Bilateral diffuse uveal melanocytic proliferation (BDUMP) is a paraneoplastic syndrome resulting in profound bilateral vision loss, with approximately 30 cases reported in the world’s literature. In 1990, Gass et al1 described 5 characteristic signs of the disease: multiple subretinal round red patches in the retinal pigment epithelium (RPE), early fluorescence of these lesions on fluorescein angiography, multiple elevated pigmented and nonpigmented uveal melanocytic tumors with diffuse uveal tract thickening, exudative retinal detachments, and rapid cataract development. The histopathologic findings include diffuse uveal infiltration by benign hypopigmented spindle cells and occasional epithelioid cells. There is focal infiltration of the choroid by heavily pigmented melanocytes with sparing of the choriocapillaris.1–4 Destruction of the RPE occurs in areas overlying the infiltrate.1
Treatment for BDUMP has been largely unsuccessful. Modalities have included corticosteroids, ocular surgery, ocular radiation, and treatment of the underlying malignant neoplasm.1–3,5 While some have shown transient vision improvement or stabilization, we describe a new treatment for this visually devastating condition that resulted in vision improvement and stability with continued treatment until the patient’s death.
Report of a Case
A 72-year-old man had bilateral decreased, dim vision for 1 month. Four months prior, he was diagnosed as having metastatic bronchogenic carcinoma, for which he was taking sorafenib.
Best-corrected visual acuities were 20/40+2 OD and 20/50+2 OS. Anterior segment examination results were normal. Dilated examination revealed clear media, a normal disc, and attenuated arterioles in each eye. In the right eye, there was a small pigmented lesion, a localized exudative retinal detachment, and an area of orange-brown giraffe-type pigmentation. In the left eye, 7 slightly elevated pigmented lesions with extensive giraffe-type pigmentation were present. The oval spots within the giraffe-type pigmentation appeared mildly hyperpigmented and were hypoautofluorescent on fundus autofluorescent photography and hyperfluorescent on fluorescein angiography in both eyes. The pigmented tumors appeared dark on indocyanine green angiography. Optical coherence tomography revealed subretinal fluid in the area of the inferotemporal retinal detachment, which extended to the fovea in the right eye. There was also subfoveal fluid in the left eye (Figure 1). B-scan ultrasonography showed diffuse choroidal thickening and discrete nodules with medium to high internal reflectivity. Electroretinographic results were normal. Goldmann visual fields revealed scotomas corresponding to the pigmented tumors and a generalized decreased peripheral visual field.
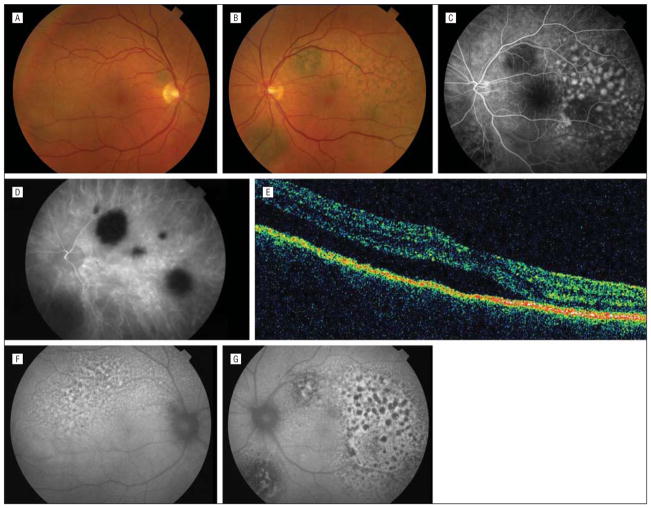
Figure 1.
Initial manifestation. A, Color fundus photograph of the right eye showing a pigmented round lesion and area of serous retinal detachment. B, Color fundus photograph of the left eye showing multiple pigmented round lesions and areas of giraffe-type pigmentation. C, Fluorescein angiogram of the left eye showing early fluorescence corresponding to areas of giraffe-type pigmentation and blockage of the choroid. D, Indocyanine green angiogram of the left eye showing round areas of hypofluorescence corresponding to pigmented tumors. E, Optical coherence tomographic scan of the right eye showing subfoveal subretinal fluid. Fundus autofluorescent photographs of the right (F) and left (G) eyes showing areas of hyperautofluorescence and hypoautofluorescence corresponding to giraffe-type pigmentation.
