Abstract
The unicellular, diazotrophic cyanobacterium Cyanothece sp. ATCC 51142 demonstrated important modifications to photosystem II (PSII) centers when grown under light/dark N2-fixing conditions. The properties of PSII were studied throughout the diurnal cycle using O2-flash-yield and pulse-amplitude-modulated fluorescence techniques. Nonphotochemical quenching (qN) of PSII increased during N2 fixation and persisted after treatments known to induce transitions to state 1. The qN was high in cells grown in the dark, and then disappeared progressively during the first 4 h of light growth. The photoactivation probability, ε, demonstrated interesting oscillations, with peaks near 3 h of darkness and 4 and 10 h of light. Experiments and calculations of the S-state distribution indicated that PSII displays a high level of heterogeneity, especially as the cells prepare for N2 fixation. We conclude that the oxidizing side of PSII is strongly affected during the period before and after the peak of nitrogenase activity; changes include a lowered capacity for O2 evolution, altered dark stability of PSII centers, and substantial changes in qN.
Many species of bacteria and cyanobacteria are capable of N2 fixation. Nitrogenase catalyzes the energetically expensive reduction of N2 to NH3 and is rapidly and irreversibly inhibited when extracted from cells in the presence of free O2 (Fay and Cox, 1967; Haystead et al., 1970). The N2-fixation trait is particularly interesting in cyanobacteria because of the apparent incompatibility of an O2-sensitive nitrogenase complex in a prokaryotic organism capable of splitting water to produce O2. Cyanobacteria have developed elegant strategies to protect nitrogenase from inactivation by O2, including spatial separation, temporal separation, and the induction of enzyme systems to destroy reactive O2 byproducts (Fay, 1992). The strategies devised by nonheterocystous, unicellular cyanobacteria that permit N2 fixation and O2 evolution in the same cell are quite sophisticated (Bergman et al., 1997). A number of these unicellular, diazotrophic species fix N2 and evolve O2 at different times during the diurnal cycle. Such temporal separation has been seen in strains classified as Gloeothece sp. (Mullineaux et al., 1981) and Synechococcus sp. (Mitsui et al., 1986; Grobbelaar et al., 1987).
Synechococcus Miami BG 43511 and BG 43522 (Mitsui et al., 1987) can be synchronized by growth under light/dark cycles so that they continue to cycle nitrogenase even during continuous light. Under these conditions, O2 evolution and nitrogenase cycled in a reciprocal way such that O2 evolution was low when nitrogenase activity was high. A similar reciprocal relationship between the two metabolic processes has been demonstrated in Synechococcus RF-1 (Grobbelaar et al., 1987), in which nitrogenase activity was found to be highest in the dark and O2 evolution highest in the light. However, in that study little was done to analyze the specific changes that occurred during photosynthesis.
We have begun to study diurnal metabolic rhythms and their control in the unicellular, diazotrophic cyanobacterium Cyanothece sp. ATCC 51142. It has been demonstrated previously that this strain possesses extraordinary diurnal metabolic periodicities that resemble circadian rhythms (Reddy et al., 1993; Schneegurt et al., 1994). When cultures were grown in the absence of fixed N2, either under 12-h light/12-h dark cycles or continuous light, the cells fixed N2 with peaks of activity approximately every 24 h. The peak of N2 fixation activity was very narrow, with a half-width of about 2.5 h, reaching a maximum about D4 (Schneegurt et al., 1994). The burst of N2 fixation was accompanied by intense respiratory activity (Colón-López et al., 1997; Meunier et al., 1997).
The nonheterocystous, filamentous, diazotrophic cyanobacterium Plectonema boryanum also shows metabolic rhythms under N2-fixing conditions, and its photosynthetic behavior has been studied (Misra and Desai, 1993; Misra and Tuli, 1994). Photosynthetic activity measurements and thermoluminescence and 77 K fluorescence spectra suggested that PSI activity increased and PSII activity decreased during the period of N2 fixation, suggesting that PSII was specifically regulated and that electron transport under diazotrophic growth conditions was regulated by the redox state of the secondary quinone acceptor, QB. Furthermore, it was proposed that the PSII QB was involved in PSII down-regulation. However, none of this previous work analyzed the phenomenon at the level of the water-oxidation mechanism (the S-state cycle).
We have determined that the physiology of Cyanothece sp. ATCC 51142 involves important changes in PSII reaction center oligomerization, phycobilisome attachment, gene transcription, protein accumulation, and activity throughout the 24-h cycles (Meunier et al., 1997; M.S. Colón-López and L.A. Sherman, unpublished observations). However, the fate of the S-state cycle and Mn2+ and O2 production under flashing light throughout these structural changes has not yet been investigated. PSII is a logical target for down-regulation during periods of N2 fixation, since O2 is produced by the PSII centers and nitrogenase is sensitive to O2. Accordingly, we found that PSI, but not PSII, contributes to N2 fixation if the cultures are illuminated during the normally dark N2-fixation period in Cyanothece sp. ATCC 51142 (Meunier et al., 1997). In the current study, we analyzed O2-flash-yield experiments and qN data and related them to our previous results to understand PSII function under these conditions. Our results suggest that the properties of the oxidizing side of PSII are strongly modified by variable dark-inactivation processes and photoactivation that can be extremely rapid.
Photoactivation is the process by which Mn2+ becomes bound to the PSII reaction center and forms into a structure that is active in the oxidation of water and in utilizing charge separations as an energy source (Cheniae and Martin, 1972; Engels et al., 1994; Gleiter et al., 1995; Burnap et al., 1996). Each step between PSII charge separations is identified by an S-state, and different S-states are denoted by Sx, where “x” ranges from 0 to 4. Upon reaching the state S4, O2 is released and the process starts again from the S0 state (Kok et al., 1970). The concept of S-states has been expanded to include the steps required to oxidize Mn2+ up to the S0 state. These steps are also known as the super-reduced S-states S−1, S−2, and S−3 (Bader et al., 1983; Kretschmann and Witt, 1993; Messinger and Renger, 1993; Messinger et al., 1997).
The reduction of the Mn2+ structure produces deactivations of S-states and can result in the loss of O2-evolving activity and the loss of Mn2+ (Cheniae and Martin, 1972; Burnap et al., 1996). Deactivations result in a loss of PSII efficiency and a randomization of PSII centers among S-states. The susceptibility to spontaneous deactivations is limited to the S3 and S2 states in most wild-type organisms; however, in some cyanobacterial PSII mutants, as well as in Cyanothece sp. (see Results), deactivations lead to the loss of O2-evolving activity. A mechanistic model of the S-state mechanism was recently proposed that explicitly incorporates photoactivation and deactivation probabilities (Meunier et al., 1996).
We will provide experimental evidence, using O2-flash yields, that the water-splitting mechanism is subjected to severe disturbances during the adaptive changes of the photosynthetic apparatus and cycling physiology in Cyanothece sp. ATCC 51142. We postulate that these disturbances are common in diazotrophic cyanobacteria that are unicellular or filamentous but nonheterocystous, and that the data presented here are representative of a class of PSII centers optimized for functioning under harsher conditions than those normally seen in higher plants. Some of this research was presented in preliminary form at the Tenth International Photosynthesis Congress (Meunier et al., 1995b).
