 5.
Diagram of the molecular organization of nectin- and cadherin-based adherens junctions. Core transmembrane proteins cadherin and nectin are shown associated with cytoplasmic proteins, in particular cadherin with catenin anchored to the cytoskeleton, and nectin with cytoskeletal-associated afadin (aka AF6). Afadin is also shown associated with Rap1, which receives signals originating from catenin and c-Src and transmits signals ultimately to Rac and the cytoskeleton. (Reprinted from Ogita and Takai 2006, with permission from John Wiley and Sons)
Acknowledgments
Supported in part by grants from the Canadian Institutes of Health Research (MOP 106598) to J.I.N., and from the National Institutes of Health (NS31027, NS44010, NS44395) to J. E. Rash with a subaward to J.I.N. We thank B. McLean for excellent technical assistance.
References
- Aijaz S, D’Atri F, Citi S, Balda MS, Matter K. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev Cell. 2005;8:777–786. doi: 10.1016/j.devcel.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Akoyev V, Takemoto DJ. ZO-1 is required for protein kinase C gamma-driven disassembly of connexin43. Cell Signal. 2007;19:958–967. doi: 10.1016/j.cellsig.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alev C, Urschel S, Sonntag S, Zoidl G, Fort AG, Höher T, Matsubara M, Willecke K, Spray DC, Dermietzel R. The neuronal connexin36 interacts with and is phosphorylated by CaMKII in a way similar to CaMKII interaction with gluamate receptors. Proc Natl Acad Sci (USA) 2008;105:20964–20969. doi: 10.1073/pnas.0805408105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R, Llinas R. Electrotonic coupling between neurons in the rat mesencephalic nucleus. J Physio. 1971;212:45–63. doi: 10.1113/jphysiol.1971.sp009309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SM, Kim N, Gumpert AM, Segretain D, Falk MM. Acute internalization of gap junctions in vascular endothelial cells in response to inflammatory mediator-induced G-protein coupled receptor activation. FEBS Lett. 2008;582:4039–4046. doi: 10.1016/j.febslet.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian S, Fam SR, Hall RA. GABAb receptor association with the PDZ scaffold MUPP1 alters receptor stability and function. J Biol Chem. 2007;282:4162–4171. doi: 10.1074/jbc.M607695200. [DOI] [PubMed] [Google Scholar]
- Baude A, Bleasdale C, Dalezios Y, Somogyi P, Klausberger T. Immunoreactivity for GABAa receptor alpha1 subunit, somatostatin and connexin36 distinguishes axoaxonic, basket and bistratified interneurons of the rat hippocampus. Cereb Cortex. 2007;17:2094–2107. doi: 10.1093/cercor/bhl117. [DOI] [PubMed] [Google Scholar]
- Becamel C, Figgs A, Poliak S, Dumuis A, Peles E, Bockaert J, Lubbert H, Ullmer C. Interaction of serotonin 5-hydroxytryptamine type 2C receptors with PDZ10 of the multi-PDZ domain protein MUPP1. J Biol Chem. 2001;276:12974–12982. doi: 10.1074/jbc.M008089200. [DOI] [PubMed] [Google Scholar]
- Bennett MVL. Gap junctions as electrical synapses. J Neurocytol. 1997;26:349–366. doi: 10.1023/a:1018560803261. [DOI] [PubMed] [Google Scholar]
- Bennett MVL, Zukin SR. Electrical coupling and neuronal synchronization in the mammalian brain. Neuron. 2004;41:495–511. doi: 10.1016/s0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- Boettner B, Govek EE, Cross J, Van Aelst L. The junctional multidomain protein AF-6 is a binding partner of the Rap1A GTPase and associates with the actin cytoskeletal regulator profilin. Proc Natl Acad Sci USA. 2000;97:9064–9069. doi: 10.1073/pnas.97.16.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettner B, Herrmann C, Van Aelst L. Ras and Rap1 interaction with AF-6 effector target. Methods Enzymol. 2001;332:151–168. doi: 10.1016/s0076-6879(01)32199-7. [DOI] [PubMed] [Google Scholar]
