Abstract
Background
Non-invasive imaging of inflammation to measure the progression of autoimmune diseases, such as rheumatoid arthritis (RA), and to monitor responses to therapy is critically needed. V-Sense, a perfluorocarbon (PFC) contrast agent that preferentially labels inflammatory cells, which are then recruited out of systemic circulation to sites of inflammation, enables detection by 19F MRI. With no 19F background in the host, detection is highly-specific and can act as a proxy biomarker of the degree of inflammation present.
Methods
Collagen-induced arthritis in rats, a model with many similarities to human RA, was used to study the ability of the PFC contrast agent to reveal the accumulation of inflammation over time using 19F MRI. Disease progression in the rat hind limbs was monitored by caliper measurements and 19F MRI on days 15, 22 and 29, including the height of clinically symptomatic disease. Naïve rats served as controls. The capacity of the PFC contrast agent and 19F MRI to assess the effectiveness of therapy was studied in a cohort of rats administered oral prednisolone on days 14 to 28.
Results
Quantification of 19F signal measured by MRI in affected limbs was linearly correlated with disease severity. In animals with progressive disease, increases in 19F signal reflected the ongoing recruitment of inflammatory cells to the site, while no increase in 19F signal was observed in animals receiving treatment which resulted in clinical resolution of disease.
Conclusion
These results indicate that 19F MRI may be used to quantitatively and qualitatively evaluate longitudinal responses to a therapeutic regimen, while additionally revealing the recruitment of monocytic cells involved in the inflammatory process to the anatomical site. This study may support the use of 19F MRI to clinically quantify and monitor the severity of inflammation, and to assess the effectiveness of treatments in RA and other diseases with an inflammatory component.
Keywords: Inflammation, Monocytes, Macrophages, Magnetic resonance imaging (MRI), Biofunctional imaging, Perfluorocarbon, Contrast agent, Arthritis
Background
Rheumatoid arthritis is a systematic, chronic, debilitating disease which affects approximately 0.5-1% of the world population [1,2]. Inflammation of the synovial membrane is a hallmark of the disease, with the disease eventually progressing to cartilage and osseous degradation. There is no known cure, however therapeutic treatments are available and recent advances in the imaging of the disease and associated inflammation have allowed earlier diagnosis and intervention [3,4], with the possibility for increased mobility and quality of life for patients through disease management [5-7].
Imaging for arthritis, or inflammation in general, can be classified as either anatomical imaging, where the manifestations of the disease on the body are observed, or biofunctional imaging, where the biological processes involved in the disease are observed [8]. In the case of RA, imaging for clinical diagnosis is limited to anatomical imaging of bone erosion (MRI, CT), inflammation of the synovial membrane (ultrasound, MRI), and joint effusion and tissue swelling (x-ray) [9,10]. These techniques, though useful, often only elucidate the disease after permanent damage is done, limiting the applicability of early intervention therapies [11]. Biofunctional imaging of arthritis focuses on metabolic activity, cellular infiltrates, and cytokine production [10], which often occur prior to the onset of permanent anatomical damage due to the disease. It may serve as an indicator of the presence of disease and severity, enabling earlier diagnosis and treatment [3]. By example, in RA patients with clinically stable disease, synovitis may persist, leading to disease progression [12]. Methodologies for non-invasive detection and localization of inflammation in RA include PET/CT [13], ultrasound [14], optical (fluorescence) imaging [15], and MRI.
MRI is of particular interest due to its high spatial resolution, which allows precise anatomical visualization of bone degradation, and its current role in the diagnosis of RA [16]. Furthermore, the safety profile of MRI makes it amenable to repetitive imaging sessions, an important consideration for use in a prolonged, chronic disorder. MRI images of macrophage infiltration associated with inflammation have been obtained in a variety of disease states using transition metal and super paramagnetic iron oxide (SPIO) contrast agents [17]. Gadolinium has been used as a blood-pool marker of sites of inflammation [18,19] and quantitative methods using the reagent have been developed [20-22]. SPIO nanoparticles are phagocytosed by circulating monocytes/macrophage, and provide MR contrast when those cells aggregate at the site of inflammation [23-27]. Unfortunately, metal-based MR contrast agents operate by either increasing or decreasing the signal obtained in the MRI image, effectively convoluting the anatomical image with cell-level information and impeding normal observation of disease progression with the technique. The use of an alternate nucleus, such as fluorine MRI (19F MRI) avoids this difficulty by specific detection of the fluorine atom, providing a signal which varies in a direct relationship with the amount 19F present, without any background signal from host tissue, and without distorting the anatomical 1H image.
