Abstract
Zinc, a redox inactive metal, has been long viewed as a component of the antioxidant network, and growing evidence points to its involvement in redox-regulated signaling. These actions are exerted through several mechanisms based on the unique chemical and functional properties of zinc. Overall, zinc contributes to maintain the cell redox balance through different mechanisms including: i) the regulation of oxidant production and metal-induced oxidative damage; ii) the dynamic association of zinc with sulfur in protein cysteine clusters, from which the metal can be released by nitric oxide, peroxides, oxidized glutathione and other thiol oxidant species; iii) zinc-mediated induction of the zinc-binding protein metallothionein, which releases the metal under oxidative conditions and act per se scavenging oxidants; iv) the involvement of zinc in the regulation of glutathione metabolism and of the overall protein thiol redox status; and v) a direct or indirect regulation of redox signaling. Findings of oxidative stress, altered redox signaling, and associated cell/tissue disfunction in cell and animal models of zinc deficiency, stress the relevant role of zinc in the preservation of cell redox homeostasis. However, while the participation of zinc in antioxidant protection, redox sensing, and redox-regulated signaling is accepted, the involved molecules, targets and mechanisms are still partially known and the subject of active research.
Introduction
Multiple biological macromolecules and physiological cell events involve zinc as a structural component or as a major regulator. As a consequence, zinc is a metal that is essential for several aspects of normal human development [1] and health [2].
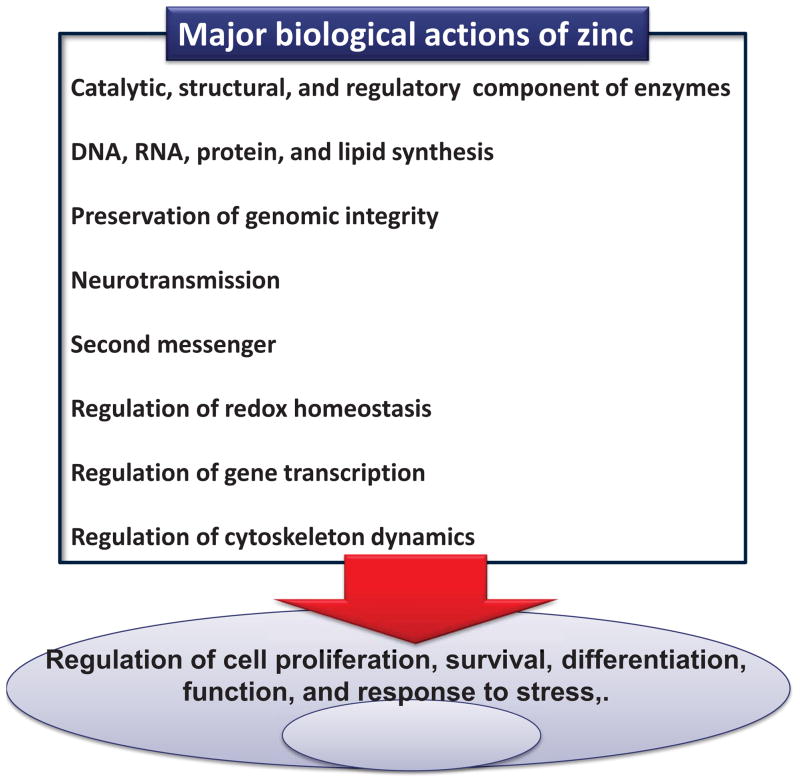
It is difficult to build a comprehensive list of the biological actions of zinc. This is due in part to the large number of proteins and enzymes that contain zinc, which explains the relevance of zinc in numerous cell processes. Furthermore, different zinc biological actions can superimpose, and the regulation of zinc at different levels/molecules can converge in one biological action. Fig. 1 shows a very general list of major zinc biological actions. Based on the Protein Data bank it was recently estimated that zinc is a component of more than 2700 enzymes, including hydrolases, transferases, oxido-reductases, ligases, isomerases and lyases [3]. Aproximately in 70% of these enzymes, zinc has a catalytic function, but it can also have a structural role, act as a substrate, or as a regulator of enzyme activity [3]. This large number of zinc enzymes explains the requirement of zinc in DNA, RNA, protein and lipid synthesis. Zinc also has a major role in the preservation of genomic stability [4]. This action involves, among other factors, the antioxidant effects of zinc, its participation in DNA repair and in the DNA damage response, and in the synthesis of molecules (e.g. methionine) that are required for DNA methylation [4]. Zinc can participate in neurotransmission [5], being stored and released from vesicles located at the synaptic endings of select glutamatergic neurons. This review will subsequently discuss aspects of other listed roles of zinc in biological systems: a second messenger action of rapidly available zinc pools, indirect and direct modulation of gene transcription, the regulation of cell redox homeostasis and redox-sensitive signals, and the requirement of zinc to preserve tubulin polymerization dynamics and function.
Figure 1.
Biological actions of zinc.
Zinc only exists in biological systems as Zn2+ given its complete d shell. However, zinc deficiency is often associated with a condition of oxidative stress. This can be explained by several mechanisms underlying zinc actions which will be discussed in this review. Overall, a large amount of zinc proteins that are modulated by or contain zinc can directly or indirectly affect the cell redox balance. The events involved in the regulation by zinc of the cell oxidant/antioxidant balance are multiple and interconnected, and although not completely understood, they are driving intense and challenging new research. Some of those mechanisms involve: i) the modulation of oxidant production and oxidative damage by cellular zinc availability [6]; ii) the capacity of zinc to reversibly bind to cysteine and histidine residues in zinc protein motifs which are proposed to act as redox switches [7]; iii) the direct and indirect involvement of the main cellular zinc binding protein, metallothionein (MT), which can per se scavenge oxidants, or release zinc in a redox-regulated manner [8, 9]; iv) the regulation by zinc of glutathione (GSH) metabolism, and of the overall thiol redox status [10]; v) a direct or indirect capacity of zinc to regulate the activity of proteins involved in cell signaling. Although zinc release from proteins can potentially modulate different cell signals and events, this review will focus on those related to the maintenance of cell redox homeostasis.
Cellular zinc pools
Eukaryotic cells contain large amounts of zinc (approximately 100 μM). Intracellular zinc pools can be classified in: the zinc tightly bound to macromolecules, zinc enclosed in high amounts inside vesicles (e.g. synaptic vesicles in neuronal glutamatergic terminals); a pool of exchangeable zinc, and a pool of zinc bound to MT. These pools are tightly regulated by specific transport proteins, zinc responsive transcription factors, and by MT and other zinc-sensing proteins that can dynamically bind and release zinc [11].
Zinc can be found in proteins either as a structural component, or in the catalytic site of select enzymes. Zinc is also part of particular protein domains that allow selective protein-protein interactions, and interactions between the protein and other macromolecules such as DNA and RNA (reviewed in [12]). Proteomic analysis looking for proteins containing zinc binding patterns and domains observed that approximately 10% of proteins in eukaryotic cells contain zinc [13].
Most of cellular zinc is bound with high affinity to proteins and other cellular components including GSH, cysteine, histidine and diphosphate molecules. On the other hand, the amount of cellular exchangeable zinc (also named “free”, “labile”, “loosely bound” zinc) is relatively very small. The use of probes that can be targeted to different cellular compartments has provided a significant advancement in the knowledge of the cellular localization and availability of the free zinc pools. Using an excitation ratiometric fluorescent biosensor based on carbonic anhydrase, Bozym et al. [14] measured 5–10 pM zinc in the cytoplasm and nucleus of resting PC12 rat pheochromocytoma cells. In primary cultures of rat hippocampal neurons, mitochondria were found to be a relevant reservoir of zinc, containing 180 nM zinc in the resting state [15]. Mitochondrial zinc is released to the cytosol when cells are stimulated with glutamate, and taken up by mitochondria upon glutamate and exogenous zinc supplementation, revealing a rapidly available mitochondrial zinc pool that could be important in signaling events [15]. In pancreatic 3 cells, while the measured cytosolic zinc concentration is 0.4 nM, in the secretory vesicles free zinc ranges between 1 and 10 μM [16]. In HeLa cells, the concentration of zinc in the endoplasmic reticulum and Golgi is very low (0.9 and 0.6 pM, respectively), increasing when cytosolic zinc increases, and being dynamically affected by variations in calcium homeostasis [17]. Fluctuations in cellular labile zinc are observed in association with cell apoptosis [18], meiosis [19], and proliferation [20]. With regard to the later, intracellular zinc concentrations vary in PC12 cells at different stages of the cell cycle [20], an event that is affected when zinc availability is low [21].
The above evidence shows the existence of different pools of free zinc that can vary dynamically, supporting a major role for the readily available zinc pools in the regulation of cell signaling, cell function and fate. In this regard and as discussed below, zinc is increasingly viewed as a second messenger. Given that free zinc concentrations vary with changes in the oxidant/antioxidant cell balance, this zinc pool can behave as a redox sensor.
The antioxidant actions of zinc
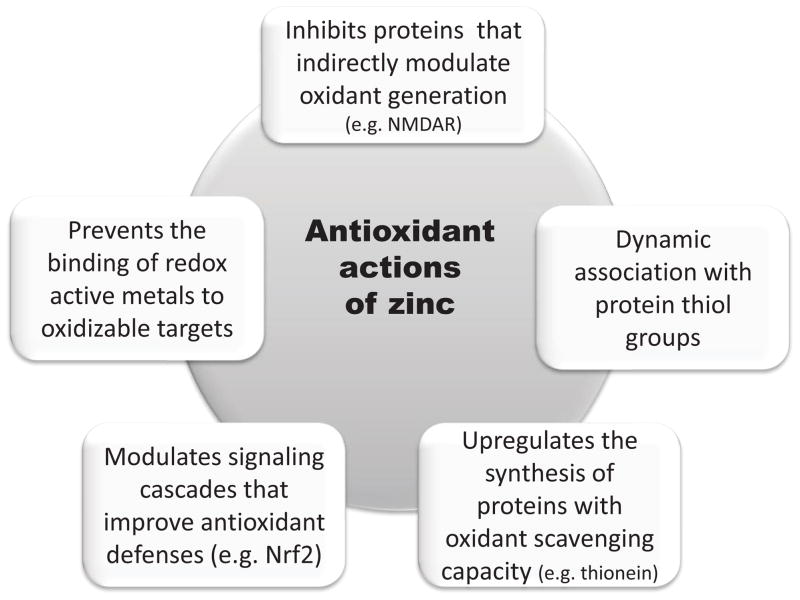
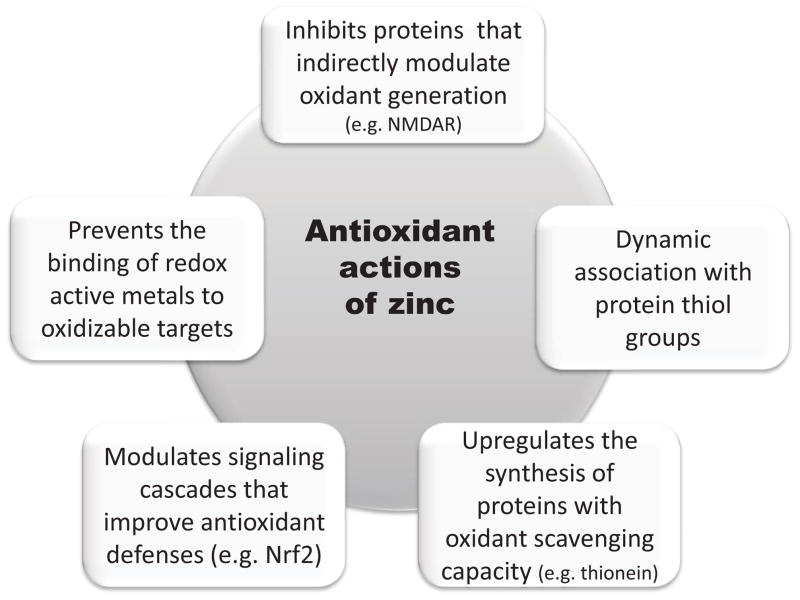
The occurrence of oxidative stress in association with a condition of zinc deficiency, and the prevention of oxidative damage by zinc supplementation has been observed in different cells and tissues [22–31]. A decrease in zinc availability is associated with an increase in cellular oxidants [6, 32], alterations in the antioxidant defense components [26, 28, 33], and increased tissue oxidation parameters [10, 25, 28, 30]. These and other similar findings generated the concept of zinc as part of the cell/tissue antioxidant defense network. The underlying mechanisms to such “antioxidant action” could encompass both cell or tissue-specific mechanisms, and others that are of general nature. Fig. 2 summarizes described mechanisms through which zinc can exert antioxidant actions in biological systems.