- Boettner B, Harjes P, Ishimaru S, Heke M, Fan HQ, Qin Y, Van Aelst L, Gaul U. The AF-6 homolog canoe acts as a Rap1 effector during dorsal closure of the Drosophila embryo. Genetics. 2003;165:159–169. doi: 10.1093/genetics/165.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron E. Cellular functions of the Rap1 GTP-binding protein: a pattern emerges. J Cell Sci. 2003;116:435–440. doi: 10.1242/jcs.00238. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Walsh JP, Hull CD, Howard SG, Buchwald NA, Levine MS. Dye-coupling in the neostriatum of the rat: I. Modulation by dopamine-depleting lesions. Synapse. 1989;4:229–237. doi: 10.1002/syn.890040308. [DOI] [PubMed] [Google Scholar]
- Chai Z, Goodenough DA, Paul DL. Cx50 requires an intact PDZ-binding motif and ZO-1 for the formation of functional intercellular channels. Mol Biol Cell. 2011;22:4503–4512. doi: 10.1091/mbc.E11-05-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AE, Selheim F, De Rooij J, Dremier S, Schwede F, Dao KK, Martinez A, Maenhaut C, Bos JL, Genieser H-G, Doskenland SO. cAMP analog mapping of Epac1 and cAMP kinase. J Biol Chem. 2003;278:35394–35402. doi: 10.1074/jbc.M302179200. [DOI] [PubMed] [Google Scholar]
- Ciolofan C, Li X, Olson O, Kamasawa N, Yasumura T, Morita M, Rash JE, Nagy JI. Association of Connexin36 and ZO-1 with ZO-2 and the MsY3 transcription factor ZO-1-associated nucleic acid-binding protein (ZONAB) in mouse retina. Neuroscience. 2006;140:433–451. doi: 10.1016/j.neuroscience.2006.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi S, Sabanay H, Kendrick-Jones J, Geiger B. Cingulin: characterization and localization. J Cell Sci. 1989;93:107–122. doi: 10.1242/jcs.93.1.107. [DOI] [PubMed] [Google Scholar]
- Citi S, Paschoud S, Pulimeno P, Timolati F, De Robertis F, Jond L, Guillemot L. The tight junction protein cingulin regulates gene expression and RhoA signaling. Ann NY Acad Sci. 2009;1165:88–98. doi: 10.1111/j.1749-6632.2009.04053.x. [DOI] [PubMed] [Google Scholar]
- Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu Rev Neurosci. 2004;27:393–418. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- Cordenonsi M, D’Atri F, Hammar E, Parry DA, Kendrick-Jones J, Shore D, Citi S. Cingulin contains globular and coiled-coil domains and interacts with ZO-1, ZO-2, ZO-3, and myosin. J Cell Biol. 1999;147:1569–1582. doi: 10.1083/jcb.147.7.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curti S, Hoge G, Nagy JI, Pereda A. Synergy between electrical coupling and membrane properties promotes strong synchronization of neurons of the mesencephalic trigeminal nucleus. J Neurosci. 2012a;32:4341–4359. doi: 10.1523/JNEUROSCI.6216-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curti S, Hoge G, Nagy JI, Pereda A. Electrical transmission between mammalian neurons is supported by a small fraction of gap junction channels. J Membrane Biol. 2012b doi: 10.1007/s00232-012-9449-z. [DOI] [PubMed] [Google Scholar]
- D’Atri F, Citi S. Cingulin interacts with F-actin in vitro. FEBS Lett. 2001;507:21–24. doi: 10.1016/s0014-5793(01)02936-2. [DOI] [PubMed] [Google Scholar]