19F MRI with the use of a perfluorocarbon (PFC) contrast agent has emerged as a powerful technique through the in situ labeling of circulating macrophage and monocytes. Labeled inflammatory cells traffic to sites of inflammation where they accumulate and render those tissues detectable by 19F MRI. This approach has been used as an indicator of inflammation in a variety of disease models including experimental autoimmune encephalitis [28], allograft rejection [29,30], inflammatory bowel disease [31], abscess visualization [32], pulmonary inflammation [33], and post-ischemia inflammation in the heart and brain [34]. Un-inflamed tissues (with the exception of the reticuloendothelial system) lack 19F signal, and therapeutic intervention can modulate the 19F intensity [31,33], indicating the specificity of this approach for inflamed tissues. Furthermore, the 19F signal correlates with the degree of macrophages present in the inflammatory site [28]. However, no correlation to disease severity has yet been established through co-measurement of 19F MRI and with a clinical marker.
Here, we employ a well-known model of RA, with a facile, independent measurement of clinical disease progression (ankle diameter), to validate the ability of 19F MRI to ascertain disease presence and severity. The current study has two aims: (1) to evaluate the ability of 19F MRI to quantitatively measure disease severity relative to standard measurements, and (2) to determine whether serial imaging with 19F MRI reflects the course of disease progression or response to therapy.
Methods
Animals, arthritis model and treatment
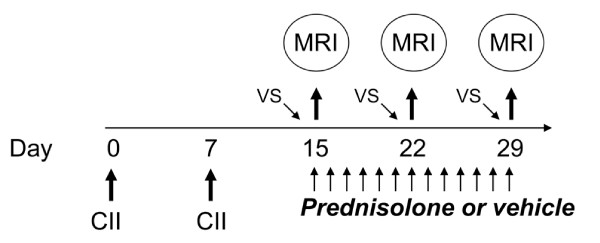
All animal studies were conducted with Institutional IACUC approval at a contract research organization (Covance Laboratories, Greenfield, IN). Female Lewis rats were purchased from Charles River, and housed under specific pathogen-free (SPF) conditions. The rat collagen induced arthritis (CIA) model was conducted as previously described [35,36]. Briefly, Lewis rats received two weekly doses of Type II bovine collagen (CII, 2 mg/mL) in incomplete Freund’s adjuvant, intradermally, leading to the onset of arthritis with clinical symptoms appearing by day 14 after the initial immunization, peak swelling in the limb joints by day 21 and arthritic damage by day 28. Naïve animals served as controls, and were housed until day 28. The development of arthritis and severity was measured by paw swelling in the hind limbs using caliper measurements every 3-4 days. To determine whether 19F MRI could be used to monitor the effect of drug therapy on established inflammation, CIA animals were randomized upon disease onset (day 14) into cohorts of vehicle control or drug treatment for monitoring by serial imaging. Therapy consisted of daily prednisolone (Sigma Chemical Company, St. Louis, MO; 10 mg/kg, selected as a model therapeutic due to its efficacy in treating existing disease) or vehicle control (1% hydroxyethylcellulose, 0.25% tween-80, 0.05% antifoam), dosed daily by oral gavage (3.4 ml/kg). The PFC contrast agent was administered prior to each imaging session to evaluate the active inflammatory response at the time of imaging. A schema of the study design is shown in Figure 1.
Figure 1 .
Study schema for serial monitoring of arthritic inflammation & disease. Six rats were injected intradermally with Type II collagen (CII) in incomplete Freund’s adjuvant on days 0 and 7. Animals were randomized into study cohorts (3 per group) on day 13, and all injected with PFC contrast agent. Two days later animals were imaged by 1H 19F MRI, and placed on daily vehicle or prednisolone for the duration of the study. MRI was repeated at weekly intervals 48 hours after contrast agent administration. Clinical measurements of ankle swelling and animal weight were conducted from day 15 (after the initial collagen immunization) to study end, every 3 to 4 days.
Magnetic Resonance Imaging
For in vivo imaging studies V-Sense, a sterile PFC-containing emulsion (20% (v/v) of perfluoropolyether, VS-1000H, Celsense, Inc., Pittsburgh, PA) was used as a 19F contrast agent to detect macrophage activity [37]. Forty-eight hours prior to imaging, a single 1.5 mL dose was administered intravenously through the tail vein.