Figure 2.
Mechanisms involved in the “antioxidant” actions of zinc.
Zinc in the modulation of oxidant production
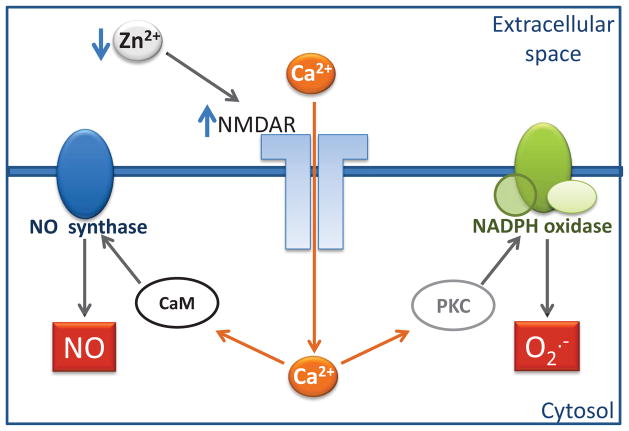
Zinc deficiency is associated with high steady state levels of nitric oxide (NO) and H2O2 in PC12 cells, H2O2 in human IMR-32 neuroblastoma cells [34], and of nitric oxide (NO) in glioblastoma cells [30]. There is very limited information on the mechanisms underlying a direct inhibitory effect of zinc on oxidant production. We recently described that the modulation by zinc of the N-methyl-D-aspartate (NMDA) receptor (NMDAR) affects NO and superoxide anion production in neuronal cells, as summarized in Fig. 2. The NMDAR is inhibited by zinc [35, 36], being highly sensitive to minor changes in zinc concentrations [37, 38]. Zinc binds to two independent low affinity and high affinity sites [39, 40] located inside and outside, respectively, of the receptor channel pore. NMDAR activation leads to the opening of the pore and to the transport of calcium and other cations from the extracellular space into the cytosol. The incubation of PC12 cells, differentiated into a neuronal phenotype, in zinc depleted medium leads to a rapid increase in the concentration of cellular calcium [6]. This increase is prevented by the specific NMDAR antagonist MK-801, which also prevents the increase in ROS and RNS. Accordingly, increases in calcium were also associated with zinc deficiency in human neuroblastoma IMR-32 cells [41] and in hippocampal slices from zinc deficient mice [42]. In zinc deficient neuronal cells, the NMDAR-dependent increase in calcium promotes the activation of NADPH oxidase via activation of protein kinase C, and the calcium-calmodulin-dependent activation of nitric oxide synthase [6]. The activation of these enzymes leads to an increased production of ROS and RNS in the zinc deficient neuronal cells (Fig. 3) [41]. The activation of NADPH oxidase as a consequence of zinc deficiency was also observed in hepatic stellate cells [32].
Figure 3. Zinc availability regulates NO and superoxide anion production in neuronal cells through the modulation of the NMDAR.
The neuronal NMDAR is blocked by zinc (Zn2+) at two different and independent sites. A decrease in extracellular zinc levels leads to the release of that inhibition, and to the influx of calcium (Ca+) through the NMDAR channel. The increase in cellular calcium activates protein kinase C (PKC) which activates NADPH oxidase. NADPH oxidase catalyzes the formation of superoxide anion which can be subsequently converted into other reactive species. On the other hand, calcium can bind to caldmodulin (CaM) and activate NO synthase leading to an increased generation of NO. This figure summarizes findings from Aimo et al. [6].
The capacity of neurons to respond to small changes in extracellular zinc levels with a regulated production of NO, superoxide anion and derived species, which acting as second messengers, can be of relevance in the regulation of physiological processes. On the other hand, if zinc levels are chronically low, overactivation of the NMDAR can lead to oxidative stress and to alterations in neuronal function and fate. The inhibition of the NMDAR by zinc is one example of an indirect “antioxidant” action exerted through the regulation of a receptor protein, of intracellular calcium levels, and subsequently of oxidant production. The capacity of zinc to modulate the activity of numerous proteins could underlie an indirect action of zinc modulating ROS and RNS generation.
Zinc-redox active metals interactions
Another indirect mechanism to regulate oxidative damage of cell components and oxidant generation, is the capacity of zinc to compete with redox active metals (iron, copper) for membrane binding sites. Membrane-associated iron and copper can catalyze the generation of radicals from lipid peroxides. Thus, replacement of these metals by the redox inactive zinc would prevent the formation of highly reactive oxidants. While not affecting membrane physical properties, zinc preferentially binds to negative charges in liposomes, preventing Fe2+-initiated lipid oxidation [43]. The competition with Fe2+ for membrane binding sites as a mechanism of zinc-mediated antioxidant action is supported by a positive correlation between the degree of lipid oxidation and the amount of Fe2+ bound to the membrane, which is ultimately determined by the concentration of zinc (Fig. 4A). In support of this mechanism, zinc is not effective preventing lipid oxidation initiated by lipid- or water-soluble azo compounds or by ultraviolet radiation [43]. Zinc also prevents the oxidation of negatively charge liposomes triggered by aluminum. Although without redox capacity, aluminum can enhance Fe2+-induced lipid oxidation through the promotion of changes in membrane physical properties that increase the efficiency of the oxidative chain reaction [44]. On the other hand, zinc is not effective preventing Fe2+-induced oxidative inactivation of the enzymes glutamine synthase and glucose-6-phosphate dehydrogenase [44]. Fe2 rapidly oxidizes, via hydroxyl radical formation, specific aminoacids in the active sites of both enzymes irreversibly inactivating them [45, 46]. Thus, at least in these particular enzymes, zinc is unable to compete with Fe2 for protein binding sites located in the catalytic domain.
Figure 4. Zinc inhibits Fe2+ binding to liposomes and lipid oxidation, acting in conjunction with hydrophilic and lipofilic antioxidants.
A- Correlation between the capacity of zinc (Zn) (15–250 μM) to inhibit Fe2+ (25 μM) binding to liposomes (phosphatidylcholine:phosphatidylserine 60:40 mol%) and to inhibit lipid oxidation (measured as 2-thiobarbituric acid-reactive substances). B- Zinc (Zn) (25 μM), (−)-epicatechin (EC) (0.5 μM) and 3-tocopherol (AT) (0.01 mol%) jointly act inhibiting Fe2+ (25 μM)-initiated lipid oxidation in liposomes. Adapted from [43].
Very importantly, zinc and hydrophilic ((−)-epicatechin) and lipophilic (3-tocopherol) antioxidants act synergistically in the prevention of Fe2+–triggered liposome oxidation [43] (Fig. 4B). These findings support a role for zinc as a component of the cell antioxidant network through the protection of membrane lipids from redox active metals-induced oxidation.
Free zinc, zinc-thiol interactions, and associated antioxidant effects
As further discussed below, the capacity of zinc to bind to thiol groups is another relevant mechanism which contributes to its antioxidant action. Zinc binds to sulfhydryl groups and protects them from oxidation. Zinc bound to thiols can be released by NO, H2O2, oxidized GSH, and other oxidant species, and participate in antioxidant protective responses. In this regard, a decrease in the cellular reduced environment is associated with an increase in the cellular content of labile zinc [47]. On the other hand, metals with affinity for thiols (e.g. Cd2+, Pb2+, Hg2+) [48] can compromise the amount of zinc bound to thiols which is readily available.
The pool of free zinc released under different conditions, including oxidative stress, is relevant in sustaining the cell redox homeostasis through different mechanisms including the capacity of zinc: to regulate antioxidant protective responses (e.g. transcription factor NF-E2-related factor 2 (Nrf2) [49], to prevent the binding of redox active metals to target molecules [43]; to prevent the activation of oxidant-generating molecules [6], and to upregulate the synthesis of the cysteine-rich thionein, which generate additional thiol groups with capacity to interchange zinc or to act as a direct oxidant scavenger [50]. In support of the above, zinc deficiency both in cells in culture and in animal models increases the susceptibility to metal–induced oxidative stress [51]; causes the activation of redox signaling [52], and reduces the capability of cells to respond to prooxidant stressors by upregulating cellular GSH [53]. As discussed below, a role for zinc in the upregulation of antioxidant genes via Nrf2 can be a major mechanism underlying the antioxidant actions of zinc [53–55].
The above evidence support the concept that, although redox inactive, zinc is a major component of the cell antioxidant network that maintains homeostasis. This review will subsequently focus on the interactions of zinc with protein and non protein (GSH) thiols, and on the relevance of these interactions to the cell redox balance and to redox signaling.
The interactions of zinc with protein thiols and redox sensing
Ten percent of the human genome is estimated to encode for zinc proteins [56], although this number is proposed to be significantly underestimated [12]. The interactions of zinc with protein ligands occur at the imidazole nitrogen in histidine, carboxylate oxygen in aspartic and glutamic acid, and thiol sulfur in cysteine. The nature, dynamic, and influence of these interactions on the functional role of zinc has been thoroughly reviewed before [12]. This review will only address the interactions of zinc with thiols given that they are central to the proposed role for zinc in redox sensing.
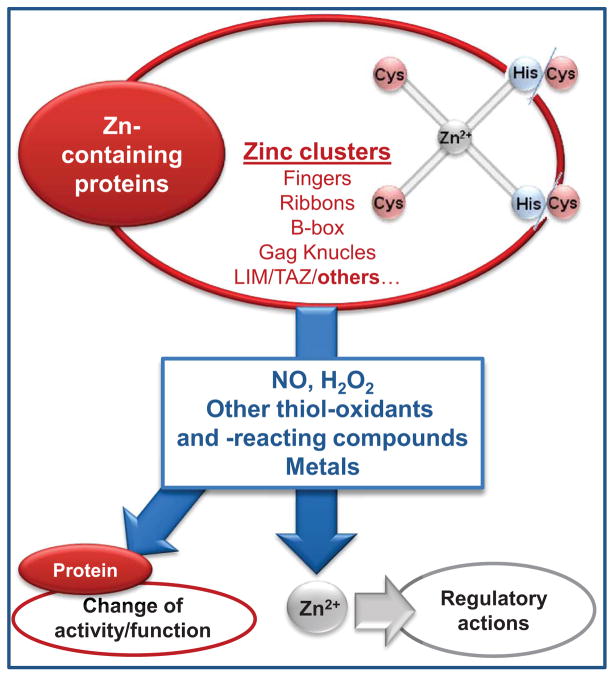
Different arrangements of zinc binding domains exist in proteins. Among them, zinc finger domains have major physiological relevance, as demonstrated by their presence in 3% of the genes in the human genome [57]. Protein zinc finger domains contain zinc coordinated tetrahedrally to cysteine and histidine residues, obtaining a chemical distinct identity through variations in select aminoacids that provide specificity in the recognition of DNA and RNA [58]. Besides the classical involvement of these protein domains in the regulation of transcription, DNA replication and repair, several other relevant regulatory functions have been described. Among them, zinc fingers have been proposed to act as biological redox switches sensing the cell redox status and triggering select responses (reviewed in [7]). A similar role is proposed for several other zinc binding domains, such as ribbons, gag knuckle, treble clef finger, and RING fingers, with a vast majority having cysteine as a coordinating aminoacid.