MATERIALS AND METHODS
Growth Conditions and Chlorophyll Determination
Cyanothece sp. ATCC 51142 (formerly strain BH68) was grown as previously described in ASP2 medium without NaNO3, with shaking at 100 or 125 rpm, at 30°C, under cool-white fluorescent illumination of approximately 50 μE m−2 s−1 (Reddy et al., 1993). Continuous-light-grown, stationary-phase cultures were subcultured by dilution to a concentration of 106 cells mL−1. Duplicate flasks were subcultured 12 h apart using the same stock culture to permit 24-h experiments to be performed in 12 h. After dilution, each flask was illuminated for 12 h. This protocol, the determination of cell number, and nitrogenase measurements were as described previously (Schneegurt et al., 1994). The chlorophyll concentration was determined using the freeware “Chlorophyll” (available for downloading at http://bilbo.bio.purdue.edu/~pmeunier/download.html), according to the cyanobacterial method of Arnon et al. (1974). For measuring the chlorophyll concentration in whole cells, a spectrophotometer (model DU-7, Beckman) was modified by adding cellophane tape (no. 810, Scotch 3M Magic Tape) to the light path before and after the cuvette to correct for light scattering by the cells. This yielded a fast and reproducible assay that was linear from 0.01 absorbance unit up to about 0.5 absorbance unit, and that was within 10% of chlorophyll determinations performed using 80% acetone extraction.
O2 Evolution/Flash-Yield Experiments
O2 yields after light flashes were measured using a bare Pt electrode onto which cells were deposited by centrifugation. Signals were amplified using an electronic circuit based upon the design of Meunier and Popovic (1988). Cells (40 μg of chlorophyll) were harvested by centrifugation at 10,000g in a rotor (model JA-20, Sorvall) for 10 min, and resuspended in 200 μL of buffer of the same osmolarity as the culture medium, but containing only 340 mm NaCl and 10 mm Hepes, pH 7.5. The 200 μL was then centrifuged for 5 min at 1,000 rpm on the electrode in a swing-out rotor (model HB4-A, Sorvall). The slow centrifugation speed was essential to apply a uniform layer of cells on the electrode.
Photoactivation treatments consisted of 3 min of 10-Hz flashes. To diminish photoinhibition phenomena, a red filter (similar to RG-610) was interposed between the sample and the lamp. The filter was removed for the subsequent experiments to obtain a saturating intensity. The typical protocol was to keep the cells in the dark for about 15 min, during which time the sample was prepared and centrifuged onto the electrode. The electrode was polarized just before the experiment, and 15 flashes were given at a 3-Hz frequency. These cells were then left on the electrode for an additional 3 min, with flashes given at a 10-Hz frequency without recording the signal. The cells were then briefly left in the dark for 10 s, and flashes were given again at a 3-Hz frequency.
Analysis of O2 Yields
We tried to analyze the O2 yields after dark adaptation from the same experiment as shown in Figure 4 for the 36-h period from 144 to 180 h. However, O2 evolution in Cyanothece sp. was much more complex than in Synechocystis sp. PCC 6803. Applying the correction previously defined for the slow respiratory O2 transients (Meunier et al., 1996) produced worse fittings, since part of the slow signals oscillated with a periodicity of 4 and was of PSII origin (Fig. 1). Moreover, large corrections (up to 5 times greater than those used with Synechocystis sp.) were necessary to completely remove the slow O2 yields from the data set in the Cyanothece sp. experiments. Therefore, we used the averaged, smaller correction applied to Synechocystis sp. PCC 6803 data (ratio of 3.0; see Meunier et al., 1995a). This produced the best agreement between the Cyanothece sp. data and S-state models.
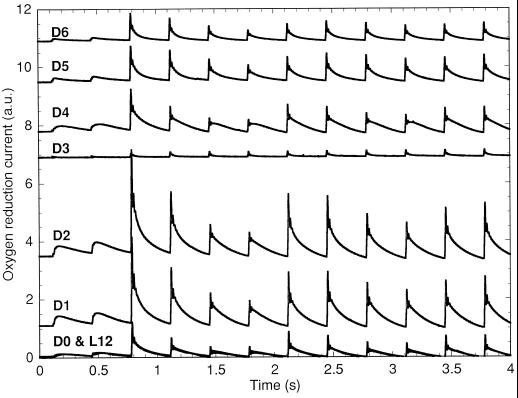
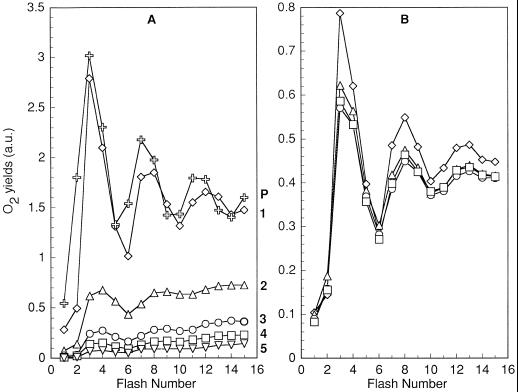
Figure 4.
O2 yields from Cyanothece sp. ATCC 51142 using cells harvested at L12 to D6 during growth under N2-fixing conditions in 12-h light/12-h dark conditions. The samples were stimulated by Xe flashes given at 3 Hz starting 0.1 s after the start of recording. Since the L12 to D0 boundary involved a change in culture flasks, the synchronicity of the two cultures at that point was verified by performing both recordings (12 h apart). The two recordings are superimposed on the graph and are indistinguishable. An arbitrary constant was added to the recordings for readability (a.u., arbitrary unit).
Figure 1.
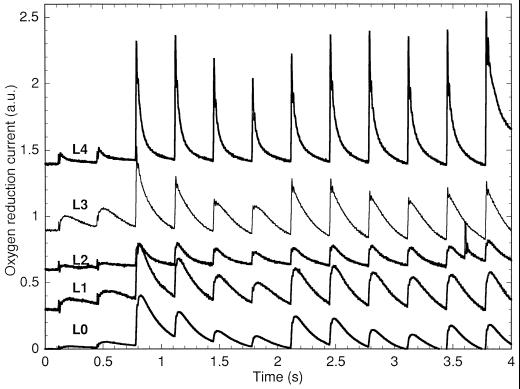
O2 yields from Cyanothece sp. ATCC 51142 using cells harvested at L0 to L4 during growth under N2-fixing conditions in 12-h light/12-h dark conditions. The samples were stimulated by Xe flashes given at 3 Hz starting 0.1 s after the start of recording. An arbitrary constant was added to the recordings for readability (a.u., arbitrary units).
The O2-yield data after dark adaptation were analyzed as described in the appendix of Meunier et al. (1996) to find the five eigenvalues relating to a generic five-step S-state mechanism. The photoactivation probability, ε, was calculated from the first (greatest) eigenvalue, λ1, as λ1 − 1. This was a very reliable determination for the following reasons: (a) λ1 has the lowest uncertainty (least sensitivity to experimental error) by a factor of approximately 10 compared with the other eigenvalues; (b) its determination did not depend on any specific model; and (c) the only assumption was that the data could be fitted by a five-step model.