- D’Atri F, Nadalutti F, Citi S. Evidence for a functional interaction between cingulin and ZO-1 in cultured cells. J Biol Chem. 2002;277:27757–27764. doi: 10.1074/jbc.M203717200. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guaninenucleotide- exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- Derangeon M, Bourmeyster N, Plaisance I, Pinet-Charvet C, Chen Q, Duthe F, Popoff MR, Sarrouilhe D, Hervé JC. RhoA GTPase and F-actin dynamically regulate the permeability of Cx43-made channels in rat cardiac myocytes. J Biol Chem. 2008;283:30754–30765. doi: 10.1074/jbc.M801556200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derangeon M, Spray DC, Bourmeyster N, Sarrouilhe D, Herve JC. Reciprocal influence of connexins and apical junction proteins on their expressions and functions. Biochim Biophys Acta. 2009;1788:768–778. doi: 10.1016/j.bbamem.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet K, Suzuki A, Ohno S, Vestweber D. Junctional adhesion molecules (JAMs): more molecules with dual functions? J Cell Sci. 2004;117:19–29. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Døskeland SO, Blank JL, Bos JL. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol. 2002;4:901–906. doi: 10.1038/ncb874. [DOI] [PubMed] [Google Scholar]
- Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- Fanning AS, Ma TY, Anderson JM. Isolation and functional characterization of the actin binding region in the tight junction protein ZO-1. FASEB J. 2002;16:1835–1837. doi: 10.1096/fj.02-0121fje. [DOI] [PubMed] [Google Scholar]
- Flores CE, Li X, Bennett MVL, Nagy JI, Pereda AE. Connexin35 and Zonula Occludens-1 directly interact: implications for the regulation of electrical transmission. Proc Natl Acad Sci USA. 2008;105:12545–12550. doi: 10.1073/pnas.0804793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CE, Nannapaneni S, Davidson KG, Yasumura T, Bennett MV, Rash JE, Pereda AE. Trafficking of gap junction channels at a vertebrate electrical synapse in vivo. Proc Natl Acad Sci USA. 2012;109:E573–E582. doi: 10.1073/pnas.1121557109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuduka T, Kosaka T, Singer W, Galuske RAW. Gap junctions among dendrites of cortical GABAergic neurons establish a dense and widespread intercolumnar network. J Neurosci. 2006;26:3434–3443. doi: 10.1523/JNEUROSCI.4076-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol. 2003;81:1–44. doi: 10.1016/s0079-6107(02)00037-8. [DOI] [PubMed] [Google Scholar]
- Guillaume JL, Daulat AM, Maurice P, Levoye A, Migaud M, Brydon L, Malpaux B, Borg-Capra C, Jockers R. The PDZ protein MUPP1 promotes Gi coupling and signaling of the Mt1 melatonin receptor. J Biol Chem. 2008;283:16762–16771. doi: 10.1074/jbc.M802069200. [DOI] [PubMed] [Google Scholar]
- Guillemot L, Foglia A, Paschoud S, Pulimeno P, Citi S. The cytoplasmic plaque of tight junctions: a scaffolding and signalling center. Biochim Biophys Acta. 2008;1778:601–613. doi: 10.1016/j.bbamem.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Hampson ECGM, Vaney DI, Weiler R. Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina. J Neurosci. 1992;12:4911–4922. doi: 10.1523/JNEUROSCI.12-12-04911.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton GI. Function-related plasticity in hypothalamus. Ann Rev Neurosci. 1997;20:375–397. doi: 10.1146/annurev.neuro.20.1.375. [DOI] [PubMed] [Google Scholar]
- Hatton GI, Yang QZ. Ionotropic histamine receptors and H2 receptors modulate supraoptic oxytocin neuronal excitability and dye coupling. J Neurosci. 2001;21:2974–2982. doi: 10.1523/JNEUROSCI.21-09-02974.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Weiler R, Vaney DL. Endogenous dopaminergic regulation of horizontal cell coupling in the mammalian retina. J Comp Neurol. 2000;418:33–40. doi: 10.1002/(sici)1096-9861(20000228)418:1<33::aid-cne3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Herve J-C, Derangeon M, Sarrouilhe D, Geipmans BNG, Bourmeyster N. Gap junctional channels are parts of multiprotein complexes. Biochem Biophys Acta. 2011;1818:1844–1865. doi: 10.1016/j.bbamem.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Hinrichsen CF. Coupling between cells of the trigeminal mesencephalic nucleus. J Dent Res. 1970;49(suppl):1369–1373. doi: 10.1177/00220345700490063701. [DOI] [PubMed] [Google Scholar]