Zinc is highly abundant, has no redox capacity, its association with sulfur in cysteines is stable under the reducing environment of the cell, and the nature of sulfur-zinc binding allows a rapid association/dissociation of the metal. All these characteristics support the redox sensitive regulatory capacity of labile zinc [7]. Although zinc is not redox active, the redox nature of thiol groups that upon oxidation causes zinc release from MT, and essentially from any protein containing cysteine-zinc clusters [59], confers zinc a redox activity.
As summarized in Fig. 5, several molecules can release zinc from sulfur binding domains in MT and other zinc proteins, and this can have direct and indirect consequences on protein function and on the regulation of cellular processes. The reaction of NO, H2O2, oxidized GSH, and other oxidant species with zinc sulfur clusters can release zinc [9, 60] and confer a dynamic nature to thiol-bound zinc [61]. However, this may not occur in all oxidative modifications of zinc clusters. In this regard, while nitrosative stress-induced damage of the 1alpha,25-dihydroxyvitamin D(3) (1alpha,25(OH)(2)D(3)) receptor (VDR) and retinoid X receptor (RXR) zinc fingers was repaired in breast adenocarcinoma cells, oxidative stress (H2O2, O2·−, tert-butyl hydroperoxide, singlet oxygen)-mediated damage caused their irreversible destruction [62]. Metals can also release zinc from zinc fingers. Replacement of zinc from zinc fingers by different metals (e.g. Al3+, Cd2+, Co2+, Cu2+, Hg2+, Ni2+, Pb2+) has been described for several proteins (reviewed in [63]). Such replacements, can either not affect or modify the protein function (e.g. altered binding of transcription factors to DNA), or lead to cysteine oxidation.
Figure 5. Release of zinc from zinc-sulfur binding domains.
Zinc can bind tetrahedrally to binding domains in proteins through Cys4 or Cys2His2 interactions. Different molecules including NO, H2O2, oxidized GSH, metals, and molecules that react with thiols or have the capacity to oxidize them, cause the release of free zinc. The released zinc can have different regulatory actions, while the modified protein may or may not undergo changes in activity/function.
MT, a family of low molecular weight and cysteine-rich proteins (20 cysteine residues from a total of 61–68 aminoacids), is the most abundant zinc storage protein. Although MT mostly binds zinc, it can also bind several other metals (e.g. Cu1+/2+, Cd2+, Hg2+, Pb2+, Fe2+, Co2+). MT contains zinc cluster domains with varying binding affinity and is a major regulator of cell free zinc content [59]. Cysteine thiolates in MT bind zinc tetrahedrally forming Zn3S9 and Zn4S11 clusters. It has been widely demonstrated that MT zinc can be interchanged between the 3 and 3 domains of MT, and also between MT and proteins (enzymes, transcription factors) that contain zinc clusters (reviewed in [59]) The zinc-thiol interactions in MT are redox regulated and zinc can be released upon reaction with NO, H2O2, and other oxidant species (reviewed in [9]). The low molecular weight, cysteine-containing, and abundant GSH can also be a source of rapidly available zinc. Oxidized GSH interacts with MT through an interchange zinc-thiol/disulfide causing zinc release [64]. The presence of interchangeable zinc in MT, the multiple situations and molecules that promote zinc release from MT, and findings that MT dynamically translocates within cell compartments that require additional zinc amounts [65, 66] point to a major role of MT as a source of rapidly available zinc. This and the direct antioxidant action of MT is further supported by findings in MT I and II knock out mice. Genetic deficits of MT I and II increase the susceptibility to the deleterious effects of both zinc deficiency and toxicity [67]. Furthermore, embryonic cells from MT I and II knock out mice are more susceptible to oxidative stress [68].
Oxidation and other modifications of zinc-thiolate clusters may not affect protein function, exert a regulatory action on the protein activity, or cause a loss of protein function/activity. The released zinc would be available to modulate cell redox homeostasis, cell signaling, and as a consequence to regulate different cellular events including the upregulation of antioxidant defenses, cell proliferation, survival and differentiation [21, 54, 63].
Zinc, redox sensing, and cell signaling
There is a general agreement that zinc can have an important role in the modulation of cell signaling (Reviewed in [11, 69]). However, the underlying mechanisms, the zinc pools involved, the effects of zinc in different cell types, and other aspects of this modulation are complex in nature and need further investigation. For example, cellular oxidative stress and upregulation of select signaling cascades (e.g. protein kinase B (Akt)) are caused by both zinc deficiency and excess ([21, 70]. This review will subsequently focus on potential targets underlying the influence of zinc on redox signaling.
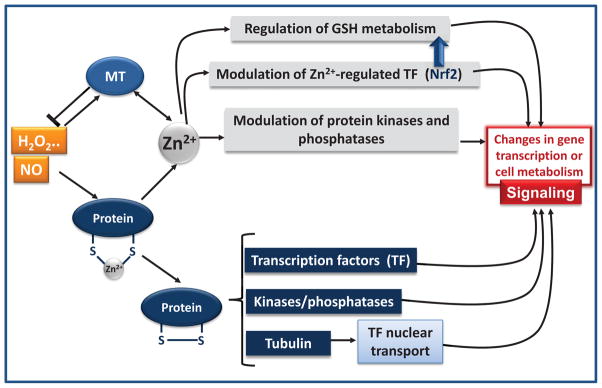
The interactions of zinc with ROS and RNS in the regulation of cell signaling can occur at different levels (summarized in Fig. 6). As previously discussed, modifications of zinc-thiol bonding by ROS and RNS can release zinc [9], which may or may not affect the activity of the protein. If the protein is a transcription factor, it can modulate its capacity to bind to DNA (e.g. transcription factors Sp1, Egr-1, glucocorticoid receptor) [71]; if the protein is a kinase or phosphatase involved in signaling, it can activate or inhibit the pathway; in the case of tubulin, thiol oxidation can affect the nuclear transport of signaling proteins into the nucleus. The released zinc can also act at different levels, including a direct modulation of signaling cascades, the regulation of GSH homeostasis, and the activation/inhibition of kinases and phosphatases (Fig. 6).
Figure 6. Zinc in the regulation of redox signaling.
NO, H2O2, oxidized GSH, and other oxidant species can modify zinc (Zn2+) thiolates leading to zinc release from MT and other proteins. Those species can exert reversible or irreversible chemical modifications of thiol residues (the formation of a disulfide is exemplified in this figure). If the protein is involved in signaling (e.g. transcription factor (TF), kinase, phosphatase, tubulin) a change in activity would lead to changes in signaling modulation. On the other hand, the released zinc can per se regulate transcription factors and the activity of phosphatases and kinases involved in signaling. A direct modulation of transcription factor Nrf2 by zinc will affect GSH synthesis, which would indirectly affect cell redox homeostasis and redox signaling. Zinc is part of the antioxidant network protecting biological systems from oxidative stress. Protein thiol-bound zinc participates in redox sensing. Zinc regulates proteins directly or indirectly involved in redox homeostasis. Zinc can modulate redox signaling through multiple mechanisms. NO and oxidants release zinc bound to sulfur clusters.
RNS, ROS, Zn and signaling
It has been proposed that NO-mediated release of zinc bound to sulfur clusters, can be one of the mechanisms through which NO can regulate cell signaling (Reviewed in [72]). In vitro exposure to NO sources causes nitrosylation of cysteine thiols, disulfide formation, and zinc release from MT [60], and other zinc binding proteins [73]. Once zinc is released, the capacity of the protein to regulate transcription can be modified. For example, NO-mediated zinc release from LAC9, a bacterial transcription factor containing zinc finger motifs, causes the inhibition of LAC9 binding to DNA [60]. The in vitro treatment with NO donors of the VDR and RXR receptors also reversibly affects their binding to DNA 1alpha,25(OH)(2)D(3) response elements [73].
A major target of zinc-dependent regulation is the Kelch-like ECH-associated protein (Keap1)/Nrf2 stress sensing system. Nrf2 is activated by electrophiles, and also by other biotic and physical stimuli (e.g. heat, U.V., sheer stress) [74]. Nrf2 activation is modulated by Keap1, which is a sensor of environmental stress. Under basal conditions, Nrf2 resides in the cytosol bound to Keap1, interaction that favors Nrf2 degradation by the proteasome. Under stress conditions this downregulation decreases, Nrf2 levels increase, and its nuclear translocation and binding to DNA antioxidant response elements promotes the expression of antioxidant and drug metabolizing genes [75, 76]. It has been recently shown that Keap1 senses stress through three different elements that respond to NO, zinc and alkenals, respectively [49]. Zinc released from proteins would act as an intracellular second messenger mediating Nrf2/Keap1 sensing of environmental stressors, while changes in basal zinc levels are proposed to physiologically modulate Nrf2/Keap1 [49]. In line with this, we observed in zinc deficient IMR-32 neuroblastoma cells and embryonic day 19 brain, a decreased expression of the catalytic and modulatory glutamate cysteine ligase (GCL) subunits, coincident with a decreased nuclear Nrf2-DNA binding. GCL subunit expression is in part regulated by Nrf2 [53]. Consistent with this, adult rats fed severely zinc deficient diets present in the olfactory epithelium a low expression of GSH S-transferase [77], another Nrf2-regulated protein [78].
Increasing evidence point to a NO/zinc-regulation of G-protein coupled receptors (GPCR). Recently, the zinc finger-containing protein RGSZ2 has been proposed as a redox switch transforming a redox signal into a zinc signal [79]. RGSZ2 binds to nNOS inhibiting NO production, and also binds, through its interaction with the HINT1 protein, to the GPCR mu-opioid receptor (MOR). MOR can activate nNOS and cause a transient increase in NO generation which leads to zinc release [80]. Not only MOR activation, but activation of other GPCR, causes a NO/nNOS-dependent release of zinc from RGSZ2, which leads to the recruitment of the redox-sensing proteins protein kinase C (PKC) gamma and Raf-1 to the GPCR and their subsequent activation [79].
MT is a major regulator of zinc homeostasis, acting as a zinc reservoir that upon thiol oxidation by NO, H2O2, oxidized GSH, and other thiol reactants (reviewed in [9]), makes zinc available for different cellular targets. The interaction NO/oxidants with zinc also occurs at the level of MT expression. NO triggers a MT-dependent transient release of zinc in the nucleus, which leads to an increased expression of MT I and II [66]. NO causes the nuclear translocation of transcription factor MTF-1 [81], which binds to metal responsive elements present in the promoter of MT I and II, leading to increased MT expression [82]. This effect is proposed to be triggered by NO-mediated release of zinc from MT, rather than through a direct effect of NO on MTF-1 [81]. In vitro and in vivo studies indicate that MT can act as a free radical scavenger [9]. In vitro, MT scavenges hydroxyl radical, superoxide anion, and peroxynitrite [83], preventing hydroxyl radical-and superoxide anion-induced DNA damage [84, 85], and being 800-fold more efficient than GSH in preventing hydroxyl radical-mediated DNA degradation [85]. In vivo, mice overexpressing MT are protected from pro-oxidant conditions, such as ischemia/reperfusion [86] and doxorubicin cardiotoxicity [87]. Although a free radical scavenging action of MT is supported by numerous studies, its physiological relevance is still controversial.