The findings were diagnostic of BDUMP. Treatment with sorafenib continued. Because we believed that a circulating growth factor might be responsible for the findings in BDUMP, plasmapheresis was initiated 3 times per week for the ophthalmic abnormalities.
After 12 sessions, best-corrected visual acuities were 20/20−2 OD and 20/25−2 OS. There was disappearance of serous detachments, thinning of the choroid, a decrease in giraffe-type pigmentation, and increased visibility of underlying pigmented tumors. Plasmapheresis was decreased to once weekly. After 17 sessions, visual symptoms were entirely resolved, and best-corrected visual acuities were 20/20 OD and 20/25+2 OS (Figure 2).
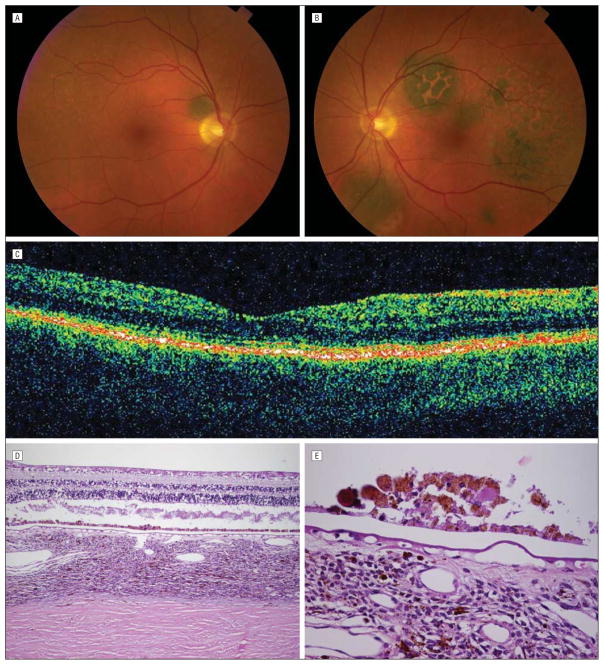
Figure 2.
Six months later, after treatment with plasmapheresis, resolution of symptoms, and return of visual acuity to 20/20 OU. A, Color fundus photograph of the right eye showing increased visibility of round, darkly pigmented tumor and resolution of subretinal fluid. B, Color fundus photograph of the left eye showing increased visibility of darkly pigmented tumors and decrease in overlying orange pigment. C, Optical coherence tomographic scan showing resolution of subretinal fluid. D, Low-power photomicrograph of the retina and choroid showing artifactual retinal detachment, diffusely thickened choroid with increased uveal melanocytic cells, areas of normal retinal pigment epithelium, and areas of hyperplastic retinal pigment epithelium (hematoxylin-eosin). E, High-power photomicrograph of hyperplastic retinal pigment epithelium, also showing some of the uveal melanocytic cells, which were mainly spindle cells with no atypia (hematoxylin-eosin).
Seven months after the initial ophthalmologic visit, the patient became too ill to continue plasmapheresis treatments. Visual acuity was 20/20 OU. Once plasmapheresis was stopped, there was a relapse of fundus abnormalities and subretinal fluid returned; 1 month prior to the patient’s death, best-corrected visual acuities declined to 20/200 OD and 20/30 OS. Death occurred 13 months after the onset of visual symptoms.
The globes were sectioned and examined with hematoxylin-eosin (Figure 2). Serum was tested for antiretinal autoantibodies by Western blotting and was positive for autoantibodies against 33- and 34-kDa human retinal proteins. These autoantibodies were tested against proteins extracted from the patient’s lung tumor, and no specific staining was noted. Against whole rat eye sections, they showed positive staining of photoreceptors (especially outer segments), some ganglion cells, and the cytoplasm of some choroid and iris cells.