- Hinrichsen CF, Larramendi LM. The trigeminal mesencephalic nucleus. II. Electron microscopy. Am J Anat. 1970;127:303–319. doi: 10.1002/aja.1001270306. [DOI] [PubMed] [Google Scholar]
- Holz GG, Chepurny OG, Schwede F. Epac-selective cAMP analogs; new tools with which to evaluate the signal transduction properties of cAMP-regulated guanine nucleotide exchange factors. Cell Signal. 2008;20:10–20. doi: 10.1016/j.cellsig.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WF, Johnston D. Noradrenergic enhancement of longterm potentiation at mossy fiber synapses in the hippocampus. J Neurophysiol. 1988;59:667–687. doi: 10.1152/jn.1988.59.2.667. [DOI] [PubMed] [Google Scholar]
- Hormuzdi SG, Filippov MA, Mitropoulon G, Monyer H, Bruzzone R. Electrical synapses: a dynamic signaling system that shapes the activity of neuronal networks. Biochem Biophys Acta. 2004;1662:113–137. doi: 10.1016/j.bbamem.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Hunter AW, Gourdie RG. The second PDZ domain of zonula occludens-1 is dispensable for targeting to connexin 43 gap junctions. Cell Commun Adhes. 2008;15:55–63. doi: 10.1080/15419060802014370. [DOI] [PubMed] [Google Scholar]
- Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell. 2005;16:5686–5698. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction–associated MAGUKs, ZO- 1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeansonne B, Lu Q, Goodenough DA, Chen YH. Claudin-8 interacts with multi-PDZ domain protein 1 (MUPP1) and reduces paracellular conductions in epithelial cells. Cell Mol Biol. 2003;49:13–21. [PubMed] [Google Scholar]
- Kamasawa N, Furman CS, Davidson KGV, Sampson JA, Magnie AR, Gebhardt BR, Kamasawa M, Yasumura T, Zumbrunnen JR, Pickard GE, Nagy JI, Rash JE. Abundance and ultrastructural diversity of neuronal gap junctions in the OFF and ON sublaminae of the inner plexiform layer of rat and mouse retina. Neuroscience. 2006;142:1093–1117. doi: 10.1016/j.neuroscience.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra MRH, Bube N, Bos JL. Rap1: a key regulator in cellcell junction formation. J Cell Sci. 2006;120:17–22. doi: 10.1242/jcs.03306. [DOI] [PubMed] [Google Scholar]
- Kothmann WW, Li X, Burr GS, O’Brien J. Connexin 35/36 is phosphorylated at regulatory sites in the retina. Vis Neurosci. 2007;24:363–375. doi: 10.1017/S095252380707037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky G, Medina I, Krapininsky L, Gapon S, Clapham DE. SynGAP-MUPP1-CaMKII synaptic complexes regulate p38 MAP kinase activity and NMDA receptor-dependent synaptic AMPA receptor potentiation. Neuron. 2004;43:563–574. doi: 10.1016/j.neuron.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Laing JG, Chou B, Steinberg TH. ZO-1 alters the plasma membrane localization and function of Cx43 in osteoblastic cells. J Cell Sci. 2005;118:2167–2176. doi: 10.1242/jcs.02329. [DOI] [PubMed] [Google Scholar]
- Landisman CE, Connors BW. Long-term modulation of electrical synapses in the mammalian thalamus. Science. 2005;310:1809–1813. doi: 10.1126/science.1114655. [DOI] [PubMed] [Google Scholar]
- Li X, Olson C, Lu S, Kamasawa N, Yasumura T, Rash JE, Nagy JI. Neuronal connexin36 association with zonula occludens- 1 protein (ZO-1) in mouse brain and interaction with the first PDZ domain of ZO-1. Eur J Neurosci. 2004a;19:2132–2146. doi: 10.1111/j.l460-9568.2004.03283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Olson C, Lu S, Nagy JI. Association of connexin36 with zonula occludens-1 in HeLa cells, bTC-3 cells, pancreas and adrenal gland. Histochem Cell Biol. 2004b;122:485–498. doi: 10.1007/s00418-004-0718-5. [DOI] [PubMed] [Google Scholar]