Zinc and glutathione
Besides the capacity of oxidized GSH to mobilize zinc from MT and other zinc-thiol clusters, zinc per se can affect GSH metabolism. GSH is involved in the detoxification of oxidants, both enzymatically and non-enzymatically; as both carrier and storage of cysteine, in the elimination of xenobiotics through direct conjugation, and acting as a buffer of intracellular redox homeostasis [88, 89]. In cells, GSH steady state levels are regulated through its synthesis, utilization and export. Interactions between zinc and GSH metabolism are relevant to the protection of cells against oxidative stress-mediated damage. For example, NO protects endothelial cells against damage induced by H2O2, in association with a zinc-dependent activation of transcription factor Nrf2 [55], which upregulates GCL expression and GSH synthesis [54, 55].
A role for zinc on GSH metabolism is suggested by the frequent finding of GSH deficits in association with zinc deficiency in different cells and tissues [23, 28, 32–34]. GSH concentration was lower in the brain of embryonic day 19 fetuses from rat dams fed marginal zinc diets compared to controls [53], and in zinc deficient neuronal cells [34, 53]. In both systems, decreased levels of GSH are associated with a decreased expression of GCL, the enzyme that catalyzes the rate-limiting step of de novo GSH synthesis. The protein and mRNA levels of the GCL catalytic and modulatory subunits were lower in zinc deficient fetal brain and neuronal cells, in association with an impaired Nrf2 nuclear translocation [53]. GCL expression is regulated by transcription factors Nrf2, AP-1 and NF-3B, [90]. Given the presence of a zinc sensing cluster in Keap1/Nrf2 [49], a decrease in labile zinc as a consequence of zinc deficiency could explain the associated decrease in Nrf2 activation. Furthermore, zinc deficiency also inhibits NF-3B transcriptional activity [91, 92]. Besides a regulation at the transcriptional level, zinc deficiency also causes an increased cleavage of the full length GCL catalytic subunit [53]. This cleavage is catalyzed by caspase 3, a zinc enzyme that is activated in conditions of zinc deficiency in different cells and tissues [93]. Thus, zinc can regulate GSH synthesis by modulating GCL both at transcriptional and posttranslational levels. Nevertheless, utilization, recycling, export, and availability of substrates for synthesis could also contribute to GSH deficits in zinc deficiency.
Thus, the modulation of cellular GSH by zinc, and the relevance of the balance oxidized/reduced GSH in the release of zinc from MT points to a major role of GSH/zinc interactions in the physiological regulation of redox homeostasis. Overall these interactions can: i) have an effect on the cell thiol redox status, ii) modulate redox sensitive signaling, and iii) determine the capacity of cells to respond to oxidative stressors.
Phosphatases
Although zinc released from sulfur clusters can affect the activity of multiple proteins (e.g. enzymes, receptors, transcription factors), this review will focus on those that are involved in redox signaling. Cell signaling is largely driven by phosphorylation/dephosphorylation reactions which can mediate both activation and inactivation of signaling proteins. Phophatases cleave phosphate groups from Ser/Thr or Tyr residues in proteins. Zinc can directly bind to phosphatases activating or inhibiting their activity. Indirectly, oxidative stress associated with decreased zinc availability, could inhibit the activity of redox sensitive phosphatases. For example, the physiological production of H2O2 triggered by the binding of ligands to select receptors (e.g. epidermal growth factor and insulin receptor), inhibits phosphatases allowing a longer phosphorylation of signaling intermediates, and a more prolonged activation of the cascade.
Protein tyrosine phosphatases (PTPs) can be both directly regulated by zinc and by changes in the redox state. PTP1B, one example of this type of regulation, contains a cysteine group in its catalytic site in a conserved sequence HCX5R(S/T) [94]. The environment within this motif decreases the pKa of the cysteine group giving it a higher nucleophilicity and higher susceptibility to oxidation. The oxidation of this cysteine to sulfenic acid, which is rapidly converted into sulphenyl-amide, leads to a major conformational change that renders the binding site unavailable to substrates [95]. The formation of the sulphenyl-amide prevents further irreversible oxidation to sulphonic acid, turning cysteine redox changes reversible and a mechanism of dynamic enzyme modulation [95]. PTP1B was initially described to be inhibited by micromolar concentrations of zinc [96]. However, it was subsequently shown to be also inhibited by nanomolar zinc concentrations, being the IC50 values for PTP1B and SHP-1 (PTP1C) 17 and 93 nM, respectively [97]. These findings support a role for zinc in the in vivo modulation of PTP1B. Thus, while zinc deficiency could inhibit PTP1B as a consequence of increased H2O2 production [6], physiological decreases in cellular zinc levels would provide a direct tonic modulation of PTP1B activity by zinc. Other PTP, the receptor PTP beta, is inhibited by zinc at picomolar concentrations [98]. The above evidence points to a physiological role for zinc in the modulation of PTPs involved in signaling modulation. In this regard, the inhibition of PTP1B by zinc can enhance the cellular response to insulin, which is supported by the described insulin-like actions of zinc [99, 100].
The human dual specificity phosphatase YVH1 or DUSP12 present a unique mechanism of intertwined regulation by zinc and redox state [101]. YVH1 is proposed to regulate major cellular processes including cell survival, ribosome biogenesis and cellular DNA content [102, 103]. YVH1 possesses a phosphatase catalytic domain containing a redox sensitive cysteine group with a similar structure and catalytic dynamics to that of PTPs [94], and a zinc-coordinating C terminal domain. In conditions of oxidative stress, the formation of a disulfide bond ejects zinc from the zinc-coordinating domain, and at the catalytic site causes YVH1 inactivation [101]. The zinc domain is proposed to act as a redox sensor in conditions of oxidative stress to prevent the irreversible YVH1 inactivation [101]. When oxidant levels return to basal levels, the restoration of zinc to its binding motif is needed for complete enzyme activation.
Other important phosphatase regulated by zinc and involved in cell signaling is the Ser/Thr phosphatase PP2A [104]. Although the involved mechanisms are not completely understood, indirect evidence points to a regulatory action of zinc on PP2A. PP2A activity is inhibited by 1 μM zinc in vitro [104], and this inhibition is proposed to underlie the increase in the Ser/Thr phosphorylation of Akt in cardiac myoblasts treated with zinc [105]. This mechanism could explain the cardioprotective actions of a zinc ionophore in reperfusion injury [106]. The release of zinc at synaptic terminals is proposed to cause tau hyperphosphorylation through the zinc-mediated inhibition of PP2A [107]. Of significant relevance for Alzheimer’s disease, hyperphosphorylated tau accumulates in neurofibrillary tangles, in association with alterations in brain zinc homeostasis [108].
MAPKs
The family of mitogen activated kinases (MAPKs), which include ERK1/2 (extracellular signal-regulated kinases), ERK5, p38 and JNK (Jun N-terminal kinases), is one of a select group of intracellular signaling proteins linking receptor activation to nuclear and cytosolic targets. While ERK1/2 are sensitive mostly to mitogenic signals, p38 and JNK respond to stress stimuli. Zinc can indirectly modulate the activity of the MAPKs. The modulation of MAPKs by zinc is supported by findings in animal and cell models of zinc deficiency, as well as in mice with deficits in zinc transporters. In the rat brain and neuronal cells zinc deficiency differentially affects ERK1/2, p38 and JNK1/2 [109, 110].
Zinc can influence ERK activity through several mechanisms (reviewed in [111]). Those mechanisms include the previously described capacity of zinc to inhibit phosphatases that can dephosphorylate ERK (e.g. PP2A) [104, 112], zinc-mediated inhibition of kinases that phosphorylate receptors which activate ERK [113], a direct activation by zinc of receptors (e.g. GPR39) with ERK1/2 as a downstream target [114]; and the activation by zinc-dependent enzymes which generate active ligands (eg. pro-brain-derived neurotrophic factor (BDNF) conversion to BDNF by metalloproteases [113]) that trigger ERK1/2-associated cascades. An indirect regulation of ERK by zinc can also occur through the previously discussed activation of select GPCR leading to the sequential nNOS activation, NO generation, zinc release, Raf-1 recruitment to the GPCR and to the activation of Raf-1/MEK/ERK [79]. In support of a physiological role for zinc on ERK modulation, ERK1/2 is inhibited in conditions of zinc deficiency in fibroblasts [115], human neuroblastoma IMR-32 cells [21, 110], primary cultures of cortical neurons [21], and in the rat developing brain [109]. Furthermore, in knockout mice for the zinc transporter Znt3, the phosphorylation of ERK1/2 is decreased in association with an increase in ERK1/2-directed tyrosine phosphatase activity [116]. ERK inhibition can in part explain the negative impact of zinc deficits on cell proliferation and survival [21, 93, 115, 117–121], and as a consequence on development [1]. On the other hand, zinc release as a consequence of GSH depletion-induced oxidative stress causes increased ERK1/2 activation through the inhibition of ERK1/2 phosphatase activity [112].
MAPKs p38 and JNK are activated by zinc deficiency. Zinc deficiency causes high levels of p38 and JNK phosphorylation and/or activity in association with oxidative stress in different cells and tissues including testes [122], developing rat brain [109], and human neuroblastoma cells [110]. Antioxidant compounds and enzymes that prevent zinc deficiency-induced oxidant increase and GSH decrease also prevent p38 and JNK activation, and the downstream activation of transcription factor AP-1 [34, 110]. Furthermore, inhibitors of the NMDA receptor not only prevent zinc deficiency-induced and NMDA-mediated production or reactive oxygen and nitrogen species, but also AP-1 activation [6]. This indicates that the increased oxidant production occurring as a consequence of decreased cellular zinc is the main factor triggering p38 and JNK activation. The regulation of p38 and JNK by oxidant species is not completely understood, but in part involve the activation of upstream MAPK kinase kinases (e.g. ASK-1 [123]), and the inactivation of MAPK phosphatases (e.g. MKP-1 [124]). On the other hand, zinc supplementation also differentially regulates MAPKs. In T-lymphocytes zinc increases p38 phosphorylation, while not affecting ERK and JNK. Different mechanisms were proposed to be involved in p38 activation by high zinc levels, including the inactivation of phosphatases and a direct or indirect activation of upstream kinases [125].
Thus, the complex regulation of MAPKs by zinc will depend, among other factors, on the extent of cellular zinc decrease or increase, on the cell type, on the existence of different stimuli, and on the particular cell network of kinases and phosphatases.
Protein kinase C
PKC constitutes a family of Ser/Thr protein kinases involved in signaling pathways that modulate important cellular processes, e.g. growth, cell death, differentiation, response to stress. PKC is a redox sensitive kinase (reviewed in [126]). PKC contains cysteine-rich regions in both the catalytic and regulatory domains, the later containing two pairs of zinc fingers in the C1 region [127, 128]. Zinc fingers participate in the interactions of PKC with membrane diagylglycerol, and of its mimetic phorbol esters, and consequently, in the regulation of PKC cellular localization and activity. In this regard, the oxidation of PKC’s zinc fingers by oxidants leads to zinc release and to the loss of zinc finger structure. As a consequence, PKC is activated in a cofactor-independent manner given the release of a self-inhibitory mechanism. Phorbol esters and mild pro-oxidant conditions cause zinc release from 3T3 and insect cells, which was identified to originate from PKC [129]. In fact, activated PKCalpha contains less zinc than its resting form. The incubation of recombinant peptides encompassing different PKC protein regions with 1.3-diolein, phorbol esters and oxidants causes a stoichiometric release of zinc [129].
Stressing the relevance of zinc in PKC regulation, not only oxidants but also zinc availability can affect PKC activity. In zinc deficient 3T3 cells, a low cytosolic classical PKC activity was observed which was not due to the translocation of the enzyme to the membrane [130]. However, a decrease in labile and total zinc causes different effects on PKC isoforms, downregulating PKCalpha (protein levels and activity), and causing a caspase-dependent cleavage and activation of PKCdelta [130]. The later could be potentially involved in zinc deficiency-associated induction of apoptotic cell death [130].