Although the other eigenvalues were consistent with our cyanobacterial model (Meunier et al., 1996), the low amplitudes of O2 evolution and the low signal-to-noise ratio after dark adaptation made the determination of the other S-state transition probabilities ambiguous at times, especially the separation between “true” misses and the effect of deactivations. We found from the simulation of theoretical sequences that the signal-to-noise ratio needed to be significantly greater than 100 (1% error) for fitting this model to data. However, the signal-to-noise ratio after dark adaptation was often at or below 100. Therefore, we did not use the cyanobacterial S-state model (Meunier et al., 1996) in this study.
Heterogeneous Photoactivation Model
The O2-yield data after a photoactivation treatment (3 min of 10-Hz flashes with a red filter similar to RG-610) had much greater amplitudes and provided a high signal-to-noise ratio (on average around 400). Nevertheless, the data were much better fitted by a generic six-step model equation than with a four- or five-step one (Meunier et al., 1995b). This was puzzling since there should not have been a significant number of centers in S−2 and S−1 after the photoactivation treatment. The 30-s dark adaptation between the photoactivation treatment and the following measurements were also unlikely to yield a significant number of centers in S−2 and S−1 at all times, since even S3 was still present in large amounts. We found that the requirement for a six-step analysis was much better explained by the combination of two heterogeneous four-step S-state models than by super-reduced S-states. Equation 8 in Meunier et al. (1996) was modified for a six-step mechanism by adding the term c6λ6n-1:
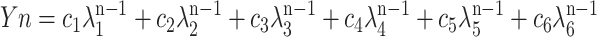 |
1 |
where the symbols have the same meanings as previously described. Fitting Equation 1 resulted in a numerically more robust determination of the eigenvalues than searching for roots in the characteristic equation after multivariable regression (as described in the appendix of Meunier et al., 1996). The eigenvalues λ3, λ4, λ5, and λ6 were all imaginary numbers. No consistent six-step S-state model could reproduce these eigenvalues. However, the properties of the O2 yields could be satisfactorily explained by the combination of two four-step S-state models:
 |
2 |
where d and κ serve the same function as c and λ in the second four-step S-state model. Moreover, when λ1 and λ2 are close enough to κ1 and κ2, Equation 2 provides an exact solution to interpreting Equation 1 as the sum of two S-state models:
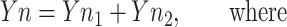 |
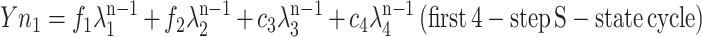 |
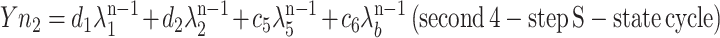 |
and where f1 + d1 = c1 and f2 + d2 = c2. From these two sets of four eigenvalues, the S-state transition probabilities of two four-step homogeneous S-state cycles with backward transitions were calculated (Meunier, 1993). This model is derived from a previously published S-state model (Kok et al., 1970), in which the apparent misses (failures to advance the S-state mechanism before the next flash) do not distinguish between true photochemical misses or single hits followed by deactivations. The possible occurrence of a miss followed by a deactivation is accounted for by the backward transition probability, so the backward transition probability is always smaller than the miss probability.
The exact number of centers that contributed to each cycle is proportional to the constants f1 and d1, whereas only their sum (c1) could be determined. However, most of the time the variables c5 and c6 were large enough relative to c3 and c4 to estimate the proportion of centers in the second (inefficient) cycle to more than 10%. Theoretical sequences generated by adding two four-step S-state cycles were tested as described previously (Meunier et al., 1995b) and were found to also require a six-step model for analysis. By contrast, the bicycle model (Shinkarev and Wraight, 1995) required only a four-step model that returned the averaged properties of the two theoretical cycles.
Fluorescence Measurements
PAM fluorescence was measured using the Walz fluorometer ED-101 cuvette kit (Heinz-Walz, Effeltrich, Germany) with the optional 590-nm excitation light (model L-590–102, Heinz-Walz), and by adding a small magnet and stirrer to keep the sample agitated. In the experiments reported here, the actinic light was the original red LED that came with the PAM instrument, and had an intensity of 68 μE m−2 s−1. Transitions to state 1 were induced by adding 10 μm DCMU and illuminating the samples with the red actinic light. The final FM(D1) level (D for DCMU and 1 for state 1) was measured after the state transition was completed, as indicated by a stabilization of the fluorescence level. It was verified previously that this treatment induced substantial changes in 77 K fluorescence (Meunier et al., 1997). However, because (a) state transitions in cyanobacteria involve important changes in energy distribution; (b) the resting state in the dark of Cyanothece sp. ATCC 51142 can be anything between state 2 and state 1 (Meunier et al., 1997); and (c) we used 590-nm excitation flashes for the detection of fluorescence, the FO level measured in Cyanothece sp. ATCC 51142 was highly dependent on state transitions. Therefore, we contend that the FM level obtained after a transition to state 1 cannot be compared with the FO level measured in dark-adapted “resting” cyanobacteria, FO(R), because phycobilisome attachment and energy distribution changed. Therefore, after reaching state 1 the fluorescence level (after a sufficient time for the reoxidation of QA in darkness) FO(D1) is higher than FO(R) because more energy is directed to PSII centers. Consequently, the fluorescence obtained per detecting flash of the PAM instrument is higher. It follows that FM(D1) should be compared with FO(D1) (Fig. 6).
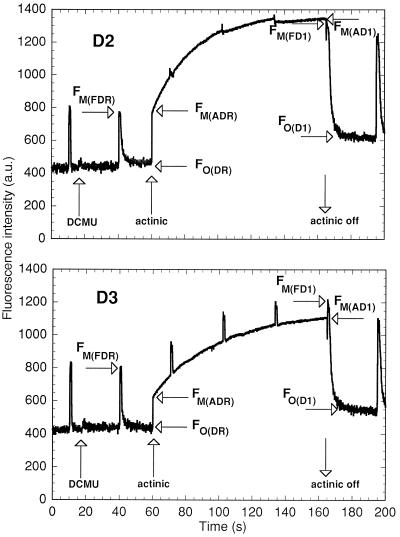
Figure 6.
PAM fluorescence monitoring of the induction of state 1 in Cyanothece sp. ATCC 51142 at D2 and D3. The fluorescence levels used in calculations are tagged as described in Methods. R, D, F, A, and 1, resting state in the dark, in the presence of DCMU, measurements with a saturating flash, measurements under actinic light, and state 1, respectively. Samples of 1 mL at 2 μg chlorophyll/mL were treated with 10 μm DCMU and the maximum intensity of the red actinic light of the PAM fluorimeter to induce state 1, as per the protocol in Meunier et al. (1997). The actinic light went off just before the flash.
