Abstract
A decrease in zinc (Zn) levels increases the production of cell oxidants, affects the oxidant defense system and triggers oxidant sensitive signals in neuronal cells. However, the underlying mechanisms are still unclear. This work tested the hypothesis that the increase in neuronal oxidants that occurs when cellular Zn decreases is mediated by the activation of the NMDA receptor. Differentiated PC12 cells were cultured in control, Zn-deficient or Zn-repleted media. The incubation in Zn deficient media led to a rapid increase in cellular calcium levels, which was prevented by a NMDA receptor antagonist (MK-801). Cellular calcium accumulation was associated with NADPH oxidase and nitric oxide synthase (NOS) activation, an increase in cell oxidant levels, and an associated activation of a redox-sensitive signal (AP-1). In cells incubated in the Zn deficient medium, NADPH oxidase activation was prevented by MK-801 and by a protein kinase C inhibitor. The rise in cell oxidants was prevented by inhibitors of NADPH oxidase, of the NOS and by MK-801. A similar pattern of inhibitor action was observed for zinc deficiency-induced AP-1 activation. Results demonstrate that a decrease in extracellular Zn leads to an increase in neuronal oxidants through the activation of the NMDAR that leads to calcium influx and to a calcium-mediated activation of protein kinase C/NADPH oxidase and NOS. Changes in extracellular Zn concentrations can be sensed by neurons, which using reactive oxygen and nitrogen species as second messengers, can regulate signaling involved in neuronal development and function.
Keywords: zinc, zinc deficiency, neuron, NMDA receptor, NADPH oxidase, oxidants, NOS, AP-1
Introduction
A significant portion of the population, especially infants, pregnant women and the elderly, are at risk of Zn deficiency, not only in developing countries, but also in developed nations [1]. Dietary Zn deficiency is associated with retarded growth and development, impaired reproduction, poor immunity, reduced neurosensory function and altered behavior [2, 3]. As Zn homeostasis in the brain is closely related to neuronal activity, an adequate supply of Zn is essential for normal brain function and the prevention of neurological diseases. In this regard, oxidative stress could, in part, mediate the deleterious effect of Zn deficiency on brain development and/or function.
Several studies have shown that in neurons, Zn is a selective inhibitor of the N-methyl-D-aspartate (NMDA) receptor (NMDAR) [4, 5], an action that may explain many of the adverse effects associated with Zn deficiency as this could result in NMDAR-mediated excitotoxicity. NMDAR is blocked by Zn at two independent sites [6, 7]. A low-affinity Zn site is located inside the channel pore and a high-affinity site is likely located outside the channel pore. The high sensitivity of the NMDAR to Zn allows even small variations in Zn concentrations to modulate NMDAR activation[8, 9]. When activated, NMDAR allows cations such as sodium, potassium and calcium (Ca) to pass through the channel, which can trigger different cell responses.
It has been consistently observed, both in vitro and in vivo, that Zn deficiency is characterized by oxidant production and increased oxidative damage to cell components [10-12]. For instance, increased lipid, protein and DNA oxidation was found in testes from Zn-deficient rats as compared to control animals [13]. In neurons, reactive oxygen species (ROS) decisively contribute to cellular signaling, affecting almost all aspects of cell function including gene expression, proliferation, migration and death [14]. Although it has been previously shown in Zn deficient neurons that there is an increased ROS production and an associated triggering of select neuronal signaling [12, 15], there is limited information on the biochemical mechanisms linking a low neuronal Zn availability to ROS generation.
Initially, studies suggested that the mechanism by which the activation of the NMDAR induces oxidative stress was through mitochondrial depolarization and subsequent oxidant generation. Recently, it was shown [16] that in neuronal cells, the primary mechanism by which stimulation of the NMDAR by NMDA induces oxidative stress involves the activation of NADPH oxidase, a superoxide (O2⋅−) producing enzymatic complex. The ability of NADPH oxidase to produce ROS rapidly makes it the main source of ROS production involved in cell signaling [17]. NADPH oxidase is a multi-subunit enzyme that consists of cytosolic components and membrane components. Upon activation, the cytosolic components translocate to the membrane to form the enzymatic complex, which transfers electrons from NADPH to O2 to produce superoxide anion (O2⋅−). Although NADPH oxidases were thought to be confined to cells of hematopoietic origin, several studies have reported its presence in neuronal cells [18-20]. At least five isoforms of NOX have been found so far, which vary in tissue or cell type localization and in their regulation and function. Noteworthy, several NOX isoforms can coexist within an individual cell or tissue. For instance, low levels of NOX4 and NOX5 (the only NOX isoform containing Ca binding sites) have been found in adult human brain tissue [21]. NOX4 has been detected in mouse cortical neurons, in pyramidal cells of the hippocampus and in Purkinje cells of the cerebellum [22]. Furthermore, NOX1, NOX2 and NOX3 were detected in rat brain tissue [23]. The mechanisms involved in the activation of NADPH oxidase are still unclear. However, it has been observed that high levels of intracellular Ca and protein kinase C (PKC) activation can cause NADPH oxidase activation [16, 24, 25]. Another oxidant-generating enzyme, nitric oxide synthase (NOS), is also Ca sensitive. NOS catalizes the oxidation of L-arginine into citrulline and nitric oxide. Nitric oxide is a diffusible gas molecule that can act as a signaling molecule, interact and modify cellular components and regulate ROS levels through its reaction with O2⋅−to render highly reactive nitrogen species (RNS) such as peroxinitrite. Thus, NOS has been implicated in modulating physiological functions such as learning, memory, and neurogenesis, as well as being involved in the pathophysiology of several human diseases [26].
Given our findings that a decrease in Zn concentration can induce a rapid increase in neuronal oxidants, this study investigated whether a decrease in extracellular Zn levels could increase neuronal oxidant production through the activation of the NMDAR. We investigated the effect of Zn deficiency on NMDAR activation, Ca influx, Ca-mediated NADPH oxidase and NOS activation and subsequent oxidants generation.
Since Ca, ROS and RNS play major roles in the modulation of neuronal signals, the association of cellular Ca and ROS variations with extracellular Zn and the activation of transcription factor activator protein 1 (AP-1) was also investigated. AP-1 is particularly relevant given the role of this redox sensitive transcription factor in critical developmental events (neuronal proliferation, differentiation, apoptosis) [27, 28].
Materials and methods
Materials
PC12 cells were obtained from the American Type Culture Collection (Rockville, MA). Cell culture media and reagents, and LipofectAMINE™ 2000 were obtained from Invitrogen Life Technologies (Carlbad, CA, USA). The oligonucleotide containing the consensus sequences for AP-1 (5′-CGC TTG ATG AGT CAG CCG GAA-3′), the reagents for the EMSA assay and the Luciferase Assay System were obtained from Promega (Madison, WI, USA). The PathDetect AP-1 cis-reporting system was obtained from Stratagene (La Jolla, CA, USA). Polyclonal antibodies for p67phox and NMDAR type 1 (NR1) were from Upstate (Charlottesville, VA, USA) and the antibody for β-tubulin was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Fura-2-AM, Fluo-4-AM, 5-(and-6)-carboxy-2′7′-dichlorodihydrofluorescein diacetate (DHDCFDA), 4, 5-diaminofluorescein-diacetate (DAF-2), and propidium iodide were obtained from Molecular Probes (Eugene, OR, USA). The ECL western blotting system was from Amersham Pharmacia Biotech Inc. (Piscataway, NJ, USA). Apocynin (APO) was obtained from Calbiochem (San Diego, CA, USA). Lipoic acid, diphenyleneiodonium chloride (DPI), MK-801 hydrogen maleate (MK-801), 8-(diethylamino)octyl 3,4,5-trimethoxybenzoate hydrochloride (TMB-8), 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis (acetoxymethyl) ester (BAPTA-AM), Ro-32-0432 (Ro), N(G)-nitro-L-arginine-methyl ester (L-NAME), 7S nerve growth factor (7S NGF) and all other reagents were from the highest quality available and were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture and incubations
All cell culture experiments were performed using PC12 differentiated cells. PC12 cells are derived from pheochromocytomas and can be differentiated into neuronal cells in the presence of nerve growth factor [29, 30], where they acquire morphological, electrophysiological and functional characteristics similar to neuronal cells. PC12 cells were cultured in DMEM high glucose supplemented with 10 % (v/v) horse serum, 5 % (v/v) fetal bovine serum and antibiotics (50 U/ml penicillin, 50 μg/ml streptomycin) in poly-L-lysine coated plates. The cells were then induced to differentiate by growing in DMEM supplemented with 10% FBS and 50 ng/ml 7S NGF. The medium was replaced every other day during 7 days.