MRI was conducted using a Varian 7T DirectDrive MRI spectrometer (Agilent Technologies, Santa Clara, CA) equipped with VnmrJ 2.2 C acquisition software, RHEL 4.u.3 OS, the Magnex 205/120/HD gradient set, a 35 mm i.d. transmit/receive volume coil, tunable for 1H or 19F imaging (m2m Imaging Corp., Cleveland, OH) and a physiological monitoring system (Small Animal Instruments, Inc. Stony Brook, NY). Rats were anesthetized with 5% isoflurane and maintained with an anesthesia nose cone at 1.5% isoflurane in oxygen. Prone rats were positioned with hind limbs extended and an external reference tube (containing 1:15 dilution of the PFC contrast agent in 1% agarose gel, and a known number of 19F atoms (i.e., spins/mm3) to enable quantitative measurement of fluorine content) placed between the legs, affixed to the animal cradle, and guided into the RF coil. Respiration and body temperature were monitored throughout image acquisition, and bore temperature was maintained below 30C.
The 1H image was obtained with a fast spin-echo sequence, multislice (21 slices, 1 mm thick), and high-resolution axial images along the length of the hind limbs, rostral and caudal to the ankles. The acquisition parameters were: repetition time/echo time (TR/TE) = 2000/22 ms, using a rapid acquisition with refocused echo (RARE) sequence, RARE factor = 8, 256 × 256 matrix, field of view (FOV) = 40 x 40 mm2, 2 averages, total acquisition time 2.1 minutes. For 19F images, a RARE sequence was used with TR/TE = 1800/10.1 ms, RARE factor = 8, 128 × 64 matrix zerofilled to 256 x 256, FOV 40 x 40 mm2, 128 averages, 21 slices and a total acquisition time of 30.7 minutes. The Larmor frequencies of 1H and 19F differ by ~6%.
19F MRI data analysis
Each MR imaging session included a reference tube containing a known dilution of the PFC contrast agent prepared at a concentration of 2.76 x 1017 spins/mm3. Voxel Tracker™ software (Celsense, Inc.) was used to correct for the effects of the Rician noise distribution inherent in MRI and to compare signal intensity from a region of interest to that of the reference tube to allow determination of the total amount of fluorine [38-40]. Regions of interest (ROIs) were drawn in the (1) noise region (2) reference material regions (3) right/left leg in each image slice. In most instances, no signal was observed in the first or last slice, indicating that the majority of the signal was within the area of analysis.
Histology
On day 29, after the final scan, animals were anesthetized by CO2 inhalation and euthanized by cervical dislocation, and ankles and knees fixed in 10% neutral buffered formalin, then decalcified prior to embedding in paraffin. Sections were stained with hematoxylin/eosin to assess inflammation/cellular infiltration within the joints. Images were captured with an Olympus Provis light microscope, and sections were also digitized with a microscope slide scanner.
Statistical analysis