- Li X, Kamasawa N, Ciolofan C, Olson CO, Lu S, Davidson KGV, Yasumura T, Shigemoto R, Rash JE, Nagy JI. Connexin45- containing neuronal gap junctions in rodent retina also contain connexin36 in both apposing hemiplaques, forming bihomotypic gap junctions, with scaffolding contributed by zonula occludens-1. J Neurosci. 2008a;28:9769–9789. doi: 10.1523/JNEUROSCI.2137-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Penes M, Odermatt B, Willecke K, Nagy JI. Ablation of Cx47 in transgenic mice leads to the loss of MUPP1, ZONAB and multiple connexins at oligodendrocyte-astrocyte gap junctions. Eur J Neurosci. 2008b;28:1503–1517. doi: 10.1111/j.1460-9568.2008.06431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lu S, Nagy JI. Direct association of connexin36 with zonula occludens-2 and zonula occludens-3. Neurochem Int. 2009;54:393–402. doi: 10.1016/j.neuint.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lynn BD, Nagy JI. The effector and scaffolding proteins AF6 and MUPP1 interact with connexin36 and localize at gap junctions that form electrical synapses in rodent brain. Eur J Neurosci. 2012;35:166–181. doi: 10.1111/j.1460-9568.2011.07947.x. [DOI] [PubMed] [Google Scholar]
- Liu X-B, Jones EG. Fine structural localization of connexin36 immunoreactivity in mouse cerebral cortex and thalamus. J Comp Neurol. 2003;466:457–467. doi: 10.1002/cne.10901. [DOI] [PubMed] [Google Scholar]
- Llinas R, Baker R, Sotelo C. Electrotonic coupling between neurons in cat inferior olive. J Neurophysiol. 1974;37:560–571. doi: 10.1152/jn.1974.37.3.560. [DOI] [PubMed] [Google Scholar]
- Lorenowicz MJ, Fernandez-Borja M, Kooistra MRH, Bos JL, Hordijk PL. PKA and Epac1 regulate endothelial integrity and migration through parallel and independent pathways. Eur J Cell Biol. 2008;87:779–792. doi: 10.1016/j.ejcb.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Lorger M, Moelling K. Regulation of epithelial wound closure and intercellular adhesion by interaction of AF6 with actin cytoskeleton. J Cell Sci. 2006;119:3385–3398. doi: 10.1242/jcs.03027. [DOI] [PubMed] [Google Scholar]
- McCracken CB, Hamby SM, Patel KM, Morgan D, Vrana KE, Roberts DC. Extended cocaine self-administration and deprivation produces region-specific and time-dependent changes in connexin36 expression in rat brain. Synapse. 2005a;58:141–150. doi: 10.1002/syn.20194. [DOI] [PubMed] [Google Scholar]
- McCracken CB, Patel KM, Vrana KE, Paul DL, Roberts DCS. Amphetamine withdrawal produces region-specific and timedependent changes in connexin36 expression in rat brain. Synapse. 2005b;56:39–44. doi: 10.1002/syn.20127. [DOI] [PubMed] [Google Scholar]
- Moreno AP, Lau AF. Gap junction channel gating modulated through protein phosphorylation. Prog Biophys Mol Biol. 2007;94:107–119. doi: 10.1016/j.pbiomolbio.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Coupled networks of parvalbumin-immunoreactive interneurons in the rat basolateral amygdala. J Neurosci. 2005;25:7366–7376. doi: 10.1523/JNEUROSCI.0899-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JI, Dudek FE, Rash JE. Update on connexins and gap junctions in neurons and glia in the mammalian nervous system. Brain Res Rev. 2004;47:191–215. doi: 10.1016/j.brainresrev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Niessen CM, Gottardi CJ. Molecular components of the adherens junction. Biochim Biophys Acta. 2008;1778:562–571. doi: 10.1016/j.bbamem.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogita H, Takai Y. Nectins and nectin-like molecules: roles in cell adhesion, polarization, movement, and proliferation. IUBMB Life. 2006;58:334–343. doi: 10.1080/15216540600719622. [DOI] [PubMed] [Google Scholar]