Zn deficient serum was prepared as previously described [31]. The Zn deficient serum was subsequently diluted with DMEM to a final concentration of 3 mg protein/ml to match the protein concentration of the control non-deficient media. The Zn concentration of the deficient medium was adjusted to 1.5 μM (1.5 Zn medium). In order to control for any other molecules that may be lost during the Zn deficient serum preparation, an aliquot of the Zn deficient medium was supplemented with ZnCl2 to obtain a final concentration of 15 mM (15 Zn medium).
After 7 days of differentiation, the media was removed and replaced with control medium or media containing 1.5 or 15 μM Zn. Cells were harvested at different time points.
Determination of intracellular calcium levels
Cells were loaded with 5 mM Fura-2-AM containing the non-ionic detergent Pluronic F-127 for 60 min. After loading, the media was removed; the cells were washed twice with phosphate buffered saline (PBS) and incubated in control, 1.5 Zn or 15 Zn media. After 1 h of incubation, the media was removed; cells were rinsed with PBS without calcium and measured as previously described [32]. Results are expressed as the ratio of the fluorescence measured with λexc 340 nm to λexc 380 nm.
Determination of cellular calcium variations by scanning laser confocal microscopy
In order to image increases in intracellular Ca in a time-lapse manner, PC12 cells (3 × 105 cells/coverslip) cultured on polylysine-treated glass coverslips (22 mm diameter) were incubated in control media and loaded with 2 μM of Fluo-4-AM for 20 min at 37°C. After loading, cells were washed twice with control media The coverslips were placed in glass culture dishes and the different media to be tested were added to the cells on the stage of an Olympus BX61WI fixed stage upright microscope equipped with a Peltier stage that maintained the media at 37°C. Water immersion fluorescence objective lenses (10× or 20×) were then used to image cells attached to the coverslip using interference contrast optics. Once representative fields of view were identified, cells were immediately imaged using the scanning laser mode of the Olympus Fluoview 500 scanning laser confocal microscope, which was equipped with four lasers (blue diode 405nm, argon 488nm, krypton 568nm, and HeNe 633nm) and four photomultiplier tubes (PMTs). For intracellular Ca imaging, the argon laser was used for excitation with the emission at 510 nm for Fluo-4 detection as well as for simultaneous transmitted light (interference contrast) imaging.
In order for each experiment to have an internal control, image capture of cells prior to each experimental manipulation was immediately followed by a rapid change in media; the control media was removed and the medium to be tested [control media or chelated media containing 1.5 μM Zn with or without the inhibitors] was added, with continued imaging over a 5 min. period of time. Image capture was conducted every 30 sec during this time period. A different set of cells was used for each experimental condition. For all experiments, both laser intensity and PMT voltage were identical so comparisons in Fluo-4 intensity could be conducted. Fluo-4 fluorescence images were overlayed on the interference contrast light images (using the Fluoview and Tiempo software) in order to localize regions of Ca increase. All experiments were repeated at least 3 times for a given group of cells.
Evaluation of the concentration of intracellular oxidants
The concentration of intracellular oxidants was estimated using the probe DHDCFDA. DHDCFDA is a non-fluorescent probe which enters the cell where it can be oxidized to a fluorescent derivative (DCF) by endogenous oxidants. Cells were incubated in control media, or chelated media containing 1.5 or 15 μM Zn. After 1-24 h of treatment, DCF were measured as previously described [33]. Results are expressed as a ratio of DCF to propidium iodide fluorescence, relative to the average of control cells.
For imaging the increase in ROS production in a time-lapse manner, the fluorescent DCF in cells of all treatments was imaged using scanning laser confocal microscopy as described above for Fluo-4. The argon laser was used for excitation and the fluorescence channel was overlaid on the interference contrast image.
Determination of cellular RNS variations by scanning laser confocal microscopy
The levels of intracellular RNS were evaluated using the nitric oxide-sensitive probe DAF-2. In the presence of NO, the relatively non-fluorescent DAF-2 is converted into a highly green fluorescent triazole compound, DAF-2T. As RNS such as peroxinitrite can also react with the non-fluorescent probe DHDCFDA leading to the formation of the fluorescent compound DCF, RNS contribution to DCF fluorescence was evaluated using the NOS inhibitor L-NAME.
Confocal imaging settings were as described above for Fluo-4 and DHDCFDA. DAF-2 (10 μM) was preloaded during 20 min, after which cells were washed with the control media. Cell images were captured prior to experimental manipulation and immediately after the change of media; the control medium was removed and the medium to be tested [control medium or chelated medium containing 1.5 μM Zn with or without the inhibitors] was added. For all experiments, both laser intensity and PMT voltage were identical so comparisons in either DAF-2 or DCF intensity could be conducted. DAF-2 and DCF fluorescence images were overlayed on the interference contrast light images (using the Fluoview and Tiempo software) in order to localize regions of RNS increase.
Electrophoretic Mobility Shift Assay (EMSA)
To prepare total fractions, after 2 h of treatment, cells were rinsed with PBS and scraped with lysis buffer (50 mM Tris pH 7.5, 150 mM NaCl, 0.1 % (v/v) Triton X-100, 1 % (v/v) Igepal, 2 mM EDTA, 2 mM EGTA, 50 mM NaF, 2 mM Na3VO4, 0.5 mM PMSF, 0.5 mM DTT, 10 μg/ml leupeptin, 10 μg/ml antipain, 5 μg/ml aprotinin, 1 μg/ml pepstatin A and 10 μM chymostatin). Samples were incubated for 30 min at 4 °C and then centrifuged at 10,000 × g for 20 min at 4 °C. The supernatant was stored at −80 °C and protein concentration was determined [34] immediately before starting the assay.
For the EMSA, the oligonucleotide containing the consensus sequence for AP-1 was end labeled with [γ-32P] ATP using T4 polynucleotide kinase. Samples were incubated with the labeled oligonucleotide (20,000-30,000 cpm) for 20 min at room temperature in binding buffer [5× binding buffer: 50 mM Tris-HCl buffer, pH 7.5, containing 20% (v/v) glycerol, 5 mM MgCl2, 2.5 mM EDTA, 2.5 mM DTT, 250 mM NaCl and 0.25 mg/ml poly(dI-dC)]. The products were then separated by electrophoresis on a 6% (w/v) non-denaturing polyacrilamide gel using 0.5 × TBE (45 mM Tris/borate, 1 mM EDTA) as the running buffer. The gels were dried and the radioactivity quantitated in a Phosphoimager 840 (GE Healthcare, Piscataway, NJ, USA).
Transfections
Cells were seeded in 6-well plates. After 24 h in culture, cells were transfected with the pAP-1-Luc plasmid and cotransfected with the β–galactosidase plasmid using LipofectAMINE™ 2000 according to the manufacturer's protocols (Invitrogen Life Technologies, Carlbad, CA, USA). After 6 h of initiating the transfection, the cell culture media was replaced with control media to recover overnight. The following day, cells were treated with the control media, or chelated media containing 1.5 μM Zn (1.5 Zn) or 15 μM Zn (15 Zn). After 6 h cells were harvested, lysed and luciferase and β–galactosidase activities were determined following the manufacturer's protocols (Promega, Madison, WI, USA).