Dunnetts’ method was used for comparisons of multiple cohorts. Two factor ANOVA was used to evaluate significance of longitudinal and treatment differences, followed by ad-hoc comparison of means using Tukey’s method. Paired T-tests were used to evaluate differences in the same animal at different time points, and unpaired T-tests were used to evaluate differences between cohorts. Error bars represent standard deviation.
Results
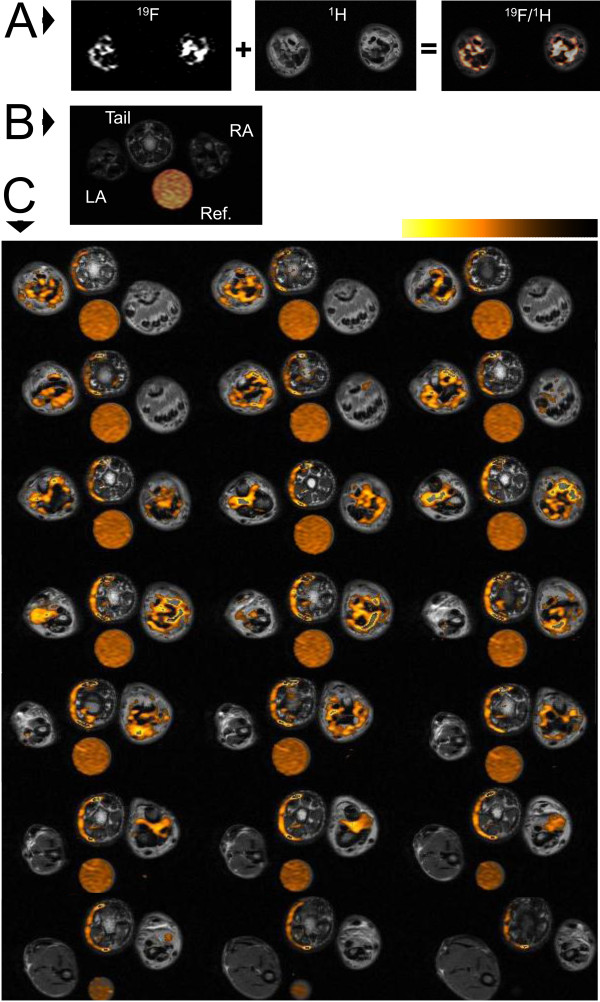
To determine whether 19F MRI could be used to image inflammation in arthritis, a cohort of rats were immunized twice with type II collagen to induce disease, and then dosed with the PFC contrast agent two days prior to 19F MRI. The time point for imaging (day 15) was selected as it coincided with nearly complete disease onset (90%) as measured by ankle swelling. Following MRI acquisition, 19F images were rendered in hot-orange pseudocolor and overlayed on the anatomical 1H image using Voxel Tracker™ software. A representative overlay is shown in Figure 2A. While all CIA rats had a strong 19F signal surrounding the bones in the ankles on day 15, consistent with the presence of inflammation at those sites, naïve rats did not have any detectable 19F in the hind limbs (Figure 2B), consistent with an absence of inflammation. Figure 2C depicts the entire panel of overlayed 1 mm axial slices through the hind limbs obtained from a single representative diseased rat.
Figure 2 .
Representative 1H and 19F MRI overlays of rat ankles.A.19F signal is rendered in hot orange scale and overlayed on a grayscale 1H image in this representative slice from an arthritic rat. B. Representative slice from a naïve rat indicating the placement of right ankle (RA) left ankle (LA), tail and reference tube (Ref). C. Complete series of 19F slices obtained through the ankles of a representative arthritic rat on day 15.
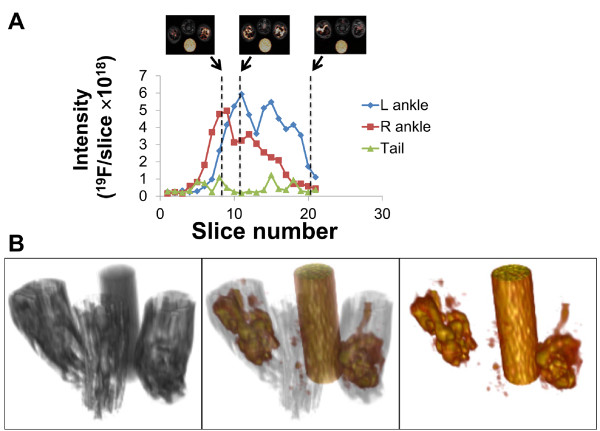
A comparison of 19F signal emanating from a region of interest with the known concentration of fluorine in the reference enabled the calculation of 19F nuclei per slice (Figure 3A) to identify the major sites of inflammatory infiltrates which have taken up 19F along the axis of the ankle. A 3D reconstruction of the distribution of 19F signal is depicted in Figure 3B.
Figure 3 .
Quantification and 3D rendering of inflammation obtained from 19F signal in rat ankles.A. ROIs were drawn around each ankle and the tail in an image stack from a representative scan of an arthritic rat (day 15) to enable the calculation of 19F spins per slice. The reference tube containing a known concentration of 19F was used in calculations. Results are represented as the amount of 19F per slice in each of the ankles or tail, with images depicted for slices 7, 9 and 17. B. 3D rendering of spatial accumulation of 19F signal in the ankles of a representative arthritic animal (day 15). 1H image is depicted on the left, and fused 19F/1H image in the middle, and 19F on the right.