- Onn SP, Grace AA. Dye coupling between rat striatal neurons recorded in vivo: compartmental organization and modulation by dopamine. J Neurophysiol. 1994;71:1917–1934. doi: 10.1152/jn.1994.71.5.1917. [DOI] [PubMed] [Google Scholar]
- Onn S-P, Grace AA. Repeated treatment with haloperidol and clozapine exerts differential effects on dye coupling between neurons in subregions of striatum and nucleus accumbens. J Neurosci. 1995;15:7024–7036. doi: 10.1523/JNEUROSCI.15-11-07024.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onn S-P, Grace AA. Alterations in electrophysiological activity and dye coupling of striatal spiny and aspiny neurons in dopamine-denervated rat striatum recorded in vivo. Synapse. 1999;33:1–15. doi: 10.1002/(SICI)1098-2396(199907)33:1<1::AID-SYN1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Onn S-P, West AR, Grace AA. Dopamine-mediated regulation of striatal neuronal and network interactions. Trends Neurosci. 2000;23:S48–S56. doi: 10.1016/s1471-1931(00)00020-3. [DOI] [PubMed] [Google Scholar]
- Ouyang X, Winbow VM, Patel LS, Burr GS, Mitchell CK, O’Brien J. Protein kinase A mediates regulation of gap junctions containing connexin35 through a complex pathway. Brain Res Mol Brain Res. 2005;135:1–11. doi: 10.1016/j.molbrainres.2004.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penes MC, Li X, Nagy JI. Expression of zonula occludens-1 (ZO-1) and the transcription factor ZO-1-associated nucleic acidbinding protein (ZONAB)-MsY3 in glial cells and colocalization at oligodendrocyte and astrocyte gap junctions in mouse brain. Eur J Neurosci. 2005;22:404–418. doi: 10.1111/j.1460-9568.2005.04225.x. [DOI] [PubMed] [Google Scholar]
- Pereda AE, Bell TD, Chang BH, Czernik AJ, Nairn AC, Soderling TR, Faber DS. Ca2+/calmodulin-dependent kinase II mediates simultaneous enhancement of gap-junctional conductance and glutamatergic transmission. Proc Natl Acad Sci USA. 1998;95:13272–13277. doi: 10.1073/pnas.95.22.13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Velazquez JL, Han D, Carlen PL. Neurotransmitter modulation of gap junctional communication in the rat hippocampus. Eur J Neurosci. 1997;9:2522–2531. doi: 10.1111/j.1460-9568.1997.tb01681.x. [DOI] [PubMed] [Google Scholar]
- Qu C, Gardner P, Schrijver I. The role of the cytoskeleton in the formation of gap junctions by Connexin 30. Exp Cell Res. 2009;315:1683–1692. doi: 10.1016/j.yexcr.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Rash JE, Staines WA, Yasumura T, Pate D, Hudson CS, Stelmack GL, Nagy JI. Immunogold evidence that neuronal gap junctions in adult rat brain and spinal cord contain connexin36 (Cx36) but not Cx32 or Cx43. Proc Natl Acad Sci USA. 2000;97:7573–7578. doi: 10.1073/pnas.97.13.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Olson CO, Pouliot WA, Davidson KGV, Yasumura T, Furman CS, Royer S, Kamasawa N, Nagy JI, Dudek FE. Connexin36, miniature neuronal gap junctions, and limited electrotonic coupling in rodent suprachiasmatic nucleus (SCN) Neuroscience. 2007a;149:350–371. doi: 10.1016/j.neuroscience.2007.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Olson CO, Davidson KGV, Yasumura T, Kamasawa N, Nagy JI. Identification of connexin36 in gap junctions between neurons in rodent locus coeruleus. Neuroscience. 2007b;147:938–956. doi: 10.1016/j.neuroscience.2007.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorig B, Sutor B. Serotonin regulates gap junction coupling in the developing rat somatosensory cortex. Eur J Neurosci. 1996;8:1685–1695. doi: 10.1111/j.1460-9568.1996.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Rorig B, Klausa G, Sutor B. Dye coupling between pyramidal neurons in developing rat prefrontal and frontal cortex is reduced by protein kinase A activation and dopamine. J Neurosci. 1995;15:7386–7400. doi: 10.1523/JNEUROSCI.15-11-07386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D, Schuchmann S, Fisahn A, Draguhn A, Buhl EH, Petrasch- Parwez E, Dermietzel R, Heinemann U, Traub RD. Axo- Axonal coupling: a novel mechanism of ultrafast neuronal communication. Neuron. 2001;31:831–840. doi: 10.1016/s0896-6273(01)00410-x. [DOI] [PubMed] [Google Scholar]