Western blot
Cytosolic and membrane fraction were prepared as described by Noh et al. [35]. Protein content was evaluated [34] immediately before loading. Samples containing 80 μg of protein were heated at 95 °C for 5 min and loaded onto SDS-PAGE. Proteins were resolved by SDS-PAGE and transferred to PVDF membranes. Molecular weight standards (Cell Signaling Technology, Beverly, MA, USA) were run simultaneously. Membranes were immunoblotted with the corresponding primary antibody overnight at 4°C, and the following day for 60 min at room temperature in the presence of the secondary antibody (HRP-conjugated). The conjugates were detected by enhanced chemiluminescence in a Phosphoimager 840. Equal loading of cell lysate was controlled by β-tubulin content for cytosolic fractions and NMDAR type 1 (NR1) for membrane fractions.
Statistical Analysis
One way analysis of variance (ANOVA) test, followed by Fisher's PLSD (protected least-squares difference) test and correlations, were performed using the routines available in Statview 5.0 (SAS Institute, Cary, NC, USA.). A p value < 0.05 was considered statistically significant. Values are given as means ± SEM.
Results
A decrease in extracellular Zn concentration causes a rapid increase in oxidant levels
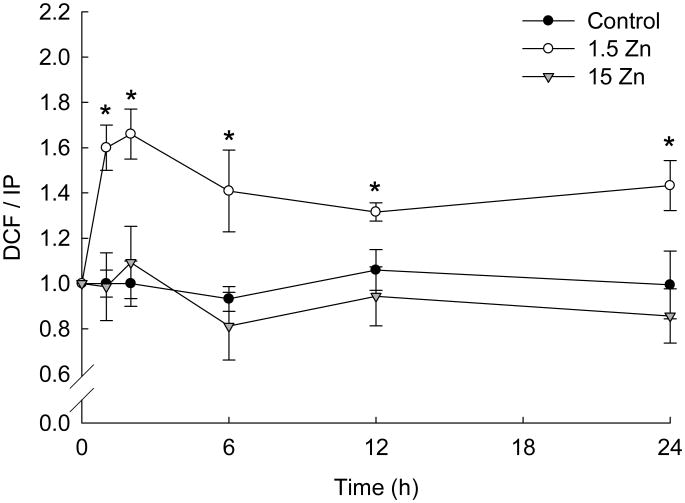
Different ROS and RNS react with the non-fluorescent probe DHDCFDA leading to the formation of the fluorescent compound DCF. After only 1 h of incubation, a significant increase in DCF fluorescence was observed in PC12 cells incubated in Zn deficient (1.5 Zn) medium (approximately 70 %) compared to cells incubated in control or Zn supplemented (15 Zn) media (Fig. 1). Cell oxidant levels remained high throughout the 24 h of incubation in the 1.5 Zn media (Fig. 1). A similar effect was observed in human neuroblastoma IMR-32 cells [33].
Figure 1. A decrease in extracellular Zn causes a rapid and persistent increase in neuronal oxidant levels.
Cell oxidant levels were evaluated with the probe DHDCFDA after incubating differentiated PC 12 cells for 1-24 h in control media (black circles), or in chelated media containing 1.5 μM Zn (1.5 Zn) (white circles) or 15 μM Zn (15 Zn) (grey triangles). DCF fluorescence was normalized to the DNA content (propidium iodide, PI, fluorescence). Data was calculated considering the value of the control group at time 0 h as 1. Results are shown as the means ± SEM of at least four independent experiments. * Significantly different compared to C and 15 Zn at the same time point (p< 0.005).
A decrease in extracellular Zn concentration triggers NADPH oxidase activation
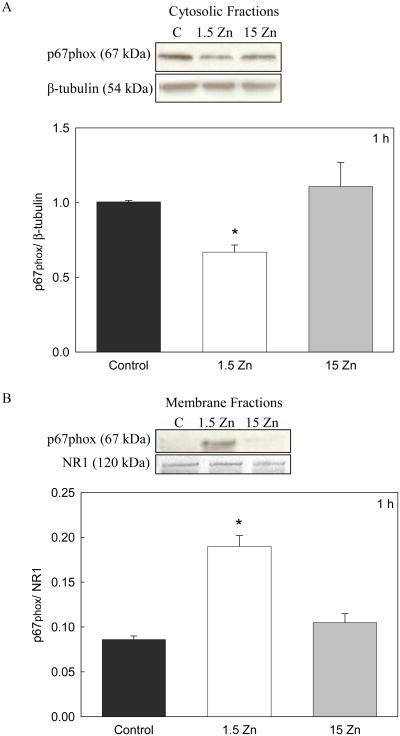
NADPH oxidase in resting cells consists of cytosolic (p47phox, p67phox and RAC) and membrane components (NOX and p22phox). When stimulated, the cytosolic components translocate to the membrane to form the active enzymatic complex. In this study, NADPH oxidase activation was evaluated as the redistribution of the p67phox component from the cytosol to the membrane. Cytosolic and membrane fractions were obtained from PC12 cells incubated in C, 1.5 Zn and 15 Zn media for 1 h. As measured by Western blot, a significant decrease in p67phox levels was observed in the cytosolic fractions, with a concomitant increase of p67phox in the membrane fraction of cells incubated in the 1.5 Zn medium compared to those incubated in control or 15 Zn media (Fig 2). These results indicate that in neuronal cells NADPH activation occurs as a consequence of a low zinc availability.
Figure 2. A decrease in extracellular Zn causes NADPH oxidase activation in neuronal cells.
Cytosolic and membrane fractions were isolated after incubating differentiated PC 12 cells during 1 h in control media, or in chelated media containing 1.5 μM Zn (1.5 Zn) or 15 μM Zn (15 Zn). A- Western blots for p67phox and P-tubulin in cytosolic fractions. B- Western blots for p67phox and NMDAR type 1 (NR1) in membrane fractions. Results are shown as means ± SEM of three independent experiments. * Significantly different compared to C and 15 Zn (p< 0.05).
A decrease in extracellular Zn concentration induces calcium influx: involvement of the NMDAR
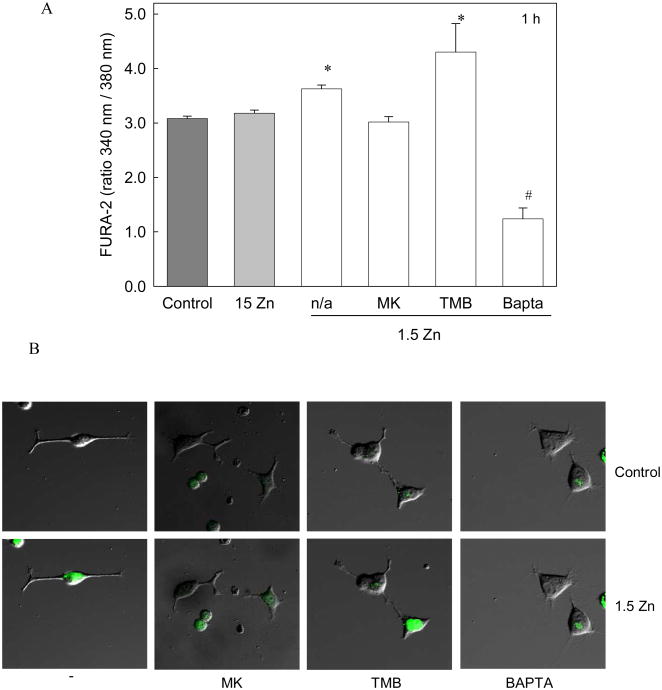
Intracellular Ca levels were measured after 1 h of incubation in the different media, using the fluorescent probe Fura-2-AM. PC12 cells incubated in 1.5 Zn media showed a significant increase in cellular Ca levels compared to those incubated in control or 15 Zn media (Fig 3 A).