We noticed that the difference FM(D1) − FO(D1) after the treatment to induce a transition to state 1 was smaller at some times during the cycle, even though the samples were all at the same chlorophyll concentration. We attributed the smaller difference to a residual qN, calculated using the maximum difference, MAX[FM(D1) − FO(D1)], measured during the cycle as the denominator:
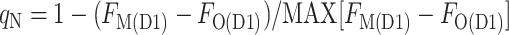 |
3 |
by contrast to the total qN, calculated as:
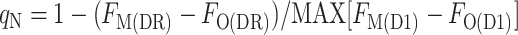 |
4 |
where FM(DR) is the FM level measured in the presence of DCMU (D) in the dark resting state (R) of the cyanobacterium, FO(DR) is the FO level measured in the presence of DCMU in the dark-resting state of the cyanobacterium, FO(D1) is the FO level measured in the presence of DCMU in state 1, and FM(D1) is the FM level measured in the presence of DCMU in state 1. The FO level was not measurably changed by the addition of DCMU, showing that the detecting light flashes had a negligible actinic effect; however, for the sake of consistency, all levels were measured in the presence of DCMU. We found two ways to measure the FM(D1) level: with a saturating flash (F), FM(FD1), or with the actinic light (A), FM(AD1). Likewise, the FM(DR) could be measured with a flash (F), FM(FDR), or with the actinic light, FM(ADR) (Fig. 6). This gives rise to four ways to calculate qN:
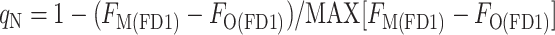 |
5a |
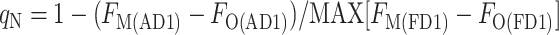 |
5b |
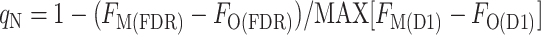 |
5c |
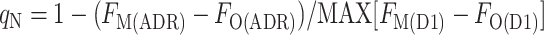 |
5d |
RESULTS
Metabolic Rhythms
The physiology of Cyanothece sp. ATCC 51142 has been detailed previously for cells grown under N2-fixing conditions with 12-h light/12-h dark or with continuous light (Schneegurt, et al., 1994; Colón-López, et al., 1997; Meunier, et al., 1997). In this study, cells were grown in 12-h light/12-h dark cycles. In such cultures nitrogenase and respiration activity peaked around D4, whereas O2 evolution peaked near L8. Samples were withdrawn every hour for the O2-flash yield or the PAM measurements to be described. We will define four main time periods: (a) early dark (D0 to the peak of nitrogenase activity); (b) late dark (D6–D12); (c) early light (L0–L6); and (d) late light (L8–L12). Although each procedure was repeated at least three times with similar results, we will present results from single experiments because the transitions are very sharp but the exact time point when they happen varies from experiment to experiment (therefore, averaging blurs the transitions).
O2 Evolution
Cyanothece sp. ATCC 51142 produced O2 under flashing light with a period-4 oscillation indicative of the S-state mechanism (Fig. 1). Samples harvested at various times showed that the S-state mechanism underwent significant changes throughout the 24-h cycle. Samples harvested at L0, the beginning of light growth, showed slow O2 signals, peaking about 45 ms after the flash, with oscillations period 4 in amplitude. However, samples harvested at later times showed increasingly faster O2 signals (peaking at 8 ms after the flash at 4 h of light) oscillating with a period 4, whereas the contribution of the slow transients to the oscillations became progressively less important.
In Synechocystis sp. PCC 6803, slow O2 signals were attributed to respiratory transients due to PSI activity (Meunier et al., 1995a). These signals persisted in the presence of the PSII herbicide DCMU, did not oscillate with a periodicity of 4, occurred in mutants with incapacitated PSII, and the deconvolution of the fast O2 transients from the slow signals resulted in greatly improved fittings with generic n-step models (Meunier et al., 1995a). Attempts to completely remove the slow signals from the data (as described in Meunier et al., 1995a) did not result in improved fittings for Cyanothece sp. ATCC 51142 data. Slower PSII O2 release has been reported in the psbO deletion mutant of Synechocystis sp. PCC 6803 (Burnap et al., 1992). In light of the qN data (see later), the slow O2 release at the beginning of the light period and increasingly faster O2 signals thereafter would be consistent with a nonoptimal conformation of the oxidizing side of PSII initially, and a subsequent photoactivation of PSII centers.
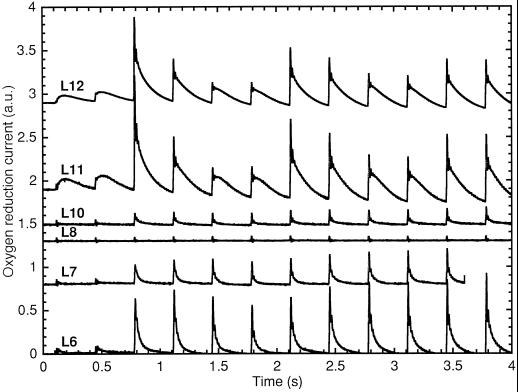
The amplitudes of the O2 yields after sample preparation in the dark increased from L0 to a maximum around L4 to L6; however, they started decreasing past L6 down to a minimum between L8 and L10, and recovered quickly afterward (Fig. 2). The dip in O2-evolution amplitudes in the middle of the afternoon was unexpected, given the rates of linear electron transport that have been observed (Meunier et al., 1997). However, a photoactivation treatment of 3 min of 10-Hz flashes followed by 30 s of darkness produced very high amplitudes, with very strong period-4 oscillations; an example of this phenomenon for L10 is presented in Figure 3. The effectiveness of the photoactivation treatment demonstrated that the apparent loss of activity from L8 to L10 after dark adaptation was reversible. Note that the amplitudes obtained after dark adaptation in Cyanothece sp. ATCC 51142 (Fig. 2) were much lower than the actual PSII capacity (e.g. Fig. 3), and the amount of inactivation in the dark was variable between repeats, whereas the trends remained the same. A striking demonstration of the highly variable dark-inactivation properties of Cyanothece sp. ATCC 51142 is shown in Figure 8.
Figure 2.
O2 yields from Cyanothece sp. ATCC 51142 using cells harvested at L6 to L12 during growth under N2-fixing conditions in 12-h light/12-h dark conditions. The samples were stimulated by Xe flashes given at 3 Hz starting 0.1 s after the start of recording. An arbitrary constant was added to the recordings for readability (a.u., arbitrary units).
Figure 3.
O2 yields from Cyanothece sp. ATCC 51142 at L10 after a dark adaptation (D) and a photoactivation treatment (P). The dark period necessary to prepare the sample and centrifuge the cells to the electrode (about 15 min) was sufficient to deactivate most Mn centers (D). The same cells were left on the electrode and photoactivated by 3 min of flashes given at 10 Hz. After 10 s of darkness, flashes were given at 3 Hz and the amplitude of the O2 yields were recorded (P). An arbitrary constant was added to the recordings for readability (a.u., arbitrary unit).
Figure 8.
Amplitudes of O2 production under flashing light from Cyanothece sp. ATCC 51142 cells harvested at L8 (A) and D8 (B). A, Curve 1, after controlling light leakages during centrifugation and sample manipulation (⋄); curve 2, after 30 s of darkness following A (▵); curves 3, 4, and 5, each after an additional 30 s of darkness following the previous experiment (○, □, and ▿); curve P, after a 3-min, 10-Hz photoactivation treatment following the experiment ( ). B, After controlling light leakages during centrifugation and sample manipulation (⋄); after a subsequent 30 s of darkness (▵); after an additional 30 s of darkness following the previous experiment (□ and ○).