Given the multiple signaling pathways regulated by PKC, the redox/zinc-mediated regulation of PKC activity could be central to an indirect action of zinc in the modulation of redox signaling, e.g. MAPKs. In this regard, activation of PKC leads to a downstream activation of ERK1/2 which provides an additional and indirect level of zinc-mediated regulation of ERK1/2 [131]
Zinc and tubulin thiols: regulation of NF-3B and NFAT nuclear transport
A role for zinc in the regulation of cell signaling is also related to the preservation of tubulin polymerization dynamics through the maintenance of an adequate intracellular thiol redox status. Microtubules are major components of the cell cytoskeleton, being essential for multiple aspects of cell physiology including structural and transport functions. Microtubules are dynamically assembled through the reversible polymerization of 3 and 3 tubulin subunits. Tubulin contains 20 reduced cysteines per 33 dimer, 8 in the 3 and 12 in the 3 subunit [132] which makes it highly susceptible to oxidation. In this regard, tubulin oxidation by peroxynitrite, H2O2, and other oxidizing agents [133–135] impairs tubulin polymerization into microtubules which can be repaired by the thioredoxin reductase [134] and the GSH/glutaredoxin reductase systems [135]. Tubulin oxidation can have deleterious consequences on cell and tissue functions, including loss of the intestinal barrier selective permeability [136], increased permeability of the vascular endothelium [137], and alterations in the transport of cellular components which, for example, can affect the modulation of gene transcription [109].
Zinc deficits cause alterations in tubulin polymerization dynamics both in adult and fetal rat brain, and in neuronal cells in culture [51, 91, 92, 138–140]. Given the rapid increase in neuronal ROS and RNS when cells are cultured in zinc deficient medium, the involvement of thiol oxidation in the observed tubulin polymerization alterations was highly feasible. In fact, simultaneous incubation of IMR-32 cells in zinc deficient media and in the presence of N-acetyl cysteine or 3-lipoic acid prevented the alterations in tubulin polymerization kinetics and the formation of tubulin oligomers with molecular weights higher than 100 kDa [10]. Similar findings were observed when cytosolic fractions from zinc deficient IMR-32 cell, rat cortical neurons and embryonic rat brain were treated with the disulfide reducing agent tris(2-carboxyethyl)phosphine. Tubulin oxidative modifications as a consequence of neuronal/brain zinc deficits involved the formation of disulfides, but not protein carbonylation or nitration [10].
Microtubules actively participate in the axonal transport of cellular components and in the cross-talk between the synapse and the nucleus [141]. A direct consequence of zinc deficiency-induced tubulin alterations in neuronal cells is an altered nuclear transport and transactivating activity of transcription factors NF-3B [91, 92] and NFAT [41]. Supplementation of IMR-32 cells with N-acetyl cysteine or 3-lipoic acid, which prevented tubulin oxidation and altered polymerization, restored the nuclear transport of NF-3B and the expression of NF-3B-dependent genes [10]. Tubulin oxidation was not observed in the liver from zinc deficient embryos, which points to the brain as a particular target of these alterations. In this regard, tubulin oxidation and its functional consequences can in part explain the adverse effects of zinc deficiency on brain development through a derangement of key developmental events, such as neurogenesis and neuronal apoptosis [142, 143].
Conclusions
Given its unique chemical characteristics, zinc is an abundant metal playing multiple roles in biological molecules and cellular events. The frequent association of oxidative stress to zinc deficiency originated the concept that zinc could be part of the antioxidant defense system. A large number of continuously growing evidence currently supports direct and indirect roles for zinc in the regulation of oxidant production, a critical relevance of thiol-bound zinc regulating the pool of free zinc which can be released by NO, H2O2 and other oxidants, and the involvement of zinc in redox signaling. The release of free zinc pulses capable of regulating cell signaling, and as a consequence cell function and fate, is of high physiological relevance and points to zinc as a central second messenger.
Alterations in zinc homeostasis with an associated deregulation of redox signaling can have significant adverse consequences given the multiple pathways involved and events affected. In the developing nervous system, zinc deficiency causes oxidative stress, impairs GSH metabolism, causes tubulin oxidation, and disrupt redox-sensitive signaling. These alterations could underlie the adverse effects of zinc deficiency on brain development, and on the observed alterations in behavior, cognition and motor performance. Furthermore, impaired Nrf2 activation as a consequence of decreased zinc availability can increase the susceptibility of neurons and of other cells to oxidant stressors. Deregulation of redox-sensitive signals (e.g. ERK) involved in the control of cell proliferation, can explain the adverse effects of zinc deficiency on tissues that undergo periods of rapid cell growth, such as the skin, the immune and reproductive system. Oxidative damage of cellular components, and redox-mediated alterations in the patterns and extent of proliferation and apoptotic cell death during development may lead to altered organ cellularity, organization and connectivity, increasing the risk for diseases later in life.
It is evident that the “antioxidant” actions of zinc, and the capacity of zinc to regulate redox signaling will depend on several factors including the particular cell/tissue, the levels of available zinc, and the array of particular signaling molecules and stimuli in the target cell/tissue. This rapidly growing field of research will help elucidate many aspect of zinc involvement in redox signaling that are currently unknown.
Acknowledgments
I dedicate this review to Dr. Lucille Hurley, my postdoctoral advisor, who was a pioneer in the field of zinc and its relevance in human development and health. Most importantly, she was a role model for many women in science. This work was supported by grants from the University of California, Davis (CA-D*-XXX-7244-H), and NIH (grant # HD 01743), USA. PO is a corresponding investigator from CONICET, Argentina.
Abbreviations
- Akt
protein kinase B
- BDNF
brain-derived neurotrophic factor
- ERK
extracellular signal-regulated kinases
- GCL
glutamate cysteine ligase
- GSH
glutathione
- GPCR
G-protein coupled receptors
- Keap1
Kelch-like ECH-associated protein
- JNK
Jun N-terminal kinases
- MAPKs
mitogen activated kinases
- MOR
mu-opioid receptor
- MT
metallothionein
- NMDA
N-methyl-D-aspartate
- NMDAR
N-methyl-D-aspartate receptor
- NO
nitric oxide
- Nrf2
transcription factor NF-E2-related factor 2
- PKC
protein kinase C
- PTPs
protein tyrosine phosphatases
- RXR
retinoid X receptor
- VDR
1alpha,25-dihydroxyvitamin D(3) (1alpha,25(OH)(2)D(3)) receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Uriu-Adams JY, Keen CL. Zinc and reproduction: effects of zinc deficiency on prenatal and early postnatal development. Birth Defects Res B Dev Reprod Toxicol. 2010;89:313–325. doi: 10.1002/bdrb.20264. [DOI] [PubMed] [Google Scholar]
- 2.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 3.Andreini C, Bertini I. A bioinformatics view of zinc enzymes. J Inorg Biochem. 2012;111:150–156. doi: 10.1016/j.jinorgbio.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 4.Sharif R, Thomas P, Zalewski P, Fenech M. The role of zinc in genomic stability. Mutat Res. 2012;733:111–121. doi: 10.1016/j.mrfmmm.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Bitanihirwe BK, Cunningham MG. Zinc: the brain’s dark horse. Synapse. 2009;63:1029–1049. doi: 10.1002/syn.20683. [DOI] [PubMed] [Google Scholar]
- 6.Aimo L, Cherr GN, Oteiza PI. Low extracellular zinc increases neuronal oxidant production through nadph oxidase and nitric oxide synthase activation. Free Radic Biol Med. 2010;48:1577–1587. doi: 10.1016/j.freeradbiomed.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kroncke KD, Klotz LO. Zinc fingers as biologic redox switches? Antioxid Redox Signal. 2009;11:1015–1027. doi: 10.1089/ARS.2008.2269. [DOI] [PubMed] [Google Scholar]
- 8.Maret W. Redox biochemistry of mammalian metallothioneins. J Biol Inorg Chem. 2011;16:1079–1086. doi: 10.1007/s00775-011-0800-0. [DOI] [PubMed] [Google Scholar]
- 9.Kang YJ. Metallothionein redox cycle and function. Exp Biol Med (Maywood) 2006;231:1459–1467. doi: 10.1177/153537020623100903. [DOI] [PubMed] [Google Scholar]
- 10.Mackenzie GG, Salvador GA, Romero C, Keen CL, Oteiza PI. A deficit in zinc availability can cause alterations in tubulin thiol redox status in cultured neurons and in the developing fetal rat brain. Free Radic Biol Med. 2011;51:480–489. doi: 10.1016/j.freeradbiomed.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukada T, Yamasaki S, Nishida K, Murakami M, Hirano T. Zinc homeostasis and signaling in health and diseases: Zinc signaling. J Biol Inorg Chem. 2011;16:1123–1134. doi: 10.1007/s00775-011-0797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maret W, Li Y. Coordination dynamics of zinc in proteins. Chem Rev. 2009;109:4682–4707. doi: 10.1021/cr800556u. [DOI] [PubMed] [Google Scholar]
- 13.Andreini C, Banci L, Bertini I, Rosato A. Zinc through the three domains of life. J Proteome Res. 2006;5:3173–3178. doi: 10.1021/pr0603699. [DOI] [PubMed] [Google Scholar]
- 14.Bozym RA, Thompson RB, Stoddard AK, Fierke CA. Measuring picomolar intracellular exchangeable zinc in PC-12 cells using a ratiometric fluorescence biosensor. ACS Chem Biol. 2006;1:103–111. doi: 10.1021/cb500043a. [DOI] [PubMed] [Google Scholar]