- Segretain D, Falk MM. Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochem Biophys Acta. 2004;1662:3–21. doi: 10.1016/j.bbamem.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Smyth JW, Vogan JM, Buch PJ, Zhang SS, Fong TS, Hong TT, Shaw RM. Actin cytoskeleton rest stops regulate anterograde traffic of connexin 43 vesicles to the plasma membrane. Circ Res. 2012 doi: 10.1161/CIRCRESAHA.111.257964. 2012 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohl G, Odermatt B, Maxeiner S, Degen J, Willecke K. New insights into the expression and function of neural connexins with transgenic mouse mutants. Brain Res Rev. 2004;47:245–259. doi: 10.1016/j.brainresrev.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sohl G, Maxeiner S, Willecke K. Expresssion and functions of neuronal gap junctions. Nat Rev Neurosci. 2005;6:191–200. doi: 10.1038/nrn1627. [DOI] [PubMed] [Google Scholar]
- Somekawa S, Fukuhura S, Nakaoka Y, Fujita H, Saito Y, Mochizuki N. Enhanced functional gap junction neoformation by protein kinase A-dependent and Epac-dependent signals downstream of cAMP in cardiac myocytes. Circ Res. 2005;97:655–662. doi: 10.1161/01.RES.0000183880.49270.f9. [DOI] [PubMed] [Google Scholar]
- Theiss C, Meller K. Microinjected anti-actin antibodies decrease gap junctional intercellular commmunication in cultured astrocytes. Exp Cell Res. 2002;281:197–204. doi: 10.1006/excr.2002.5652. [DOI] [PubMed] [Google Scholar]
- Ullmer C, Schmuck K, Figge A, Lubbert H. Cloning and characterization of MUPP1, a novel PDZ domain protein. FEBS Lett. 1998;424:63–68. doi: 10.1016/s0014-5793(98)00141-0. [DOI] [PubMed] [Google Scholar]
- Umeda K, Kenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M, Tsukita S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Urschel S, Hoher T, Schubert T, Alev C, Sohl G, Worsdorfer P, Asahara T, Dermietzel R, Weiler R, Willecke K. Protein kinase A-mediated phosphorylation of connexin36 in mouse retina results in decreased gap junctional communication between AII amacrine cells. J Biol Chem. 2006;281:33163–33171. doi: 10.1074/jbc.M606396200. [DOI] [PubMed] [Google Scholar]
- Utepbergenov DI, Fanning AS, Anderson JM. Dimerization of the scaffolding protein ZO-1 through the second PDZ domain. J Biol Chem. 2006;281:24671–24677. doi: 10.1074/jbc.M512820200. [DOI] [PubMed] [Google Scholar]
- Xia XB, Mills SL. Gap junctional regulatory mechanisms in the AII amacrine cell of the rabbit retina. Vis Neurosci. 2004;21:791–805. doi: 10.1017/S0952523804045122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Harada N, Kano K, Taya S-I, Canaani E, Matsuura Y, Mizoguchi A, Ide C, Kaibuchi K. The ras target AF-6 interacts with ZO-1 and serves as a peripheral compoment of tight junctions in epithelial cells. J Cell Biol. 1997;139:785–795. doi: 10.1083/jcb.139.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Harada N, Kawano Y, Taya S, Kaibuchi K. In vivo interaction of AF-6 with activated ras and ZO-1. Biochem Biophys Res Commun. 1999;259:103–107. doi: 10.1006/bbrc.1999.0731. [DOI] [PubMed] [Google Scholar]
- Yang QZ, Hatton GI. Histamine H1-receptor modulation of inter-neuronal coupling among vasopressinergic neurons depends on nitric oxide synthase activation. Brain Res. 2002;955:115–122. doi: 10.1016/s0006-8993(02)03374-7. [DOI] [PubMed] [Google Scholar]