The amplitudes of the O2 yields after sample preparation in the dark increased progressively from D0 (L12) to D2, but decreased precipitously to negligible levels at D3 (Fig. 4; note the different scale from Fig. 2). This corresponds to the usual time of the peak of nitrogenase activity and respiration in Cyanothece sp. ATCC 51142 (Reddy et al., 1993; Schneegurt et al., 1994; Colón-López et al., 1997). Nitrogenase activity has a very narrow peak in 12-h light/12-h dark-grown Cyanothece sp. ATCC 51142, and the mean for 27 cycles was at D4 (Colón-López et al., 1997). The amplitudes of the O2 yields only partially recovered at D4 and later, suggesting that some PSII were inhibited during N2 fixation. The transients measured from D7 to D11 are very similar to those at D6 and, for clarity, are not shown.
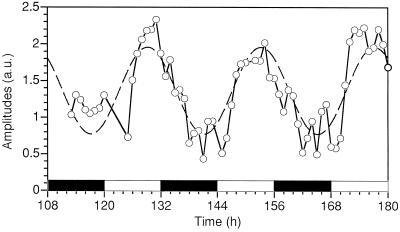
To better understand these phenomena, flash-O2 yields were measured on cells harvested every hour for a 3-d period using the photoactivation protocol used in Figure 3 (see Meunier et al., 1997 for the metabolic periodicities). After a photoactivation treatment, the experimental data (Fig. 5, solid line) showed a general trend that could be represented by a 24-h-period sine wave (dashed line). The photoactivated amplitudes on the bare Pt electrode varied in a manner similar to the results obtained using the Clark electrode (Meunier et al., 1997), and were much higher than after dark adaptation. For this experiment, we conclude that maximal activities are highest when cells are growing in the late-light phase (about L8) and lowest in the late-dark period.
Figure 5.
Average amplitudes of O2 production under flashing light in Cyanothece sp. ATCC 51142 after a photoactivation treatment and over a 3-d period starting 108 h after subculture. Black bars, Dark periods; white bars, light periods. The dashed line represents a sine wave with a fixed 24-h period, which had the phase and amplitude fitted to the data using the DeltaGraph program (Delta Point, Inc., Monterey, CA). a.u., Arbitrary units.
Fluorescence Quenching
The measurement of both 77 K and PAM fluorescence during illumination of cells in the presence of DCMU indicated that several strong state transitions were occurring in Cyanothece sp. ATCC 51142 throughout the 24-h cycles (Meunier et al., 1997). qN was proposed to be mainly due to state transitions (Campbell and Öquist, 1996). The calculation qN uses the difference between the maximum level of fluorescence, FM, and the minimal level, FO, under a test condition, compared with the maximized difference in the presence of DCMU (see Methods). However, there was an ambiguity in the evaluation of the FM levels at certain times during the cycle (Fig. 6; see Methods). The fluorescence levels measured by using the red actinic light of the PAM fluorimeter, FM(ADR) and FM(AD1), were in practice equal to those measured with a saturating flash, FM(FDR) and FM(FD1), in wild-type Synechocystis sp. PCC 6803 (not shown) and in Cyanothece sp. ATCC 51142 at D2 (Fig. 6). However, these two types of levels were significantly different from D3 (Fig. 6) until approximately L4. This means that the maximum intensity of the actinic red light in the PAM could not always maintain all of the QA in a reduced state in Cyanothece sp. ATCC 51142, even in the presence of DCMU.
We used the two ways to measure FM to calculate the residual qN after inducing a transition to state 1 (Fig. 7A). According to Campbell and Öquist (1996), negligible qN should remain in cyanobacteria after the induction of state 1. However, the residual qN measured with the flash (Eq. 5a; Fig. 7A) increased in the dark (starting from D2), up to considerable levels from D5 to D12, and decreased in the light. The amount of qN that was not due to state transitions was negligible from L5 to D1. By comparison, the residual qN measured under red actinic light of the PAM fluorimeter was greater from D4 to D12 than that measured by flashes, which implies that it was harder to maintain QA in its reduced form, and suggests a greater instability of PSII charge separations (Eq. 5b; Fig. 7A). Indeed, in PSII centers with an inactive donor side, excitation energy is quenched by increased charge recombinations between QA− and P680+, which is observable as an increased fluorescence quenching during the inactivation of O2 evolution (Krieger et al., 1992). We interpret the qN not due to state transitions between D4 and D12 as the result of an inhibition of PSII.
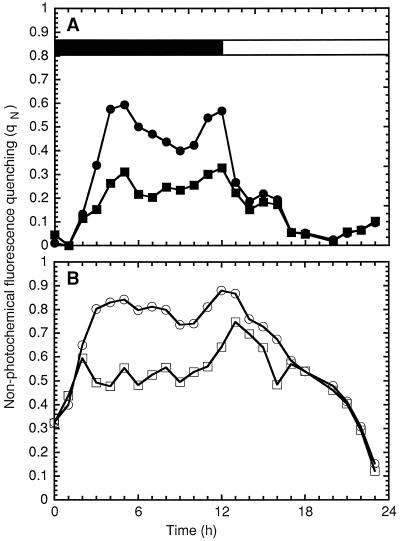
Figure 7.
Calculations of qN in 1 mL of Cyanothece sp. ATCC 51142 grown under 12-h light/12-h dark conditions using the levels of fluorescence depicted in Figure 6. Black bar, dark period; white bar, light period. A, Remaining qN after the induction of state 1 measured with flashes (▪) or with the actinic light (•). B, Total qN in the dark in the presence of DCMU measured with flashes (□) or with the actinic light (○).
The total qN determined under flashes of light (see Methods) was important during the dark period and decreased during the light period (Eq. 5c; Fig. 7B). The fluorescence quenching determined from the red actinic light (Eq. 5d; Fig. 7B) was identical, but only from L4 to D2; it was much higher from D4 to D12. The high fluorescence quenching from D4 to D12 was inconsistent with the previously reported state transitions, especially the strong state 1 observed at D6 (Meunier et al., 1997). This, plus the lower amplitudes of O2 evolution under flashing light, suggest that PSII centers were inactivated or inhibited in the dark during N2 fixation. The disappearance of that source of quenching in the light is consistent with a subsequent photoactivation or repair of PSII centers. It follows that the inactivation and photoactivation mechanisms in Cyanothece sp. ATCC 51142 are of particular interest.
The inactivation and photoactivation processes in Cyanothece sp. ATCC 51142 demonstrated unusual properties and striking differences between cells harvested at L8 and D8. Cells at L8 and D8 were harvested and kept in absolute darkness from the time of harvest until treatment; these precautions preserved activity. L8 cells illuminated with 3-Hz flashes produced an O2-evolution pattern shown in Figure 8A. A sample was then left on the electrode in the dark for 30 s, after which 15 additional flashes were given. Under these conditions, PSII O2 evolution declined more than 2-fold (Fig. 8A, curve 2). This pattern could be repeated several times until very little PSII activity remained (Fig. 8A, curves 3–5). This loss was reversible, as demonstrated by a 3-min photoactivation treatment of 10-Hz flashes prior to a flash train (Fig. 8A, curve P). The sample was in the dark for 15 min before these experiments and thus remained stable in complete darkness. This demonstrated that the dark inactivation of PSII was stimulated by exposure to 3-Hz light flashes followed by dark incubation. This property, coupled with the metabolic rhythms, made the determination of the decay times of S3 and S2 impractical (at least near L6–L10).