Figure 3. A decrease in extracellular Zn causes an increase in intracellular calcium levels in neuronal cells.
PC12 differentiated cells were incubated in control media (dark grey bar), or in chelated media containing 15 μM Zn (15 Zn) (grey bar) or 1.5 μM Zn (1.5 Zn) (white bars) in the absence (n/a) or presence of the NMDAR antagonist MK-801 (MK) 1 uM, the intracellular calcium antagonist TMB-8 (TMB) 10 μM or the calcium chelator Bapta-AM (Bapta) 10 μM. The intracellular calcium levels were determined A- after 1 h of incubation, using Fura-2-AM as described under “Materials and Methods”. Values are shown as the means ± SEM of at least 3 independent experiments.
* Significantly different compared to control, 15 Zn and 1.5 Zn + MK (p< 0.05).
# Significantly different from all groups (p< 0.001), and B- after 1 min of incubation, by confocal microscopy, , using Fluo-4-AM as described in “Materials and Methods”.
To investigate the source of cellular Ca increase, the effects of an antagonist of the NMDAR (MK-801) and an inhibitor of Ca mobilization from intracellular stores (TMB-8) were studied. After 1 h of incubation, the increase observed in PC-12 cells incubated in 1.5 Zn media was prevented by MK-801 but not by TMB-8 (Fig 3 A). Bapta-AM was used as a negative control in the determination of intracellular Ca. The same cellular response was observed in human neuroblastoma IMR-32 cells (data not shown).
Ca levels were also evaluated by scanning laser confocal microscopy using the probe Fluo-4-AM. When the control media was replaced by media containing 1.5 μM Zn, a rapid increase in Fluo-4 fluorescence was observed in the cell bodies (Fig 3 B). Similar to the results obtained with Fura-2, the increase in fluorescence observed in the cells incubated in Zn deficient media was prevented when cells were treated with MK-801, but not with TMB-8 (Fig 3 B).
A decrease in extracellular Zn concentration triggers NADPH oxidase and NOS activation increasing oxidant production through a NMDAR- mediated process
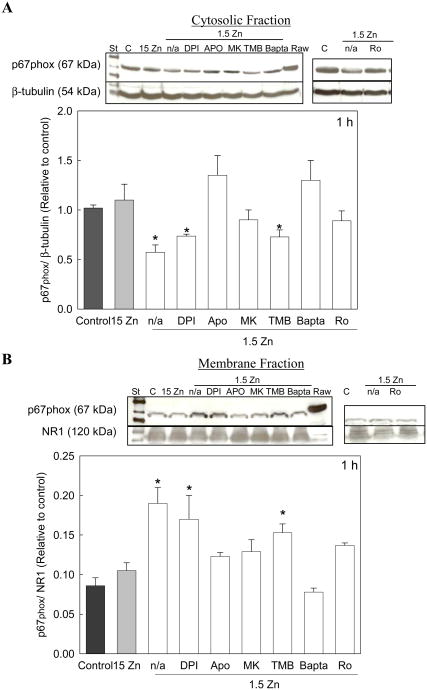
Calcium can activate NADPH oxidase, either directly or through the activation of Ca-sensitive PKC [25]. The involvement of NMDAR-dependent increase in cellular Ca on the activation of NADPH oxidase was next investigated. The effects of MK-801, TMB-8 and Bapta-AM were assessed. Both, MK-801 as well as Bapta-AM, significantly reduced the translocation of p67phox from the cytosol to the cell membrane, while TMB-8 had no effect (Fig 4). Furthermore, to investigate whether NADPH oxidase activation was mediated by PKC, we incubated the cells with Ro-320432, an inhibitor of Ca-dependent PKC. Results show that at low extracellular Zn concentrations, the presence of Ro-320432 in the cell culture media markedly decreases the translocation of p67phox from the cytosol to the cell membrane (Fig. 4).
Figure 4. A decrease in extracellular Zn triggers NADPH oxidase activation in neuronal cells.
Cytosolic and membrane fractions were isolated after 1 h exposure to control media or to chelated media containing 15 μM Zn (15 Zn) or 1.5 μM Zn (1.5 Zn) in the absence (n/a) or presence of the NADPH oxidase inhibitors DPI 0.5 μM and Apocynin (APO) 100 μM, the NMDAR antagonist MK-801 (MK) 1 μM, the intracellular calcium antagonist TMB-8 (TMB) 10 μM, the calcium chelator Bapta-AM (Bapta) 10 μM or the inhibitor of PKC; Ro-320432 (Ro) 1 μM. A- Western blots for p67phox and P-tubulin in cytosolic fractions. B- Western blots for p67phox and NMDAR type 1 (NR1) in membrane fractions. Cell extracts from activated Raw 264 macrophage cells (RAW) were used as a standard for p67phox Results are shown as means ± SEM of 3 independent experiments. * Significantly different compared to control, 15 Zn, or 1.5 Zn in the presence of APO, MK or Bapta (p< 0.05).
As a control for this assay, cells incubated in 1.5 Zn media were treated with two different NADPH oxidase inhibitors: the non specific inhibitor DPI, which inhibits enzymes such as NADPH oxidase by blocking the reduction of the cofactor flavin adenine dinucleotide, and the selective inhibitor APO, which blocks the translocation of the NADPH oxidase cytosolic components to the membrane. As expected, p67phox translocation to the membrane was inhibited by APO, while DPI had no effect (Fig. 4).
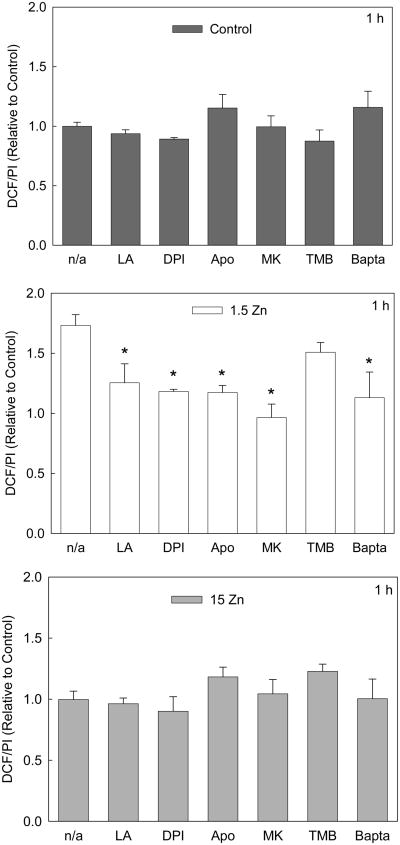
NADPH oxidase catalyzes the production of O2⋅−, which by dismutation can generate H2O2, or by reaction with nitric oxide can produce peroxynitrite. After only 1 h, the increase in oxidant levels observed in the 1.5 Zn group was inhibited when cells were incubated in the presence of the NADPH oxidase inhibitors (DPI or APO), MK-801 or Bapta-AM, but not in the presence of TMB-8 (Fig 5). Lipoic acid was used as a negative control because of its antioxidant properties. In cells incubated for 1 h with lipoic acid, the increase in DCF fluorescence associated with low extracellular Zn levels was inhibited.
Figure 5. A decrease in extracellular Zn causes a rapid increase in neuronal oxidant levels.