- 15.Dittmer PJ, Miranda JG, Gorski JA, Palmer AE. Genetically encoded sensors to elucidate spatial distribution of cellular zinc. J Biol Chem. 2009;284:16289–16297. doi: 10.1074/jbc.M900501200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vinkenborg JL, Nicolson TJ, Bellomo EA, Koay MS, Rutter GA, Merkx M. Genetically encoded FRET sensors to monitor intracellular Zn2+ homeostasis. Nat Methods. 2009;6:737–740. doi: 10.1038/nmeth.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin Y, Dittmer PJ, Park JG, Jansen KB, Palmer AE. Measuring steady-state and dynamic endoplasmic reticulum and Golgi Zn2+ with genetically encoded sensors. Proc Natl Acad Sci U S A. 2011;108:7351–7356. doi: 10.1073/pnas.1015686108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zalewski PD, Forbes IJ, Seamark RF, Borlinghaus R, Betts WH, Lincoln SF, Ward AD. Flux of intracellular labile zinc during apoptosis (gene-directed cell death) revealed by a specific chemical probe, Zinquin. Chem Biol. 1994;1:153–161. doi: 10.1016/1074-5521(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 19.Bernhardt ML, Kong BY, Kim AM, O’Halloran TV, Woodruff TK. A zinc-dependent mechanism regulates meiotic progression in Mammalian oocytes. Biol Reprod. 2012;86:114. doi: 10.1095/biolreprod.111.097253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Maret W. Transient fluctuations of intracellular zinc ions in cell proliferation. Exp Cell Res. 2009;315:2463–2470. doi: 10.1016/j.yexcr.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Adamo AM, Zago MP, Mackenzie GG, Aimo L, Keen CL, Keenan A, Oteiza PI. The role of zinc in the modulation of neuronal proliferation and apoptosis. Neurotox Res. 2010;17:1–14. doi: 10.1007/s12640-009-9067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burke JP, Fenton MR. Effect of a zinc-deficient diet on lipid peroxidation in liver and tumor subcellular membranes. Proc Soc Exp Biol Med. 1985;179:187–191. doi: 10.3181/00379727-179-42083. [DOI] [PubMed] [Google Scholar]
- 23.Oteiza PI, Clegg MS, Keen CL. Short-term zinc deficiency affects nuclear factor-kappab nuclear binding activity in rat testes. J Nutr. 2001;131:21–26. doi: 10.1093/jn/131.1.21. [DOI] [PubMed] [Google Scholar]
- 24.Oteiza PI, Clegg MS, Zago MP, Keen CL. Zinc deficiency induces oxidative stress and AP-1 activation in 3T3 cells. Free Radic Biol Med. 2000;28:1091–1099. doi: 10.1016/s0891-5849(00)00200-8. [DOI] [PubMed] [Google Scholar]
- 25.Oteiza PI, Olin KL, Fraga CG, Keen CL. Zinc deficiency causes oxidative damage to proteins, lipids and DNA in rat testes. J Nutr. 1995;125:823–829. doi: 10.1093/jn/125.4.823. [DOI] [PubMed] [Google Scholar]
- 26.Oteiza PL, Olin KL, Fraga CG, Keen CL. Oxidant defense systems in testes from zinc-deficient rats. Proc Soc Exp Biol Med. 1996;213:85–91. doi: 10.3181/00379727-213-44040. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan JF, Jetton MM, Hahn HK, Burch RE. Enhanced lipid peroxidation in liver microsomes of zinc-deficient rats. Am J Clin Nutr. 1980;33:51–56. doi: 10.1093/ajcn/33.1.51. [DOI] [PubMed] [Google Scholar]
- 28.Kraus A, Roth HP, Kirchgessner M. Supplementation with vitamin C, vitamin E or beta-carotene influences osmotic fragility and oxidative damage of erythrocytes of zinc-deficient rats. J Nutr. 1997;127:1290–1296. doi: 10.1093/jn/127.7.1290. [DOI] [PubMed] [Google Scholar]
- 29.Virgili F, Canali R, Figus E, Vignolini F, Nobili F, Mengheri E. Intestinal damage induced by zinc deficiency is associated with enhanced CuZn superoxide dismutase activity in rats: effect of dexamethasone or thyroxine treatment. Free Radic Biol Med. 1999;26:1194–1201. doi: 10.1016/s0891-5849(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 30.Ho E, Ames BN. Low intracellular zinc induces oxidative DNA damage, disrupts p53, NFkappa B, and AP1 DNA binding, and affects DNA repair in a rat glioma cell line. Proc Natl Acad Sci U S A. 2002;99:16770–16775. doi: 10.1073/pnas.222679399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho E, Courtemanche C, Ames BN. Zinc deficiency induces oxidative DNA damage and increases p53 expression in human lung fibroblasts. J Nutr. 2003;133:2543–2548. doi: 10.1093/jn/133.8.2543. [DOI] [PubMed] [Google Scholar]
- 32.Kojima-Yuasa A, Umeda K, Ohkita T, Opare Kennedy D, Nishiguchi S, Matsui-Yuasa I. Role of reactive oxygen species in zinc deficiency-induced hepatic stellate cell activation. Free Radic Biol Med. 2005;39:631–640. doi: 10.1016/j.freeradbiomed.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Tomat AL, Inserra F, Veiras L, Vallone MC, Balaszczuk AM, Costa MA, Arranz C. Moderate zinc restriction during fetal and postnatal growth of rats: effects on adult arterial blood pressure and kidney. Am J Physiol Regul Integr Comp Physiol. 2008;295:R543–549. doi: 10.1152/ajpregu.00050.2008. [DOI] [PubMed] [Google Scholar]
- 34.Mackenzie GG, Zago MP, Erlejman AG, Aimo L, Keen CL, Oteiza PI. alpha-Lipoic acid and N-acetyl cysteine prevent zinc deficiency-induced activation of NF-kappaB and AP-1 transcription factors in human neuroblastoma IMR-32 cells. Free Radic Res. 2006;40:75–84. doi: 10.1080/10715760500312305. [DOI] [PubMed] [Google Scholar]
- 35.Westbrook GL, Mayer ML. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987;328:640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- 36.Peters S, Koh J, Choi DW. Zinc selectively blocks the action of N-methyl-D-aspartate on cortical neurons. Science. 1987;236:589–593. doi: 10.1126/science.2883728. [DOI] [PubMed] [Google Scholar]
- 37.Zheng F, Gingrich MB, Traynelis SF, Conn PJ. Tyrosine kinase potentiates NMDA receptor currents by reducing tonic zinc inhibition. Nat Neurosci. 1998;1:185–191. doi: 10.1038/634. [DOI] [PubMed] [Google Scholar]
- 38.Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J Neurosci. 1997;17:5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Legendre P, Westbrook GL. The inhibition of single N-methyl-D-aspartate-activated channels by zinc ions on cultured rat neurones. J Physiol. 1990;429:429–449. doi: 10.1113/jphysiol.1990.sp018266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christine CW, Choi DW. Effect of zinc on NMDA receptor-mediated channel currents in cortical neurons. J Neurosci. 1990;10:108–116. doi: 10.1523/JNEUROSCI.10-01-00108.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mackenzie GG, Oteiza PI. Zinc and the cytoskeleton in the neuronal modulation of transcription factor NFAT. J Cell Physiol. 2007;210:246–256. doi: 10.1002/jcp.20861. [DOI] [PubMed] [Google Scholar]
- 42.Takeda A, Yamada K, Tamano H, Fuke S, Kawamura M, Oku N. Hippocampal calcium dyshomeostasis and long-term potentiation in 2-week zinc deficiency. Neurochem Int. 2008;52:241–246. doi: 10.1016/j.neuint.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 43.Zago MP, Oteiza PI. The antioxidant properties of zinc: interactions with iron and antioxidants. Free Radic Biol Med. 2001;31:266–274. doi: 10.1016/s0891-5849(01)00583-4. [DOI] [PubMed] [Google Scholar]
- 44.Zago MP, Verstraeten SV, Oteiza PI. Zinc in the prevention of Fe2+-initiated lipid and protein oxidation. Biol Res. 2000;33:143–150. doi: 10.4067/s0716-97602000000200014. [DOI] [PubMed] [Google Scholar]
- 45.Fisher MT, Stadtman ER. Oxidative modification of Escherichia coli glutamine synthetase. Decreases in the thermodynamic stability of protein structure and specific changes in the active site conformation. J Biol Chem. 1992;267:1872–1880. [PubMed] [Google Scholar]
- 46.Szweda LI, Stadtman ER. Iron-catalyzed oxidative modification of glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides. Structural and functional changes. J Biol Chem. 1992;267:3096–3100. [PubMed] [Google Scholar]
- 47.Pirev E, Calles C, Schroeder P, Sies H, Kroncke KD. Ultraviolet-A irradiation but not ultraviolet-B or infrared-A irradiation leads to a disturbed zinc homeostasis in cells. Free Radic Biol Med. 2008;45:86–91. doi: 10.1016/j.freeradbiomed.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 48.Haase H, Hebel S, Engelhardt G, Rink L. Flow cytometric measurement of labile zinc in peripheral blood mononuclear cells. Anal Biochem. 2006;352:222–230. doi: 10.1016/j.ab.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 49.McMahon M, Lamont DJ, Beattie KA, Hayes JD. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc Natl Acad Sci U S A. 2010;107:18838–18843. doi: 10.1073/pnas.1007387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng W, Benz FW, Cai J, Pierce WM, Kang YJ. Metallothionein disulfides are present in metallothionein-overexpressing transgenic mouse heart and increase under conditions of oxidative stress. J Biol Chem. 2006;281:681–687. doi: 10.1074/jbc.M506956200. [DOI] [PubMed] [Google Scholar]
- 51.Mackenzie GG, Keen CL, Oteiza PI. Zinc status of human IMR-32 neuroblastoma cells influences their susceptibility to iron-induced oxidative stress. Dev Neurosci. 2002;24:125–133. doi: 10.1159/000065691. [DOI] [PubMed] [Google Scholar]
- 52.Aimo L, Oteiza PI. Zinc deficiency increases the susceptibility of human neuroblastoma cells to lead-induced activator protein-1 activation. Toxicol Sci. 2006;91:184–191. doi: 10.1093/toxsci/kfj137. [DOI] [PubMed] [Google Scholar]
- 53.Omata Y, Salvador GA, Supasai S, Keenan AH, Oteiza PI. A decreased Zn availability affects glutathione metabolism in neuronal cells and in the developing brain. Free Radic Biol Med. 2012 doi: 10.1093/toxsci/kft022. Under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cortese MM, Suschek CV, Wetzel W, Kroncke KD, Kolb-Bachofen V. Zinc protects endothelial cells from hydrogen peroxide via Nrf2-dependent stimulation of glutathione biosynthesis. Free Radic Biol Med. 2008;44:2002–2012. doi: 10.1016/j.freeradbiomed.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 55.Cortese-Krott MM, Suschek CV, Wetzel W, Kroncke KD, Kolb-Bachofen V. Nitric oxide-mediated protection of endothelial cells from hydrogen peroxide is mediated by intracellular zinc and glutathione. Am J Physiol Cell Physiol. 2009;296:C811–820. doi: 10.1152/ajpcell.00643.2008. [DOI] [PubMed] [Google Scholar]
- 56.Andreini C, Banci L, Bertini I, Rosato A. Counting the zinc-proteins encoded in the human genome. J Proteome Res. 2006;5:196–201. doi: 10.1021/pr050361j. [DOI] [PubMed] [Google Scholar]