By contrast, cells harvested at D8 were relatively insensitive to the same treatment (Fig. 8B). After the initial measurement, PSII activity remained approximately constant after many cycles of light flashes and dark incubation. This experiment demonstrated that the effectiveness of 3-Hz flashes in triggering dark losses of activity was negligible in cells harvested at D8. However, it must be noted that the amplitudes of the O2 yields were about 3-fold lower in D8 cells than in L8 cells. This is one of the compelling reasons to conclude that many PSII centers were inhibited in the dark-grown cells.
Analysis of O2 Yields
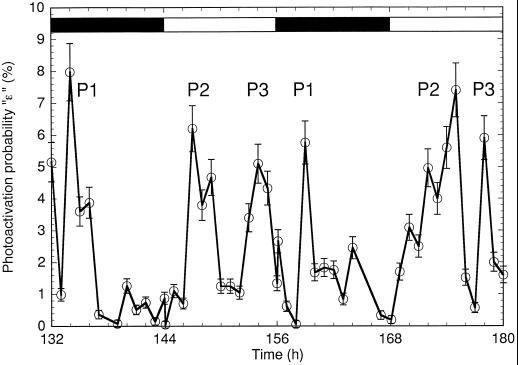
As discussed in “Materials and Methods,” the low O2 yields observed after dark adaptation resulted in a poor signal-to-noise ratio that prevented the unambiguous and reproducible fitting of S-state models. However, the photoactivation probability could be reliably recovered since it was calculated directly from the robust first eigenvalue, independently of any other probabilities or model assumptions (see Methods). The photoactivation of centers, ε, showed three repeating peaks, P1 to P3 (Fig. 9). P1 was close to the peak of N2 fixation, whereas P2 and P3 were in the first half and the second half of the light phase, respectively. Of these photoactivation peaks, only P2 was correlated with an increase in PSII activity. Clearly, the photoactivation probability after dark adaptation reflected a phenomenon other than an increase in the steady-state level of active PSII centers. However, the photoactivation treatment described in Methods was effective in lowering ε to 0, suggesting that ε could be inversely correlated with the dark stability of PSII. Indeed, P3 correlated with the reversible high dark instability of PSII (Figs. 2, 3, and 8), and P1 correlated with the inactivation of PSII centers.
Figure 9.
Photoactivation probability, ε, in dark-adapted cells of Cyanothece sp. ATCC 51142 for the last 48 h of the experiment shown in Figure 5 (○). Black bars, dark periods; white bars, light periods. P1, P2, and P3, Significant repeating periods of high photoactivation; the location of the peaks should be taken as the center of mass of the area under the curve (which is effectively spaced 24 h apart, whereas the maximum measured photoactivation probability is not necessarily spaced this way). The modeling errors were represented by the standard deviations calculated from the error matrix returned by the multivariable linear regression over a generic five-step equation. They were found to be smaller than or equal to 0.1% + 0.1 × ε, which are the values shown by the error bars. As many O2 yields as possible were used for the regression (in most cases, 14); however, the results were insensitive to the number of flashes used and to the inclusion of the first flash. The results were essentially identical to a generic four-step equation ignoring the first flash.
The O2 yields after photoactivation in Cyanothece sp. ATCC 51142 proved impossible to analyze consistently in the framework of variants of single Kok models (not shown). The six-step eigenvalue analysis of photoactivated O2 yields in Cyanothece sp. ATCC 51142 (Meunier et al., 1995b) provided a clue as to the origin of the problem by always finding two pairs of conjugated eigenvalues instead of the usual single pair. The experimental eigenvalues found for samples harvested at all times had a first eigenvalue equal to 1; therefore, the sum of all probabilities was 1 and the photoactivation probability was 0. This indicated that the photoactivation treatment had been effective in removing quickly photoactivatable PSII centers. The second, real and negative eigenvalue related to the damping mechanisms (Meunier, 1993) was also present within the usual range of values. However, two conjugated pairs of eigenvalues with large imaginary components were found instead of one conjugated pair and two more real eigenvalues. By mathematical necessity, the eigenvalues obtained from a Kok model (even extended to S−2) always contain only one pair of conjugated eigenvalues because there is only one cycle.
The two conjugated pairs suggested the presence of two S-state cycles (in different PSII centers) with significantly different properties. The consideration of PSII heterogeneity in the O2 yields permitted the successful interpretation of the eigenvalues (Table I). The combination of a dissipative or inefficient cycle with an efficient one could reproduce the O2 yields down to the level of experimental noise. The efficient centers retained similar properties, with about 9 to 11% apparent misses, 2 to 4% double-hits, and 0 to 1% backward transitions. By contrast, the inefficient centers changed throughout the cycle in number and in properties. The inefficient centers were characterized by a lower double-hit probability and higher backward transition and miss probabilities. The inefficiency was the most benign around D5, close to the previously observed strong state 1 and (presumed) oxidized state of the plastoquinone pool (Meunier et al., 1997).
Table I.
Calculated S-state transition probabilities for the two heterogeneous S-state cycles at different times during the metabolic rhythms of Cyanothece sp. ATCC 51142
| Growth Period | Cycle | S-State Transition Probabilities
|
||
|---|---|---|---|---|
| Misses (α) | Double hits (γ) | Backward transitions (δ) | ||
| L5 | Efficient | 0.11 | 0.04 | 0.00 |
| Wasteful | N.Q.a | N.Q. | N.Q. | |
| L8 | Efficient | 0.09 | 0.03 | 0.01 |
| Wasteful | 0.15 | 0.00 | 0.12 | |
| D1 | Efficient | 0.09 | 0.02 | 0.01 |
| Wasteful | 0.11 | 0.00 | 0.10 | |
| D5 | Efficient | 0.09 | 0.03 | 0.00 |
| Wasteful | 0.11 | 0.02 | 0.07 | |
| D12 (L0) | Efficient | 0.10 | 0.03 | 0.01 |
| Wasteful | 0.10 | 0.02 | 0.10 | |
The single-hit probability is equal to 1 minus the sum of the other probabilities because after the photoactivation treatment, the first eigenvalue was always very close to 1.
N.Q., Not quantifiable. The constants C5 and C6 in Equation 1 were so small that the estimated importance of the second cycle was less than 0.5% of the O2 yields, and therefore the 5th and 6th eigenvalues were ill determined.
There were too few inefficient centers to quantify their properties at L5, in agreement with our proposal that centers were photoactivated in the early light period. The numbers provided in Table I are not unique, because there are many combinations of heterogeneous cycles that could produce O2 yields close to the experimental ones. However, this interpretation is the simplest, being an exact solution, and is consistent throughout the metabolic rhythms in Cyanothece sp. ATCC 51142. Thus, PSII heterogeneity could explain the properties of the O2 yields found after photoactivation. We interpret this as evidence for a difference in the properties of freshly activated PSII (inefficient PSII) compared with the centers that were stable in the dark.
DISCUSSION
The data presented in this paper indicated that O2 evolution underwent many changes during the diurnal cycle of the diazotrophic cyanobacterium Cyanothece sp. ATCC 51142. The capacity for O2 evolution, the time required for O2 release by the S-state mechanism, and the dark stability of PSII centers changed dramatically throughout a diurnal cycle. In particular, the data reflect a substantial level of PSII heterogeneity as the cells are preparing for N2 fixation. It is evident that PSII is a main target for down-regulation during nitrogenase activity.