Cell oxidant levels were evaluated with the probe DHDCFDA after incubating differentiated PC12 cells for 1 h in control media (dark grey bars), or in chelated media containing 1.5 μM Zn (1.5 Zn) (white bars) or 15 μM Zn (15 Zn), (grey bars), in the absence or presence of the antioxidant a-lipoic acid (LA) 0.5 mM, the NADPH oxidase inhibitors DPI 0.5 μM and Apocynin (APO) 100 μM, the NMDAR antagonist MK-801 (MK) 1 μM, the intracellular calcium antagonist TMB-8 (TMB) 10 μM, the calcium chelator Bapta-AM (Bapta) 10 μM. DCF fluorescence was normalized to the DNA content (propidium iodide fluorescence, PI). Results are shown as means ± SEM of 5 independent experiments. * Significantly different compared to the value of the respective group in the absence of inhibitors or in the presence of TMB (p< 0.01).
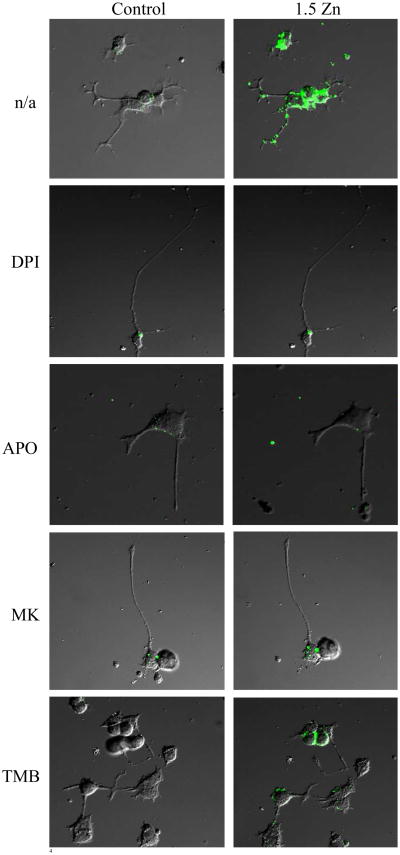
Variations in cell oxidant levels were also evaluated with the probe DHDCFDA by confocal microscopy (Fig. 6). In Zn deficient cells, DCF fluorescence was observed in the axons and close to the cell membrane rather than in the cell bodies. Similar findings to those described above using spectrofluorometry (Fig. 5), were obtained when cell oxidant levels were evaluated by fluorescence microscopy (Fig. 6).
Figure 6. A decrease in extracellular Zn causes a rapid increase in neuronal oxidant levels.
Cell oxidant levels were evaluated by confocal microscopy using the probe DHDCFDA in differentiated PC12 cells incubated for 5 min in control media (left column), or in chelated media containing 1.5 μM Zn (1.5 Zn) (right column), in the absence (n/a) or presence of the NADPH oxidase inhibitors DPI 0.5 mM and Apocynin (APO) 100 μM, the NMDAR antagonist MK-801 (MK) 1 μM or the intracellular calcium antagonist TMB-8 (TMB) 10 μM.
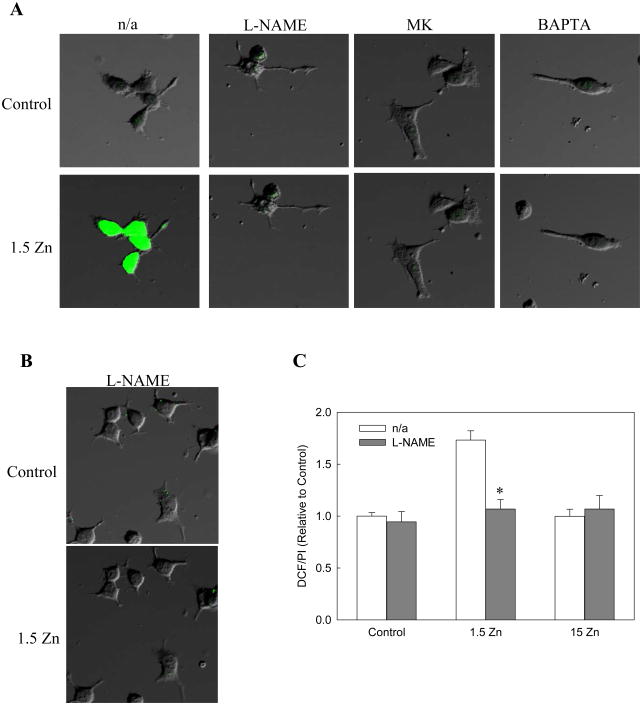
It is well known that the neuronal NOS is a Ca dependent enzyme. Therefore, the increase in Ca influx through the NMDAR, when extracellular Zn concentration decreases, may also affect the activity of this nitric oxide-generating enzyme. DAF-2, a nitric oxide-sensitive probe was used to evaluate NOS activation. A rapid increase in DAF-2 fluorescence was observed when the control media was changed to 1.5 Zn media, which was prevented by addition of L-NAME, MK-801 and BAPTA (Figure 7A).
Figure 7. A decrease in extracellular Zn causes a rapid increase in neuronal RNS levels.
Cell RNS levels were evaluated by confocal microscopy using the probe A) DAF-2 and B) DHDCFDA in differentiated PC12 cells incubated for 5 min in control media (upper row), or in chelated media containing 1.5 μM Zn (1.5 Zn) (lower row), in the absence (n/a) or presence of the NOS inhibitor L-NAME 0.5 mM,.the NMDAR antagonist MK-801 (MK) 1 μM or the calcium chelator Bapta-AM (Bapta) 10 μM. C) Cell oxidant levels were evaluated with the probe DHDCFDA after incubating differentiated PC12 cells for 1 h in control or in chelated media containing 1.5 μM Zn (1.5 Zn) or 15 μM Zn (15 Zn), in the absence or presence of the NOS inhibitor L-NAME 0.5 mM. DCF fluorescence was normalized to the DNA content (propidium iodide fluorescence, PI). Results are shown as means ± SEM of 5 independent experiments. * Significantly different compared to the value of the respective group in the absence of the inhibitor (p< 0.01).
In order to evaluate if neuronal NOS was also contributing to the increase in oxidant levels associated with a decrease in extracellular Zn concentrations, cells were incubated with the NOS inhibitor L-NAME. The increase in DCF fluorescence observed in the 1.5 Zn group was prevented when cells were incubated with L-NAME (Fig. 7B and 7C). This finding supports an increase in peroxinitrite levels (a RNS formed as a consequence of NADPH oxidase activation and increased NO formation) that is capable of oxidizing DHDCFDA. No significant changes in DCF fluorescence were observed when cells were incubated with the different inhibitors in either control or 15 Zn media (Fig. 7C).
A decrease in extracellular Zn concentration increases the activation of the oxidant sensitive transcription factor AP-1 through a mechanism involving NMDAR and NADPH oxidase
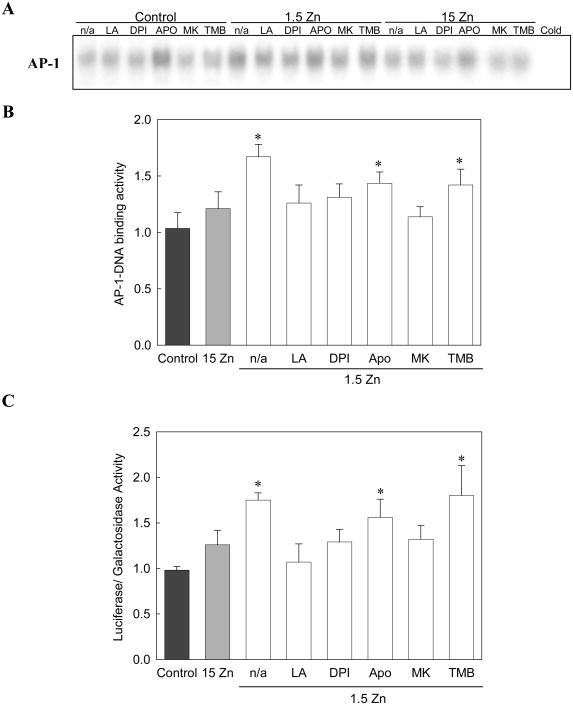
The activation of the oxidant sensitive AP-1 was next evaluated, and its relationship to NMDAR and NADPH oxidase activation was studied. AP-1 activation was determined by measuring the AP-1-DNA binding activity in total cell fractions by EMSA, and the transactivation of an AP-1-driven reporter gene (pAP-1-Luc).