Comment
Nearly all patients with BDUMP have been treated for their underlying malignant neoplasm, but our case is the first to our knowledge to demonstrate successful treatment for the eye findings with return to baseline visual acuity and resolution of visual symptoms.
Although rare, BDUMP has consistently resulted in devastating visual consequences. Usually during the year preceding death, patients with this paraneoplastic syndrome have severe bilateral vision loss. Vision decline has been attributed to destruction of photoreceptors and underlying RPE, serous retinal detachments, and, later, cataracts.1–3 In our patient, cataracts never developed, sub-retinal fluid and choroidal thickening resolved, and visual acuity improved to 20/20 following plasmapheresis. As plasmapheresis works by removing proteins from blood, our patient’s positive response suggests that circulating antibodies or growth factors may be responsible.
Histopathologic findings were consistent with BDUMP, with the exception of areas of RPE hypertrophy instead of more typical RPE loss.1,3,5 Hypertrophy of the RPE with cells dividing, accumulating, and migrating into the sub-retinal space can occur when subretinal fluid is present, and RPE acquires lipofuscin as the cells phagocytize photoreceptor outer segments. We hypothesize that the RPE hypertrophy may correspond to giraffe-type pigmentation; however, we do not have a point-by-point comparison between histopathologic specimens and fundus photographs.
As has been reported in other cases of BDUMP,1,5 our patient had circulating antiretinal autoantibodies. The significance of our patient’s 33- and 34-kDa retinal proteins to which these autoantibodies react is not known. Much is still to be learned in the field of antiretinal autoantibodies, but this raises the possibilities that patients with BDUMP can have such antibodies and that these antibodies may be at least partially responsible for the loss of photoreceptors.4
In our patient, we believe that a circulating growth factor or antibody may have been responsible for stimulation of the pathologic changes noted in BDUMP. This notion is supported by the return of good visual acuity, resolution of subretinal fluid, and decreased choroidal thickening during plasmapheresis treatments and also by the recurrence of these abnormalities with cessation of plasmapheresis.
We report a new treatment modality that has the potential to improve and stabilize vision in a disease that results in bilateral vision loss preceding death in patients with systemic malignancy. Plasmapheresis should be considered in patients with BDUMP.
Acknowledgments
Funding/Support: This work was supported in part by an unrestricted grant from Research to Prevent Blindness and grant EY13053 from the National Institutes of Health.
Footnotes
Financial Disclosure: None reported.
References
- 1.Gass JD, Gieser RG, Wilkinson CP, Beahm DE, Pautler SE. Bilateral diffuse uveal melanocytic proliferation in patients with occult carcinoma. Arch Ophthalmol. 1990;108(4):527–533. doi: 10.1001/archopht.1990.01070060075053. [DOI] [PubMed] [Google Scholar]
- 2.Barr CC, Zimmerman LE, Curtin VT, Font RL. Bilateral diffuse melanocytic uveal tumors associated with systemic malignant neoplasms: a recently recognized syndrome. Arch Ophthalmol. 1982;100(2):249–255. doi: 10.1001/archopht.1982.01030030251003. [DOI] [PubMed] [Google Scholar]
- 3.Gass JD, Glatzer RJ. Acquired pigmentation simulating Peutz-Jeghers syndrome: initial manifestation of diffuse uveal melanocytic proliferation. Br J Ophthalmol. 1991;75(11):693–695. doi: 10.1136/bjo.75.11.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ritland JS, Eide N, Tausjø J. Bilateral diffuse uveal melanocytic proliferation and uterine cancer: a case report. Acta Ophthalmol Scand. 2000;78(3):366–368. doi: 10.1034/j.1600-0420.2000.078003366.x. [DOI] [PubMed] [Google Scholar]
- 5.Saito W, Kase S, Yoshida K, et al. Bilateral diffuse uveal melanocytic proliferation in a patient with cancer-associated retinopathy. Am J Ophthalmol. 2005;140(5):942–945. doi: 10.1016/j.ajo.2005.05.048. [DOI] [PubMed] [Google Scholar]