The regulation of photosynthesis in Cyanothece sp. ATCC 51142 is mediated in part through state transitions and changes in reaction-center oligomerization (Meunier et al., 1997). The source of qN that developed in the early dark was an inhibition of PSII that lowered variable fluorescence (Fv [= FM − FO]), even in the presence of DCMU, and which led to lower amplitudes of O2 evolution. The dark inactivation of PSII centers during N2 fixation occurred while PSII centers were in the monomeric form following a transition to state 2 (Meunier et al., 1997). After dark adaptation, the amplitudes of O2 evolution were negligible at D3; however, the amplitudes after 3 min of 10-Hz flashes were comparable to one-third of the maximum PSII amplitudes. The partial recovery may be explained by a reduced state of the plastoquinone pool during the intense respiratory activity associated with N2 fixation and a re-oxidation of the plastoquinone pool by the flashes. The fact that it was partial demonstrates that some PSII were inhibited. Indeed, the previously observed sudden and strong transition to state 1 after the peak in N2 fixation, indicating an oxidation of the plastoquinone pool, appeared correlated with only a partial recovery of O2 evolution in the experiments reported here. Meanwhile, the PAM fluorescence method was unable to detect state transitions in the period from D3 to L1, due to the significant inhibition of PSII.
The photoactivation of PSII centers in the early light occurred while the cells were close to state 2, which favors excitation of PSI. Progressively, as PSII centers were photoactivated, the qN diminished and revealed a strong state 1 toward the end of the light period. However, at any given time, the PAM method was ambiguous as to the contributions to qN from state transitions or from not-yet-photoactivated centers. The analysis of the O2 yields revealed a strong photoactivation probability in the early part of the light phase, whereas the 77 K spectra in Meunier et al. (1997) indicated that most of the transition to state 1 occurred in the second half of the light phase. Thus, the photoactivation of PSII centers was prevalent in the first half of the light phase, when the centers were mainly in monomeric form.
At the beginning of the light period, when most PSII centers were inhibited, the S-state mechanism produced slow O2 signals of a period 4. As photoactivation progressed, these changed to fast signals (Figs. 1 and 2), demonstrating the simultaneous presence of PSII centers with differing properties that were interconvertible. The O2 produced was contributed by (at least) two populations of PSII centers and required two S-state cycles for modeling. The data we obtained for the interconversion and photoactivation processes suggests the repair of an inefficient, dark-unstable PSII form to an efficient, faster one.
We have developed a modified model of the PSII S-state mechanism in cyanobacteria (Meunier et al., 1996), which has formalized the significant differences in flash-yield experiments between cyanobacteria and chloroplasts. The modeling of cyanobacterial O2 evolution required explicit deactivations in the dark interval between flashes and in homogeneous probabilities, and an explicit accounting of the number of active PSII centers by photoinhibition or photoactivation (Meunier et al., 1996). The model was used to explain some of the interesting properties of Synechocystis sp. PCC 6803 wild type and the psbO deletion mutant (lacking the Mn2+-stabilizing protein MSP) (Meunier et al., 1996).
The comparison of flash-yield results among Synechocystis wild type, the psbO deletion mutant, and Cyanothece cells from different times in the diurnal cycle led to the discovery of some important similarities. O2 evolution by wild-type Synechocystis sp. PCC 6803 is very stable in the dark and remains so even after a prolonged period of centrifugation onto a bare Pt electrode (Burnap et al., 1996; Meunier et al., 1996). The psbO deletion mutant of Synechocystis sp. PCC 6803 shows very poor O2 evolution after dark incubation. However, after a 3-min flash regime at 10 Hz, the same sample showed a greatly enhanced production of O2 (i.e. high photoreactivation; Meunier et al., 1996, fig. 3). Therefore, we suggest that Cyanothece sp. ATCC 51142 cells sometimes during the light period have an O2-evolving mechanism that resembles the psbO deletion mutant of Synechocystis sp. PCC 6803. At the same time, the PSII centers of Cyanothece sp. cells growing in the dark are fewer, but possess a dark stability more similar to that of Synechocystis sp. PCC 6803 wild type. Moreover, 77 K spectra indicated changes in the oligomerization of PSII centers (Meunier et al., 1997). These observations and the replacement of the D1 protein suggest that the protein conformations, composition, and oligomerization of PSII centers are under constant flux in Cyanothece sp. ATCC 51142.
ACKNOWLEDGMENT
We are grateful to Dr. Rob Burnap of Oklahoma State University for the use of his bare Pt electrode equipment and for his suggestion to photoactivate the cells.
Abbreviations:
- DX
X h of darkness
- FM
maximum fluorescence
- FO
initial fluorescence
- LX
X h of light
- PAM
pulse-amplitude-modulated
- qN
nonphotochemical quenching
Footnotes
This work was supported by grants from the U.S. Department of Energy (no. DE-FG02-89ER14028A) and the U.S. Department of Agriculture (no. 93-37306-9238) to L.A.S.
LITERATURE CITED
- Arnon DI, McSwain BD, Tsujimoto HY, Wada K. Photochemical activity and components of membrane preparations from blue-green algae I: coexistence of two photosystems in relation to chlorophyll aand removal of phycocyanin. Biochim Biophys Acta. 1974;357:231–245. doi: 10.1016/0005-2728(74)90063-2. [DOI] [PubMed] [Google Scholar]
- Bader KP. Physiological and evolutionary aspects of the H2O/H2O2-cycle in cyanobacteria. Biochim Biophys Acta. 1994;1188:213–219. [Google Scholar]
- Bader KP, Thibault P, Schmid GH. A study on oxygen evolution and on the S-state distribution in thylakoid preparations of the filamentous blue-green alga Oscillatoria chalybea. Z Naturforsch. 1983;38c:778–792. [Google Scholar]
- Bergman B, Gallon JR, Rai AN, Stal LJ. N2fixation by non-heterocystous cyanobacteria [review] FEMS Microbiol Rev. 1997;19:139–185. [Google Scholar]
- Burnap RL, Qian M, Pierce C. The manganese-stabilizing protein of photosystem II modifies the in vivo deactivation and photoactivation kinetics of the H2O oxidation complex in Synechocystissp. PCC 6803. Biochemistry. 1996;35:874–882. doi: 10.1021/bi951964j. [DOI] [PubMed] [Google Scholar]
- Burnap RL, Shen JR, Jursinic PA, Inoue Y, Sherman LA. Oxygen yield and thermoluminescence characteristics of a cyanobacterium lacking the manganese-stabilizing protein of photosystem II. Biochemistry. 1992;31:7404–7410. doi: 10.1021/bi00147a027. [DOI] [PubMed] [Google Scholar]