For the EMSA assays, the specificity of the AP-1-DNA complex was assessed by competition with a 100-fold molar excess of unlabeled oligonucleotide containing the consensus sequence for AP-1 (Cold). The AP-1-DNA binding activity was measured in cells incubated in control media, or chelated media containing 1.5 or 15 μM Zn. When measured after 2 h of incubation (Fig. 8A), AP-1-DNA binding activity was significantly higher only in the 1.5 Zn cells. The increase observed was prevented by incubating the cells with DPI or MK-801 (Fig. 8B). TMB-8 showed no effect in the 1.5 Zn cells (Fig. 8B). APO, on the other hand, caused an increase in AP-DNA binding activity in all groups tested (control, 1.5 Zn and 15 Zn) (Fig. 8A). The same effect was observed in human neuroblastoma IMR-32 cells (data not shown)
Figure 8. A decrease in extracellular Zn triggers AP-1 activation in neuronal cells.
AP-1-DNA binding activity was measured in total fractions by EMSA. A) EMSA in total fractions isolated from cells incubated for 2 h in control media, or in chelated media containing 1.5 μM Zn (1.5 Zn) or 15 μM Zn (15 Zn), in the absence (n/a) or presence of of the antioxidant a-lipoic acid (LA) 0.5 mM, the NADPH oxidase inhibitors DPI 0.5 μM and Apocynin (APO) 100 μM, the NMDAR antagonist MK-801 (MK) 1 μM, or the intracellular calcium antagonist TMB-8 (TMB) 10 μM. To determine the specificity of the AP-1-DNA complex, samples were incubated in the presence of a 100-fold molar excess of unlabeled oligonucleotide containing the consensus sequence for AP-1 (Cold) prior to the binding assay. B) EMSA bands were quantitated and results are shown as means ± SEM of 5 independent experiments. * Significantly different compared to control, 15 Zn or 1.5 Zn in the presence of LA, DPI or MK (p< 0.05). C) Transactivation of pAP-1-Luc plasmid. Luciferase and β-galactosidase activities were evaluated after incubating the cells for 6 h in control 1.5 Zn or 15 Zn media, in the absence (n/a) or presence of 0.5 mM LA, 0.5 μM DPI, 100 μM APO, 1 μM MK or 10 μM TMB. Results were calculated as the ratio luciferase/β-galactosidase activity and expressed relative to control values. Results are shown as means ± SEM of 3 independent experiments.
* Significantly different compared to control, 15 Zn or 1.5 Zn in the presence of LA, DPI or MK (p< 0.05).
To evaluate AP-1 driven gene transactivation, a reporter gene assay (luciferase) was conducted. Cells were transfected with the pAP-1-Luc plasmid and co-transfected with a vector expressing β-galactosidase (as a control for the transfection efficiency). Results were expressed in terms of the galactosidase activity in the samples. After 6 h of incubation, the AP-1 transcriptional activity was 70 % higher in the 1.5 Zn cells compared to control or 15 Zn cells (Fig. 8C). This increase was prevented when cells incubated in 1.5 μM Zn media were simultaneously treated with DPI or MK-801, while TMB-8 had no effect (Fig. 8C). Unexpectedly, APO increased AP-1 activation in all the experimental groups. This effect may be explained by evidence showing that APO can activate AP-1 through a mechanism independent of NADPH oxidase [36].
Discussion
Zn deficiency causes oxidative stress as observed in different cells and tissues [11, 13, 37, 38]. In neuronal cells, Zn deficiency leads to a high oxidant production, glutathione depletion and the activation of oxidant-sensitive signaling cascades [10, 12]. The mechanisms underlying cell oxidant production when neuronal Zn decreases are unknown. In this study, we observed that the increase in neuronal oxidant levels occurs very rapidly, indicating that Zn deficiency-associated oxidative stress cannot be initially due to processes that require new protein synthesis, such as the proposed alterations in the expression of antioxidant enzymes [39] or of components of the respiratory chain [40]. The present results suggest that neuronal cells sense a decrease in extracellular Zn levels and rapidly respond to this change by inducing oxidant production, which are known to function as secondary messengers [14]. These events could be important, especially during brain development, when a fine regulation of processes, such as neuronal proliferation, differentiation and apoptosis, is required.
We therefore studied a possible mechanism by which neuronal cells could sense a decrease in extracellular Zn levels, and through an increase in the steady state levels of oxidants trigger redox-sensitive signals (AP-1) that regulate neurodevelopment.
Extracellular Zn modulates the activity of neuronal ion channels such as GABA, AMPA and NMDA. While the AMPA receptors are activated by Zn [41], the GABA receptors may be blocked or activated depending on the cell type [42]. We focused our study on the NMDAR because its channel is blocked by Zn at two independent regulatory sites [4, 5]. Several studies have previously shown that increasing Zn levels can block NMDAR activation decreasing seizure propensity [4, 5, 7]. Thus, we hypothesized that a decrease in extracellular Zn could release the NMDAR from its inhibition; consequently open the channel, allowing Ca influx and subsequent NADPH oxidase activation. In support of this hypothesis, we observed that a decrease in extracellular Zn levels activated the NMDAR, leading to Ca influx. This was also supported by the finding that the increase in cellular Ca was prevented by the specific NMDAR antagonist MK-801. The mobilization of Ca from intracellular stores did not contribute to the rise in Ca, as the inhibitor TMB-8 failed to prevent Ca increase in the cells exposed to Zn deficient media. The physiological relevance of NMDAR modulation by Zn is further supported by findings that Zn deficiency increased seizure susceptibility in 4-week old epileptic mice and in kainic acid-stimulated adult rats [43, 44].
We subsequently investigated whether the increase in cellular Ca levels associated to the decrease in extracellular Zn concentrations, could lead to the activation of the enzyme NADPH oxidase and subsequently to cell oxidants production.
The exposure of differentiated PC12 cells to Zn deficient media led to the activation of NADPH oxidase, measured as the translocation of p67phox from the cytosol to the cell membrane. As expected, APO inhibited the translocation of p67phox to the membrane, while DPI, that acts inhibiting a cofactor of the NADPH oxidase, had no effect. Bapta inhibited NADPH oxidase activation indicating the involvement of Ca. The inhibitory action of MK-801 indicated that Ca-mediated activation of NADPH oxidase is secondary to Ca influx through the NMDAR channels.
Up to date, at least five isoforms of NOX have been described [45]. The best characterized, NOX2 (also known as gp91), is found in phagocytes. Of the NOX isoforms, only NOX5 has a binding site for Ca and can be directly activated by Ca. This NOX isoform has been found to be present in the brain [21]. All other NOX isoforms can be activated by Ca indirectly. For instance, several studies have demonstrated that NADPH oxidase is activated by PKC in different cell types such as monocytes, cortical neurons and astrocytes [46, 47]. In this study Ro-320432 prevented the translocation of p67phox to the membrane in PC12 cells incubated in low Zn media, suggesting that in PC12 cells, the activation of NADPH oxidase is mediated by PKC.
When activated, NADPH oxidase transfers electrons from NADPH to O2 to produce O2⋅−, which can react with nitric oxide forming peroxinitrite or it can be spontaneously or enzymatically converted to H2O2. We measured DHDCFDA oxidation as a parameter of cell oxidant levels. Consistent with the findings on NADPH oxidase activation, the exposure of PC12 cells to Zn deficient media led to a rapid increase in DCF fluorescence that was prevented by both NADPH oxidase inhibitors, DPI and APO. This is consistent with previous findings that in Zn deficient hepatic stellate cells, NADPH oxidase was responsible for the observed increase in cell oxidants [37]. The present study demonstrates, for the first time, that the rapid neuronal oxidant increase, when extracellular Zn concentration decreases, is, in part, due to PKC-mediated NADPH oxidase activation, secondary to Ca influx through the NMDAR channel, since both Ro-320432 and MK-801 prevented this effect.