- 57.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann N, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, Gibbs RA, Muzny DM, Scherer SE, Bouck JB, Sodergren EJ, Worley KC, Rives CM, Gorrell JH, Metzker ML, Naylor SL, Kucherlapati RS, Nelson DL, Weinstock GM, Sakaki Y, Fujiyama A, Hattori M, Yada T, Toyoda A, Itoh T, Kawagoe C, Watanabe H, Totoki Y, Taylor T, Weissenbach J, Heilig R, Saurin W, Artiguenave F, Brottier P, Bruls T, Pelletier E, Robert C, Wincker P, Smith DR, Doucette-Stamm L, Rubenfield M, Weinstock K, Lee HM, Dubois J, Rosenthal A, Platzer M, Nyakatura G, Taudien S, Rump A, Yang H, Yu J, Wang J, Huang G, Gu J, Hood L, Rowen L, Madan A, Qin S, Davis RW, Federspiel NA, Abola AP, Proctor MJ, Myers RM, Schmutz J, Dickson M, Grimwood J, Cox DR, Olson MV, Kaul R, Shimizu N, Kawasaki K, Minoshima S, Evans GA, Athanasiou M, Schultz R, Roe BA, Chen F, Pan H, Ramser J, Lehrach H, Reinhardt R, McCombie WR, de la Bastide M, Dedhia N, Blocker H, Hornischer K, Nordsiek G, Agarwala R, Aravind L, Bailey JA, Bateman A, Batzoglou S, Birney E, Bork P, Brown DG, Burge CB, Cerutti L, Chen HC, Church D, Clamp M, Copley RR, Doerks T, Eddy SR, Eichler EE, Furey TS, Galagan J, Gilbert JG, Harmon C, Hayashizaki Y, Haussler D, Hermjakob H, Hokamp K, Jang W, Johnson LS, Jones TA, Kasif S, Kaspryzk A, Kennedy S, Kent WJ, Kitts P, Koonin EV, Korf I, Kulp D, Lancet D, Lowe TM, McLysaght A, Mikkelsen T, Moran JV, Mulder N, Pollara VJ, Ponting CP, Schuler G, Schultz J, Slater G, Smit AF, Stupka E, Szustakowski J, Thierry-Mieg D, Thierry-Mieg J, Wagner L, Wallis J, Wheeler R, Williams A, Wolf YI, Wolfe KH, Yang SP, Yeh RF, Collins F, Guyer MS, Peterson J, Felsenfeld A, Wetterstrand KA, Patrinos A, Morgan MJ, de Jong P, Catanese JJ, Osoegawa K, Shizuya H, Choi S, Chen YJ. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 58.Klug A. The discovery of zinc fingers and their applications in gene regulation and genome manipulation. Annu Rev Biochem. 2010;79:213–231. doi: 10.1146/annurev-biochem-010909-095056. [DOI] [PubMed] [Google Scholar]
- 59.Bell SG, Vallee BL. The metallothionein/thionein system: an oxidoreductive metabolic zinc link. Chembiochem. 2009;10:55–62. doi: 10.1002/cbic.200800511. [DOI] [PubMed] [Google Scholar]
- 60.Kroncke KD, Fehsel K, Schmidt T, Zenke FT, Dasting I, Wesener JR, Bettermann H, Breunig KD, Kolb-Bachofen V. Nitric oxide destroys zinc-sulfur clusters inducing zinc release from metallothionein and inhibition of the zinc finger-type yeast transcription activator LAC9. Biochem Biophys Res Commun. 1994;200:1105–1110. doi: 10.1006/bbrc.1994.1564. [DOI] [PubMed] [Google Scholar]
- 61.Aravindakumar CT, Ceulemans J, De Ley M. Nitric oxide induces Zn2+ release from metallothionein by destroying zinc-sulphur clusters without concomitant formation of S-nitrosothiol. Biochem J. 1999;344(Pt 1):253–258. doi: 10.1042/0264-6021:3440253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kroncke KD, Klotz LO, Suschek CV, Sies H. Comparing nitrosative versus oxidative stress toward zinc finger-dependent transcription. Unique role for NO. J Biol Chem. 2002;277:13294–13301. doi: 10.1074/jbc.M111216200. [DOI] [PubMed] [Google Scholar]
- 63.Hartwig A. Zinc finger proteins as potential targets for toxic metal ions: differential effects on structure and function. Antioxid Redox Signal. 2001;3:625–634. doi: 10.1089/15230860152542970. [DOI] [PubMed] [Google Scholar]
- 64.Maret W. Oxidative metal release from metallothionein via zinc-thiol/disulfide interchange. Proc Natl Acad Sci U S A. 1994;91:237–241. doi: 10.1073/pnas.91.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsujikawa K, Imai T, Kakutani M, Kayamori Y, Mimura T, Otaki N, Kimura M, Fukuyama R, Shimizu N. Localization of metallothionein in nuclei of growing primary cultured adult rat hepatocytes. FEBS Lett. 1991;283:239–242. doi: 10.1016/0014-5793(91)80597-v. [DOI] [PubMed] [Google Scholar]
- 66.Spahl DU, Berendji-Grun D, Suschek CV, Kolb-Bachofen V, Kroncke KD. Regulation of zinc homeostasis by inducible NO synthase-derived NO: nuclear metallothionein translocation and intranuclear Zn2+ release. Proc Natl Acad Sci U S A. 2003;100:13952–13957. doi: 10.1073/pnas.2335190100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kelly EJ, Quaife CJ, Froelick GJ, Palmiter RD. Metallothionein I and II protect against zinc deficiency and zinc toxicity in mice. J Nutr. 1996;126:1782–1790. doi: 10.1093/jn/126.7.1782. [DOI] [PubMed] [Google Scholar]
- 68.Lazo JS, Kondo Y, Dellapiazza D, Michalska AE, Choo KH, Pitt BR. Enhanced sensitivity to oxidative stress in cultured embryonic cells from transgenic mice deficient in metallothionein I and II genes. J Biol Chem. 1995;270:5506–5510. doi: 10.1074/jbc.270.10.5506. [DOI] [PubMed] [Google Scholar]
- 69.Oteiza PI, Mackenzie GG. Zinc, oxidant-triggered cell signaling, and human health. Mol Aspects Med. 2005;26:245–255. doi: 10.1016/j.mam.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 70.Wu W, Wang X, Zhang W, Reed W, Samet JM, Whang YE, Ghio AJ. Zinc-induced PTEN protein degradation through the proteasome pathway in human airway epithelial cells. J Biol Chem. 2003;278:28258–28263. doi: 10.1074/jbc.M303318200. [DOI] [PubMed] [Google Scholar]
- 71.Webster KA, Prentice H, Bishopric NH. Oxidation of zinc finger transcription factors: physiological consequences. Antioxid Redox Signal. 2001;3:535–548. doi: 10.1089/15230860152542916. [DOI] [PubMed] [Google Scholar]
- 72.Li H, Cao R, Wasserloos KJ, Bernal P, Liu ZQ, Pitt BR, St Croix CM. Nitric oxide and zinc homeostasis in pulmonary endothelium. Ann N Y Acad Sci. 2010;1203:73–78. doi: 10.1111/j.1749-6632.2010.05558.x. [DOI] [PubMed] [Google Scholar]
- 73.Kroncke KD, Carlberg C. Inactivation of zinc finger transcription factors provides a mechanism for a gene regulatory role of nitric oxide. FASEB J. 2000;14:166–173. doi: 10.1096/fasebj.14.1.166. [DOI] [PubMed] [Google Scholar]
- 74.Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med. 2004;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 75.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kang KW, Lee SJ, Kim SG. Molecular mechanism of nrf2 activation by oxidative stress. Antioxid Redox Signal. 2005;7:1664–1673. doi: 10.1089/ars.2005.7.1664. [DOI] [PubMed] [Google Scholar]
- 77.Kudo H, Doi Y, Nishino T, Nara S, Hamasaki K, Fujimoto S. Dietary zinc deficiency decreases glutathione S-transferase expression in the rat olfactory epithelium. J Nutr. 2000;130:38–44. doi: 10.1093/jn/130.1.38. [DOI] [PubMed] [Google Scholar]
- 78.Ikeda H, Serria MS, Kakizaki I, Hatayama I, Satoh K, Tsuchida S, Muramatsu M, Nishi S, Sakai M. Activation of mouse Pi-class glutathione S-transferase gene by Nrf2(NF-E2-related factor 2) and androgen. Biochem J. 2002;364:563–570. doi: 10.1042/BJ20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sanchez-Blazquez P, Rodriguez-Munoz M, Bailon C, Garzon J. GPCRs Promote the Release of Zinc Ions Mediated by nNOS/NO and the Redox Transducer RGSZ2 Protein. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2012.4517. [DOI] [PubMed] [Google Scholar]
- 80.Sanchez-Blazquez P, Rodriguez-Munoz M, Garzon J. Mu-opioid receptors transiently activate the Akt-nNOS pathway to produce sustained potentiation of PKC-mediated NMDAR-CaMKII signaling. PLoS One. 2010;5:e11278. doi: 10.1371/journal.pone.0011278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stitt MS, Wasserloos KJ, Tang X, Liu X, Pitt BR, St Croix CM. Nitric oxide-induced nuclear translocation of the metal responsive transcription factor, MTF-1 is mediated by zinc release from metallothionein. Vascul Pharmacol. 2006;44:149–155. doi: 10.1016/j.vph.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 82.Radtke F, Heuchel R, Georgiev O, Hergersberg M, Gariglio M, Dembic Z, Schaffner W. Cloned transcription factor MTF-1 activates the mouse metallothionein I promoter. EMBO J. 1993;12:1355–1362. doi: 10.1002/j.1460-2075.1993.tb05780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thornalley PJ, Vasak M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim Biophys Acta. 1985;827:36–44. doi: 10.1016/0167-4838(85)90098-6. [DOI] [PubMed] [Google Scholar]
- 84.Cai L, Klein JB, Kang YJ. Metallothionein inhibits peroxynitrite-induced DNA and lipoprotein damage. J Biol Chem. 2000;275:38957–38960. doi: 10.1074/jbc.C000593200. [DOI] [PubMed] [Google Scholar]
- 85.Abel J, de Ruiter N. Inhibition of hydroxyl-radical-generated DNA degradation by metallothionein. Toxicol Lett. 1989;47:191–196. doi: 10.1016/0378-4274(89)90075-1. [DOI] [PubMed] [Google Scholar]
- 86.Kang YJ, Li Y, Sun X. Antiapoptotic effect and inhibition of ischemia/reperfusion-induced myocardial injury in metallothionein-overexpressing transgenic mice. Am J Pathol. 2003;163:1579–1586. doi: 10.1016/S0002-9440(10)63514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kang YJ, Zhou ZX, Wang GW, Buridi A, Klein JB. Suppression by metallothionein of doxorubicin-induced cardiomyocyte apoptosis through inhibition of p38 mitogen-activated protein kinases. J Biol Chem. 2000;275:13690–13698. doi: 10.1074/jbc.275.18.13690. [DOI] [PubMed] [Google Scholar]
- 88.Aoyama K, Watabe M, Nakaki T. Regulation of neuronal glutathione synthesis. J Pharmacol Sci. 2008;108:227–238. doi: 10.1254/jphs.08r01cr. [DOI] [PubMed] [Google Scholar]
- 89.Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mackenzie GG, Keen CL, Oteiza PI. Microtubules are required for NF-kappaB nuclear translocation in neuroblastoma IMR-32 cells: modulation by zinc. J Neurochem. 2006;99:402–415. doi: 10.1111/j.1471-4159.2006.04005.x. [DOI] [PubMed] [Google Scholar]
- 92.Mackenzie GG, Zago MP, Keen CL, Oteiza PI. Low intracellular zinc impairs the translocation of activated NF-kappa B to the nuclei in human neuroblastoma IMR-32 cells. J Biol Chem. 2002;277:34610–34617. doi: 10.1074/jbc.M203616200. [DOI] [PubMed] [Google Scholar]
- 93.Clegg MS, Hanna LA, Niles BJ, Momma TY, Keen CL. Zinc deficiency-induced cell death. IUBMB Life. 2005;57:661–669. doi: 10.1080/15216540500264554. [DOI] [PubMed] [Google Scholar]
- 94.Zhang ZY, Wang Y, Dixon JE. Dissecting the catalytic mechanism of protein-tyrosine phosphatases. Proc Natl Acad Sci U S A. 1994;91:1624–1627. doi: 10.1073/pnas.91.5.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, Barford D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 96.Brautigan DL, Bornstein P, Gallis B. Phosphotyrosyl-protein phosphatase. Specific inhibition by Zn. J Biol Chem. 1981;256:6519–6522. [PubMed] [Google Scholar]
- 97.Haase H, Maret W. Intracellular zinc fluctuations modulate protein tyrosine phosphatase activity in insulin/insulin-like growth factor-1 signaling. Exp Cell Res. 2003;291:289–298. doi: 10.1016/s0014-4827(03)00406-3. [DOI] [PubMed] [Google Scholar]
- 98.Wilson M, Hogstrand C, Maret W. Picomolar Concentrations of Free Zinc(II) Ions Regulate Receptor Protein-tyrosine Phosphatase beta Activity. J Biol Chem. 2012;287:9322–9326. doi: 10.1074/jbc.C111.320796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Coulston L, Dandona P. Insulin-like effect of zinc on adipocytes. Diabetes. 1980;29:665–667. doi: 10.2337/diab.29.8.665. [DOI] [PubMed] [Google Scholar]