- Campbell D, Öquist G. Predicting light acclimation in cyanobacteria from nonphotochemical quenching of photosystem II fluorescence, which reflects state transitions in these organisms. Plant Physiol. 1996;111:1293–1298. doi: 10.1104/pp.111.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheniae GM, Martin IF. Effects of hydroxylamine on photosystem II. II. Photoreversal of the NH2OH destruction of O2evolution. Plant Physiol. 1972;50:87–94. doi: 10.1104/pp.50.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colón-López MS, Sherman DM, Sherman LA. Transcriptional and translational regulation of nitrogenase in light-dark- and continuous-light-grown cultures of the unicellular cyanobacterium, Cyanothecesp. ATCC 51142. J Bacteriol. 1997;179:4319–4327. doi: 10.1128/jb.179.13.4319-4327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels DH, Lott A, Schmid GH, Pistorious EK. Inactivation of the water-oxidizing enzyme in manganese stabilizing protein-free mutant cells of the cyanobacteria Synechococcus PCC 7942 and SynechocystisPCC 6803 during the dark incubation and conditions leading to photoactivation. Photosynth Res. 1994;42:227–244. doi: 10.1007/BF00018265. [DOI] [PubMed] [Google Scholar]
- Fay P. Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol Rev. 1992;56:340–373. doi: 10.1128/mr.56.2.340-373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay P, Cox RM. O2 inhibition of N2fixation in cell-free preparations of blue-green algae. Biochim Biophys Acta. 1967;143:562–569. doi: 10.1016/0005-2728(67)90061-8. [DOI] [PubMed] [Google Scholar]
- Gleiter HM, Haag E, Shen JR, Eaton-Rye JJ, Seeliger AG, Inoue Y, Vermaas WFJ, Renger G. Functional characterization of mutant strains of the cyanobacterium Synechocystissp. PCC 6803 lacking short domains within the large, lumen-exposed loop of the chlorophyll protein CP-47 in photosystem II. Biochemistry. 1995;34:6847–6856. doi: 10.1021/bi00206a008. [DOI] [PubMed] [Google Scholar]
- Grobbelaar N, Lin HY, Huang TC. Induction of a nitrogenase activity rhythm in Synechococcusand the protection of its nitrogenase against photosynthetic oxygen. Curr Microbiol. 1987;15:29–33. [Google Scholar]
- Haystead A, Robinson R, Stewart WDP. Nitrogenase activity in extracts of heterocystous blue-green algae. Arch Mikrobiol. 1970;75:235–243. doi: 10.1007/BF00408884. [DOI] [PubMed] [Google Scholar]
- Kok B, Forbush B, McGloin M. Cooperation of charges in photosynthetic O2evolution-1. A linear four step mechanism. Photochem Photobiol. 1970;11:457–475. doi: 10.1111/j.1751-1097.1970.tb06017.x. [DOI] [PubMed] [Google Scholar]
- Kretschmann H, Witt HT. Chemical reduction of the water splitting enzyme system of photosynthesis and its light-induced reoxidation characterized by optical and mass spectrometric measurements: a basis for the estimation of the states of the redox active manganese and of water in the quaternary oxygen-evolving S-state cycle. Biochim Biophys Acta. 1993;1144:331–345. [Google Scholar]
- Krieger A, Moya I, Weis E. Energy-dependent quenching of chlorophyll-A fluorescence: effect of pH on stationary fluorescence and picosecond-relaxation kinetics in thylakoid membranes and photosystem II preparations. Biochim Biophys Acta. 1992;1102:167–176. [Google Scholar]
- Messinger J, Renger G. Generation, oxidation by the oxidized form of the tyrosine of polypeptide D2, and possible electronic configuration of the redox states S0, S−1, and S−2of the water oxidase in isolated spinach thylakoids. Biochemistry. 1993;32:9379–9386. doi: 10.1021/bi00087a017. [DOI] [PubMed] [Google Scholar]
- Messinger J, Seaton G, Wydzynski T, Wacker U, Renger G. S−3state of the water oxidase in photosystem II. Biochemistry. 1997;36:6862–6873. doi: 10.1021/bi962653r. [DOI] [PubMed] [Google Scholar]
- Meunier PC. O2evolution by photosystem II: the contribution of backward transitions to the anomalous behaviour of double-hits revealed by a new analysis method. Photosynth Res. 1993;36:111–118. doi: 10.1007/BF00016276. [DOI] [PubMed] [Google Scholar]
- Meunier PC, Burnap RL, Sherman LA. Interaction of the photosynthetic and respiratory electron transport chains producing slow O2 signals under flashing light in Synechocystissp. PCC 6803. Photosynth Res. 1995a;45:31–40. doi: 10.1007/BF00032233. [DOI] [PubMed] [Google Scholar]
- Meunier PC, Burnap RL, Sherman LA. Improved 5-step modeling of the photosystem II S-state mechanism in cyanobacteria. Photosynth Res. 1996;47:61–76. doi: 10.1007/BF00017754. [DOI] [PubMed] [Google Scholar]
- Meunier PC, Colón-López MS, Sherman LA. Temporal changes in state transitions and photosystem organization in the unicellular, diazotrophic cyanobacterium Cyanothecesp. ATCC 51142. Plant Physiol. 1997;115:991–1000. doi: 10.1104/pp.115.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier PC, Popovic R. High-accuracy O2polarograph for photosynthetic systems. Rev Sci Inst. 1988;59:486–491. [Google Scholar]
- Meunier PC, Watters JW, Colón-López MS, Sherman LA. Regulation of the O2-evolving mechanism during N2 fixation in the diazotrophic cyanobacterium Cyanothecesp. ATCC 51142. In: Mathis P, editor. Research in Photosynthesis, Vol 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995b. pp. 389–392. [Google Scholar]
- Misra HS, Desai TS. Involvement of acceptor side components of PSII in the regulatory mechanism of Plectonema boryanumgrown photoautotrophically under diazotrophic condition. Biochem Biophys Res Com. 1993;194:1001–1007. doi: 10.1006/bbrc.1993.1920. [DOI] [PubMed] [Google Scholar]
- Misra HS, Tuli R. Nitrogen fixation by Plectonema boryanumhas a photosystem II independent component. Microbiology. 1994;140:971–976. [Google Scholar]
- Mitsui A, Cao S, Takahashi A, Arai T. Growth synchrony and cellular parameters of the unicellular nitrogen-fixing marine cyanobacterium, Synechococcussp. strain Miami BG043511 under continuous illumination. Physiol Plant. 1987;69:1–8. [Google Scholar]
- Miyao M, Ikeuchi M, Yamamoto N, Ono T. Specific degradation of the D1 protein of photosystem-II by treatment with hydrogen-peroxide in darkness: implications for the mechanism of degradation of the D1 protein under illumination. Biochemistry. 1995;34:10019–10026. doi: 10.1021/bi00031a025. [DOI] [PubMed] [Google Scholar]
- Mullineaux PM, Gallon JR, Chaplin AE. Acetylene reduction (nitrogen fixation) by cyanobacteria grown under alternating light dark cycles. FEMS Microbiol. 1981;10:245–247. [Google Scholar]
- Reddy KJ, Haskell B, Sherman DM, Sherman LA. Unicellular, aerobic N2-fixing cyanobacteria of the genus Cyanothece. J Bacteriol. 1993;175:1284–1292. doi: 10.1128/jb.175.5.1284-1292.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneegurt M, Sherman DM, Nayar S, Sherman LA. Oscillating behaviour of carbohydrate granule formation and dinitrogen fixation in the cyanobacterium Cyanothecesp. strain ATCC 51142. J Bacteriol. 1994;176:1586–1597. doi: 10.1128/jb.176.6.1586-1597.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkarev VP, Wraight CA. Oxygen evolution in photosynthesis: from unicycle to bicycle. Proc Natl Acad Sci USA. 1995;90:1834–1838. doi: 10.1073/pnas.90.5.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]