A decrease in extracellular Zn also led to an increase in RNS levels. The incubation of cells with L-NAME attenuated the increase in cell oxidants observed when extracellular Zn levels decrease. Furthermore, the finding that in the Zn deficient cells the increase in RNS levels is prevented by NMDAR inhibition and Ca chelation supports a NMDAR/Ca-mediated activation of NOS. In support of our findings, previous research on the inhibition of long-term potentiation found that chelation of ambient extracellular Zn induces a low-level NMDAR activation, which activates calcineurin. In turn, calcineurin dephosphorylates NOS, inducing an increase in RNS, which can contribute to MAPK p38 activation and to the inhibition of long-term potentiation [48].
Being AP-1 a redox-sensitive transcription factor [49-51] that regulates critical developmental events [27, 28], its activation was evaluated as a physiological consequence of NMDAR/NADPH oxidase/NOS activation and subsequent increased oxidant production. It has been previously shown that Fos and Jun, components of the transcription factor AP-1, can be rapidly activated in response to NMDAR activation [52]. Therefore, we examined whether NMDAR-mediated Ca influx and NADPH oxidase activation could mediate, through an increase in cellular oxidants, the activation of AP-1 associated with neuronal Zn deficiency. AP-1 was rapidly activated when PC12 cells were exposed to a Zn deficient media. The involvement of oxidants in Zn deficiency-induced AP-1 activation is supported by the inhibitory actions of an antioxidant (lipoic acid) and a NADPH oxidase inhibitor (DPI). MK-801, but not TMB-8, prevented AP-1 activation in 1.5 Zn cells, indicating the involvement of NMDAR, and the subsequent Ca influx in the oxidant-mediated AP-1 activation.
In conclusion, the evidence presented here shows that in neuronal cells, a decrease in extracellular Zn levels can activate NMDAR, and as a consequence promote Ca influx. The increase in cellular Ca levels leads to PKC-mediated activation of the enzyme NADPH oxidase, which generates ROS, and to the activation of NOS, which generates nitric oxide. The rapid activation of AP-1 upon NMDAR activation stresses the relevance of extracellular Zn variations in the regulation of neuronal signals. The above findings provide a mechanistic link between Zn deficiency caused by nutritional deficiency or as a consequence of other disease states and the oxidant-mediated signaling pathways that can affect neuronal development and physiology. Importantly, results suggest a physiological role of Zn during neurodevelopment through the neuronal sensing of variations in extracellular Zn that can be translated in oxidant-mediated activation of cell signals that regulate neuronal development and function.
Acknowledgments
Supported by grants from the University of California, Davis; University of California Davis Bodega Marine Laboratory; and NIH (grant # HD 01743), USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Briefel RR, Bialostosky K, Kennedy-Stephenson J, McDowell MA, Ervin RB, Wright JD. Zinc intake of the U.S. population: findings from the third National Health and Nutrition Examination Survey, 1988-1994. J Nutr. 2000;130:1367S–1373S. doi: 10.1093/jn/130.5.1367S. [DOI] [PubMed] [Google Scholar]
- 2.Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Annu Rev Nutr. 2004;24:401–431. doi: 10.1146/annurev.nutr.23.011702.073349. [DOI] [PubMed] [Google Scholar]
- 3.Caulfield LE, Zavaleta N, Shankar AH, Merialdi M. Potential contribution of maternal zinc supplementation during pregnancy to maternal and child survival. Am J Clin Nutr. 1998;68:499S–508S. doi: 10.1093/ajcn/68.2.499S. [DOI] [PubMed] [Google Scholar]
- 4.Westbrook GL, Mayer ML. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987;328:640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- 5.Peters S, Koh J, Choi DW. Zinc selectively blocks the action of N-methyl-D-aspartate on cortical neurons. Science. 1987;236:589–593. doi: 10.1126/science.2883728. [DOI] [PubMed] [Google Scholar]
- 6.Legendre P, Westbrook GL. The inhibition of single N-methyl-D-aspartate-activated channels by zinc ions on cultured rat neurones. J Physiol. 1990;429:429–449. doi: 10.1113/jphysiol.1990.sp018266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christine CW, Choi DW. Effect of zinc on NMDA receptor-mediated channel currents in cortical neurons. J Neurosci. 1990;10:108–116. doi: 10.1523/JNEUROSCI.10-01-00108.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng F, Gingrich MB, Traynelis SF, Conn PJ. Tyrosine kinase potentiates NMDA receptor currents by reducing tonic zinc inhibition. Nat Neurosci. 1998;1:185–191. doi: 10.1038/634. [DOI] [PubMed] [Google Scholar]
- 9.Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J Neurosci. 1997;17:5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho E, Ames BN. Low intracellular zinc induces oxidative DNA damage, disrupts p53, NFkappa B, and AP1 DNA binding, and affects DNA repair in a rat glioma cell line. Proc Natl Acad Sci USA. 2002;99:16770–16775. doi: 10.1073/pnas.222679399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canali R, Vignolini F, Nobili F, Mengheri E. Reduction of oxidative stress and cytokine-induced neutrophil chemoattractant (CINC) expression by red wine polyphenols in zinc deficiency induced intestinal damage of rat. Free Radic Biol Med. 2000;28:1661–1670. doi: 10.1016/s0891-5849(00)00285-9. [DOI] [PubMed] [Google Scholar]
- 12.Mackenzie GG, Zago MP, Erlejman AG, Aimo L, Keen CL, Oteiza PI. alpha-Lipoic acid and N-acetyl cysteine prevent zinc deficiency-induced activation of NF-kappaB and AP-1 transcription factors in human neuroblastoma IMR-32 cells. Free Radic Res. 2006;40:75–84. doi: 10.1080/10715760500312305. [DOI] [PubMed] [Google Scholar]
- 13.Oteiza PI, Olin KL, Fraga CG, Keen CL. Zinc deficiency causes oxidative damage to proteins, lipids and DNA in rat testes. J Nutr. 1995;125:823–829. doi: 10.1093/jn/125.4.823. [DOI] [PubMed] [Google Scholar]
- 14.Sauer H, Wartenberg M, Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem. 2001;11:173–186. doi: 10.1159/000047804. [DOI] [PubMed] [Google Scholar]
- 15.Zago MP, Mackenzie GG, Adamo AM, Keen CL, Oteiza PI. Differential modulation of MAP kinases by zinc deficiency in IMR-32 cells: role of H(2)O(2) Antioxid Redox Signal. 2005;7:1773–1782. doi: 10.1089/ars.2005.7.1773. [DOI] [PubMed] [Google Scholar]
- 16.Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 18.Bentley ME, Caulfield LE, Ram M, Santizo MC, Hurtado E, Rivera JA, Ruel MT, Brown KH. Zinc supplementation affects the activity patterns of rural Guatemalan infants. J Nutr. 1997;127:1333–1338. doi: 10.1093/jn/127.7.1333. [DOI] [PubMed] [Google Scholar]
- 19.Penland JG, Sandstead HH, Alcock NW, Dayal HH, Chen XC, Li JS, Zhao F, Yang JJ. A preliminary report: effects of zinc and micronutrient repletion on growth and neuropsychological function of urban Chinese children. J Am Coll Nutr. 1997;16:268–272. doi: 10.1080/07315724.1997.10718684. [DOI] [PubMed] [Google Scholar]