- 100.Jansen J, Rosenkranz E, Overbeck S, Warmuth S, Mocchegiani E, Giacconi R, Weiskirchen R, Karges W, Rink L. Disturbed zinc homeostasis in diabetic patients by in vitro and in vivo analysis of insulinomimetic activity of zinc. J Nutr Biochem. 2012 doi: 10.1016/j.jnutbio.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 101.Bonham CA, Vacratsis PO. Redox regulation of the human dual specificity phosphatase YVH1 through disulfide bond formation. J Biol Chem. 2009;284:22853–22864. doi: 10.1074/jbc.M109.038612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kozarova A, Hudson JW, Vacratsis PO. The dual-specificity phosphatase hYVH1 (DUSP12) is a novel modulator of cellular DNA content. Cell Cycle. 2011;10:1669–1678. doi: 10.4161/cc.10.10.15641. [DOI] [PubMed] [Google Scholar]
- 103.Lo KY, Li Z, Wang F, Marcotte EM, Johnson AW. Ribosome stalk assembly requires the dual-specificity phosphatase Yvh1 for the exchange of Mrt4 with P0. J Cell Biol. 2009;186:849–862. doi: 10.1083/jcb.200904110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhuo S, Dixon JE. Effects of sulfhydryl regents on the activity of lambda Ser/Thr phosphoprotein phosphatase and inhibition of the enzyme by zinc ion. Protein Eng. 1997;10:1445–1452. doi: 10.1093/protein/10.12.1445. [DOI] [PubMed] [Google Scholar]
- 105.Lee S, Chanoit G, McIntosh R, Zvara DA, Xu Z. Molecular mechanism underlying Akt activation in zinc-induced cardioprotection. Am J Physiol Heart Circ Physiol. 2009;297:H569–575. doi: 10.1152/ajpheart.00293.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Karagulova G, Yue Y, Moreyra A, Boutjdir M, Korichneva I. Protective role of intracellular zinc in myocardial ischemia/reperfusion is associated with preservation of protein kinase C isoforms. J Pharmacol Exp Ther. 2007;321:517–525. doi: 10.1124/jpet.107.119644. [DOI] [PubMed] [Google Scholar]
- 107.Sun XY, Wei YP, Xiong Y, Wang XC, Xie AJ, Wang XL, Yang Y, Wang Q, Lu YM, Wang JZ, Liu R. Synaptic released zinc promotes tau hyperphosphorylation by inhibition of PP2A. J Biol Chem. 2012 doi: 10.1074/jbc.M111.309070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bush AI. The Metal Theory of Alzheimer’s Disease. J Alzheimers Dis. 2012 doi: 10.3233/JAD-2012-129011. [DOI] [PubMed] [Google Scholar]
- 109.Aimo L, Mackenzie GG, Keenan AH, Oteiza PI. Gestational zinc deficiency affects the regulation of transcription factors AP-1, NF-kappaB and NFAT in fetal brain. J Nutr Biochem. 2010;21:1069–1075. doi: 10.1016/j.jnutbio.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zago MP, Mackenzie GG, Adamo AM, Keen CL, Oteiza PI. Differential Modulation of MAP Kinases by Zinc Deficiency in IMR-32 Cells: Role of H(2)O(2) Antioxid Redox Signal. 2005;7:1773–1782. doi: 10.1089/ars.2005.7.1773. [DOI] [PubMed] [Google Scholar]
- 111.Nuttall JR, Oteiza PI. Zinc and the ERK kinases in the developing brain. Neurotox Res. 2012;21:128–141. doi: 10.1007/s12640-011-9291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ho Y, Samarasinghe R, Knoch ME, Lewis M, Aizenman E, DeFranco DB. Selective inhibition of mitogen-activated protein kinase phosphatases by zinc accounts for extracellular signal-regulated kinase 1/2-dependent oxidative neuronal cell death. Mol Pharmacol. 2008;74:1141–1151. doi: 10.1124/mol.108.049064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hwang JJ, Park MH, Choi SY, Koh JY. Activation of the Trk signaling pathway by extracellular zinc. Role of metalloproteinases. J Biol Chem. 2005;280:11995–12001. doi: 10.1074/jbc.M403172200. [DOI] [PubMed] [Google Scholar]
- 114.Holst B, Egerod KL, Schild E, Vickers SP, Cheetham S, Gerlach LO, Storjohann L, Stidsen CE, Jones R, Beck-Sickinger AG, Schwartz TW. GPR39 signaling is stimulated by zinc ions but not by obestatin. Endocrinology. 2007;148:13–20. doi: 10.1210/en.2006-0933. [DOI] [PubMed] [Google Scholar]
- 115.Lefebvre D, Boney CM, Ketelslegers JM, Thissen JP. Inhibition of insulin-like growth factor-I mitogenic action by zinc chelation is associated with a decreased mitogen-activated protein kinase activation in RAT-1 fibroblasts. FEBS Lett. 1999;449:284–288. doi: 10.1016/s0014-5793(99)00419-6. [DOI] [PubMed] [Google Scholar]
- 116.Sindreu C, Palmiter RD, Storm DR. Zinc transporter ZnT-3 regulates presynaptic Erk1/2 signaling and hippocampus-dependent memory. Proc Natl Acad Sci U S A. 2011;108:3366–3370. doi: 10.1073/pnas.1019166108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rossi L, Migliaccio S, Corsi A, Marzia M, Bianco P, Teti A, Gambelli L, Cianfarani S, Paoletti F, Branca F. Reduced growth and skeletal changes in zinc-deficient growing rats are due to impaired growth plate activity and inanition. J Nutr. 2001;131:1142–1146. doi: 10.1093/jn/131.4.1142. [DOI] [PubMed] [Google Scholar]
- 118.Wang X, Fosmire GJ, Gay CV, Leach RM., Jr Short-term zinc deficiency inhibits chondrocyte proliferation and induces cell apoptosis in the epiphyseal growth plate of young chickens. J Nutr. 2002;132:665–673. doi: 10.1093/jn/132.4.665. [DOI] [PubMed] [Google Scholar]
- 119.Prasad AS, Beck FW, Endre L, Handschu W, Kukuruga M, Kumar G. Zinc deficiency affects cell cycle and deoxythymidine kinase gene expression in HUT-78 cells. J Lab Clin Med. 1996;128:51–60. doi: 10.1016/s0022-2143(96)90113-4. [DOI] [PubMed] [Google Scholar]
- 120.Fraker PJ. Roles for cell death in zinc deficiency. J Nutr. 2005;135:359–362. doi: 10.1093/jn/135.3.359. [DOI] [PubMed] [Google Scholar]
- 121.Truong-Tran AQ, Grosser D, Ruffin RE, Murgia C, Zalewski PD. Apoptosis in the normal and inflamed airway epithelium: role of zinc in epithelial protection and procaspase-3 regulation. Biochem Pharmacol. 2003;66:1459–1468. doi: 10.1016/s0006-2952(03)00498-2. [DOI] [PubMed] [Google Scholar]
- 122.Zhao Y, Tan Y, Dai J, Li B, Guo L, Cui J, Wang G, Shi X, Zhang X, Mellen N, Li W, Cai L. Exacerbation of diabetes-induced testicular apoptosis by zinc deficiency is most likely associated with oxidative stress, p38 MAPK activation, and p53 activation in mice. Toxicol Lett. 2011;200:100–106. doi: 10.1016/j.toxlet.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 123.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kuwano Y, Kim HH, Abdelmohsen K, Pullmann R, Jr, Martindale JL, Yang X, Gorospe M. MKP-1 mRNA stabilization and translational control by RNA-binding proteins HuR and NF90. Mol Cell Biol. 2008;28:4562–4575. doi: 10.1128/MCB.00165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Honscheid A, Dubben S, Rink L, Haase H. Zinc differentially regulates mitogen-activated protein kinases in human T cells. J Nutr Biochem. 2012;23:18–26. doi: 10.1016/j.jnutbio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 126.Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radic Biol Med. 2000;28:1349–1361. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 127.Quest AF, Bloomenthal J, Bardes ES, Bell RM. The regulatory domain of protein kinase C coordinates four atoms of zinc. J Biol Chem. 1992;267:10193–10197. [PubMed] [Google Scholar]
- 128.Hommel U, Zurini M, Luyten M. Solution structure of a cysteine rich domain of rat protein kinase C. Nat Struct Biol. 1994;1:383–387. doi: 10.1038/nsb0694-383. [DOI] [PubMed] [Google Scholar]
- 129.Korichneva I, Hoyos B, Chua R, Levi E, Hammerling U. Zinc release from protein kinase C as the common event during activation by lipid second messenger or reactive oxygen. J Biol Chem. 2002;277:44327–44331. doi: 10.1074/jbc.M205634200. [DOI] [PubMed] [Google Scholar]
- 130.Chou SS, Clegg MS, Momma TY, Niles BJ, Duffy JY, Daston GP, Keen CL. Alterations in protein kinase C activity and processing during zinc-deficiency-induced cell death. Biochem J. 2004;383:63–71. doi: 10.1042/BJ20040074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gopalakrishna R, Gundimeda U, Schiffman JE, McNeill TH. A direct redox regulation of protein kinase C isoenzymes mediates oxidant-induced neuritogenesis in PC12 cells. J Biol Chem. 2008;283:14430–14444. doi: 10.1074/jbc.M801519200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nogales E, Wolf SG, Downing KH. Structure of the alpha beta tubulin dimer by electron crystallography. Nature. 1998;391:199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- 133.Landino LM, Hagedorn TD, Kim SB, Hogan KM. Inhibition of tubulin polymerization by hypochlorous acid and chloramines. Free Radic Biol Med. 2011;50:1000–1008. doi: 10.1016/j.freeradbiomed.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Landino LM, Iwig JS, Kennett KL, Moynihan KL. Repair of peroxynitrite damage to tubulin by the thioredoxin reductase system. Free Radic Biol Med. 2004;36:497–506. doi: 10.1016/j.freeradbiomed.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 135.Landino LM, Moynihan KL, Todd JV, Kennett KL. Modulation of the redox state of tubulin by the glutathione/glutaredoxin reductase system. Biochem Biophys Res Commun. 2004;314:555–560. doi: 10.1016/j.bbrc.2003.12.126. [DOI] [PubMed] [Google Scholar]
- 136.Banan A, Choudhary S, Zhang Y, Fields JZ, Keshavarzian A. Oxidant-induced intestinal barrier disruption and its prevention by growth factors in a human colonic cell line: role of the microtubule cytoskeleton. Free Radic Biol Med. 2000;28:727–738. doi: 10.1016/s0891-5849(00)00160-x. [DOI] [PubMed] [Google Scholar]
- 137.Bernhard D, Csordas A, Henderson B, Rossmann A, Kind M, Wick G. Cigarette smoke metal-catalyzed protein oxidation leads to vascular endothelial cell contraction by depolymerization of microtubules. Faseb J. 2005;19:1096–1107. doi: 10.1096/fj.04-3192com. [DOI] [PubMed] [Google Scholar]
- 138.Hesketh JE. Impaired microtubule assembly in brain from zinc-deficient pigs and rats. Int J Biochem. 1981;13:921–926. doi: 10.1016/0020-711x(81)90019-7. [DOI] [PubMed] [Google Scholar]
- 139.Oteiza PI, Cuellar S, Lonnerdal B, Hurley LS, Keen CL. Influence of maternal dietary zinc intake on in vitro tubulin polymerization in fetal rat brain. Teratology. 1990;41:97–104. doi: 10.1002/tera.1420410110. [DOI] [PubMed] [Google Scholar]
- 140.Oteiza PI, Hurley LS, Lonnerdal B, Keen CL. Marginal zinc deficiency affects maternal brain microtubule assembly in rats. J Nutr. 1988;118:735–738. doi: 10.1093/jn/118.6.735. [DOI] [PubMed] [Google Scholar]
- 141.Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL. Fainzilber, M. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- 142.Peters JM, Wiley LM, Zidenberg-Cherr S, Keen CL. Influence of short-term maternal zinc deficiency on the in vitro development of preimplantation mouse embryos. Proc Soc Exp Biol Med. 1991;198:561–568. doi: 10.3181/00379727-198-43289. [DOI] [PubMed] [Google Scholar]
- 143.Corniola RS, Tassabehji NM, Hare J, Sharma G, Levenson CW. Zinc deficiency impairs neuronal precursor cell proliferation and induces apoptosis via p53-mediated mechanisms. Brain Res. 2008;1237:52–61. doi: 10.1016/j.brainres.2008.08.040. [DOI] [PubMed] [Google Scholar]