- 20.Dvergsten CL, Johnson LA, Sandstead HH. Alterations in the postnatal development of the cerebellar cortex due to zinc deficiency. III. Impaired dendritic differentiation of basket and stellate cells. Brain Res. 1984;318:21–26. doi: 10.1016/0165-3806(84)90058-0. [DOI] [PubMed] [Google Scholar]
- 21.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 22.Vallet P, Charnay Y, Steger K, Ogier-Denis E, Kovari E, Herrmann F, Michel JP, Szanto I. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132:233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 23.Maru Y, Nishino T, Kakinuma K. Expression of Nox genes in rat organs, mouse oocytes, and sea urchin eggs. DNA Seq. 2005;16:83–88. doi: 10.1080/10425170500069734. [DOI] [PubMed] [Google Scholar]
- 24.Tammariello SP, Quinn MT, Estus S. NADPH oxidase contributes directly to oxidative stress and apoptosis in nerve growth factor-deprived sympathetic neurons. J Neurosci. 2000;20:RC53. doi: 10.1523/JNEUROSCI.20-01-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abramov AY, Jacobson J, Wientjes F, Hothersall J, Canevari L, Duchen MR. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci. 2005;25:9176–9184. doi: 10.1523/JNEUROSCI.1632-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou L, Zhu DY. Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide. 2009;20:223–230. doi: 10.1016/j.niox.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Pennypacker KR. AP-1 transcription factor complexes in CNS disorders and development. J Fla Med Assoc. 1995;82:551–554. [PubMed] [Google Scholar]
- 28.Tong L, Toliver-Kinsky T, Taglialatela G, Werrbach-Perez K, Wood T, Perez-Polo JR. Signal transduction in neuronal death. J Neurochem. 1998;71:447–459. doi: 10.1046/j.1471-4159.1998.71020447.x. [DOI] [PubMed] [Google Scholar]
- 29.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dichter MA, Tischler AS, Greene LA. Nerve growth factor-induced increase in electrical excitability and acetylcholine sensitivity of a rat pheochromocytoma cell line. Nature. 1977;268:501–504. doi: 10.1038/268501a0. [DOI] [PubMed] [Google Scholar]
- 31.Oteiza PI, Clegg MS, Zago MP, Keen CL. Zinc deficiency induces oxidative stress and AP-1 activation in 3T3 cells. Free Radic Biol Med. 2000;28:1091–1099. doi: 10.1016/s0891-5849(00)00200-8. [DOI] [PubMed] [Google Scholar]
- 32.Mackenzie GG, Oteiza PI. Zinc and the cytoskeleton in the neuronal modulation of transcription factor NFAT. J Cell Physiol. 2007;210:246–256. doi: 10.1002/jcp.20861. [DOI] [PubMed] [Google Scholar]
- 33.Aimo L, Oteiza PI. Zinc deficiency increases the susceptibility of human neuroblastoma cells to lead-induced activator protein-1 activation. Toxicol Sci. 2006;91:184–191. doi: 10.1093/toxsci/kfj137. [DOI] [PubMed] [Google Scholar]
- 34.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 35.Noh KM, Kim YH, Koh JY. Mediation by membrane protein kinase C of zinc-induced oxidative neuronal injury in mouse cortical cultures. J Neurochem. 1999;72:1609–1616. doi: 10.1046/j.1471-4159.1999.721609.x. [DOI] [PubMed] [Google Scholar]
- 36.Lapperre TS, Jimenez LA, Antonicelli F, Drost EM, Hiemstra PS, Stolk J, MacNee W, Rahman I. Apocynin increases glutathione synthesis and activates AP-1 in alveolar epithelial cells. FEBS Lett. 1999;443:235–239. doi: 10.1016/s0014-5793(98)01723-2. [DOI] [PubMed] [Google Scholar]
- 37.Kojima-Yuasa A, Umeda K, Ohkita T, Opare Kennedy D, Nishiguchi S, Matsui-Yuasa I. Role of reactive oxygen species in zinc deficiency-induced hepatic stellate cell activation. Free Radic Biol Med. 2005;39:631–640. doi: 10.1016/j.freeradbiomed.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 38.Tomat AL, Inserra F, Veiras L, Vallone MC, Balaszczuk AM, Costa MA, Arranz C. Moderate zinc restriction during fetal and postnatal growth of rats: effects on adult arterial blood pressure and kidney. Am J Physiol Regul Integr Comp Physiol. 2008;295:R543–549. doi: 10.1152/ajpregu.00050.2008. [DOI] [PubMed] [Google Scholar]
- 39.Taysi S, Cikman O, Kaya A, Demircan B, Gumustekin K, Yilmaz A, Boyuk A, Keles M, Akyuz M, Turkeli M. Increased oxidant stress and decreased antioxidant status in erythrocytes of rats fed with zinc-deficient diet. Biol Trace Elem Res. 2008;123:161–167. doi: 10.1007/s12011-008-8095-x. [DOI] [PubMed] [Google Scholar]
- 40.Ho E, Courtemanche C, Ames BN. Zinc deficiency induces oxidative DNA damage and increases p53 expression in human lung fibroblasts. J Nutr. 2003;133:2543–2548. doi: 10.1093/jn/133.8.2543. [DOI] [PubMed] [Google Scholar]
- 41.Kwak S, Weiss JH. Calcium-permeable AMPA channels in neurodegenerative disease and ischemia. Curr Opin Neurobiol. 2006;16:281–287. doi: 10.1016/j.conb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Smart TG, Hosie AM, Miller PS. Zn2+ ions: modulators of excitatory and inhibitory synaptic activity. Neuroscientist. 2004;10:432–442. doi: 10.1177/1073858404263463. [DOI] [PubMed] [Google Scholar]
- 43.Fukahori M, Itoh M. Effects of dietary zinc status on seizure susceptibility and hippocampal zinc content in the El (epilepsy) mouse. Brain Res. 1990;529:16–22. doi: 10.1016/0006-8993(90)90806-m. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell CL, Barnes MI, Grimes LM. Diethyldithiocarbamate and dithizone augment the toxicity of kainic acid. Brain Res. 1990;506:327–330. doi: 10.1016/0006-8993(90)91273-j. [DOI] [PubMed] [Google Scholar]
- 45.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 46.Noh KM, Koh JY. Induction and activation by zinc of NADPH oxidase in cultured cortical neurons and astrocytes. J Neurosci. 2000;20:RC111. doi: 10.1523/JNEUROSCI.20-23-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siow YL, Au-Yeung KK, Woo CW, O K. Homocysteine stimulates phosphorylation of NADPH oxidase p47phox and p67phox subunits in monocytes via protein kinase Cbeta activation. Biochem J. 2006;398:73–82. doi: 10.1042/BJ20051810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Izumi Y, Tokuda K, Zorumski CF. Long-term potentiation inhibition by low-level N-methyl-D-aspartate receptor activation involves calcineurin, nitric oxide, and p38 mitogen-activated protein kinase. Hippocampus. 2008;18:258–265. doi: 10.1002/hipo.20383. [DOI] [PubMed] [Google Scholar]
- 49.Polytarchou C, Hatziapostolou M, Papadimitriou E. Hydrogen peroxide stimulates proliferation and migration of human prostate cancer cells through activation of activator protein-1 and up-regulation of the heparin affin regulatory peptide gene. J Biol Chem. 2005;280:40428–40435. doi: 10.1074/jbc.M505120200. [DOI] [PubMed] [Google Scholar]
- 50.Abate C, Patel L, Rauscher FJ, 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990;249:1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- 51.Okuno H, Akahori A, Sato H, Xanthoudakis S, Curran T, Iba H. Escape from redox regulation enhances the transforming activity of Fos. Oncogene. 1993;8:695–701. [PubMed] [Google Scholar]
- 52.Platenik J, Kuramoto N, Yoneda Y. Molecular mechanisms associated with long-term consolidation of the NMDA signals. Life Sci. 2000;67:335–364. doi: 10.1016/s0024-3205(00)00632-9. [DOI] [PubMed] [Google Scholar]