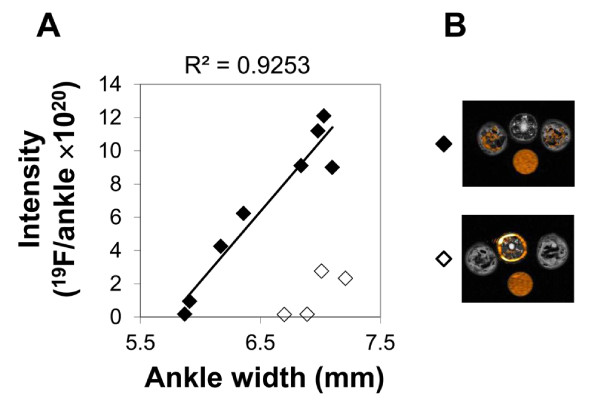
In order to correlate 19F signal with the clinical measurement, caliper measurements were obtained from diseased animals at the time of imaging. Animals imaged prior to disease onset on day 10 exhibited no swelling in the hind limbs and also had no detectable 19F signal (n = 3, data not shown). In animals with clinically evident disease imaged on day 15, four of six animals exhibited a strong linear dependence between diameter and 19F intensity (R2 = 0.93), while two of the six animals exhibited swelling with a lower, but still detectable, content of 19F. In these two animals, a very strong 19F signal was observed in the tail near the injection site (Figure 4B), in contrast with the other four animals, where little 19F was detectable in the tail. A strong correlation (R = 0.96) was observed between the caliper measurement of the ankle and the total amount of 19F signal (Figure 4A) when the animals with high signal in the tail were excluded from analysis.
Figure 4 .
Correlation between clinical measure of disease and 19F MRI imaging.A. Clinical measurements of disease severity manifested as ankle swelling were obtained through caliper measurements of ankle thickness and were plotted versus the total 19F spins/ankle as measured from the MRI images on day 15 (◆). Solid line represents the linear trend generated from data on individual ankles (n = 12). Two animals had very high levels of 19F found in the tail near the site of tail vein injection, suggesting that V-Sense was not delivered into the circulation. These two animals (◇) had significantly lower levels of 19F in each ankle than animals with similar clinical measurements (p < 0.001), and were not included in the linear regression. B. Representative slices from animals without (◆) and with (◇) high 19F in the tail are shown.
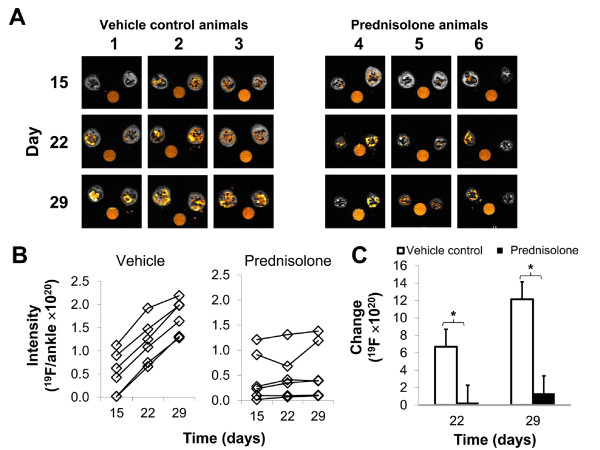
To determine whether 19F MRI could be used to evaluate the course of disease (progression, or remission), cohorts of animals were serially imaged at weekly intervals following the onset of disease. At day 15, all animals had 19F signal in one or both of their ankles, as shown in Figures 5A (average signal of all ankles 4.87 x 1019 ± 4.50 x 1019 range: 1.66 x 1018 to 1.12 x 1020). Evaluation of the 19F signal over time indicated a significant difference among groups (Additional file 1: Table S1, P = 3.47 x 10-4). Upon treatment with the steroid prednisolone, no significant increase in 19F signal was detected in the hind limbs at day 22 and only a minor increase over day 15 was observed by day 29, suggesting that the therapy was effective in eliminating the additional recruitment of inflammatory cells at later time points (Figure 5B, Additional file 1: Table S1). In strong contrast there were highly significant increases in 19F signal detected in the vehicle control cohort, consistent with progressive disease and the persisting recruitment of inflammatory phagocytic cells (Figure 5B, Additional file 1: Table S1). While the 19F intensity was variable among individual animals (Figure 5B), serial evaluation of the change in signal revealed that animals with untreated progressive disease accumulated significantly more 19F signal as compared to the prednisolone group (Figure 5C; P = 7.29 x 10-6 at day 22; P = 1.65x10-8 at day 29). Clinical evaluation of hind limb swelling corroborated the imaging observations of the effectiveness of prednisolone for reducing swelling versus the control group (Figure 6A, B). To validate the efficacy of treatment, animals were sacrificed on day 29, and ankles and knees were fixed and paraffin-embedded for histological assessments. As shown in Figure 7, vehicle control treated CIA animals had large inflammatory infiltrates in both ankles and knees, while treated animals had markedly reduced synovitis, consistent with the resolution of the inflammatory process in the treated groups as measured by both 19F MRI and clinical measurements.
Figure 5 .
Serial imaging and quantification of inflammation and therapeutic response in arthritic rats. As depicted in Figure 4, arthritic rats undergoing steroid treatment or vehicle control underwent serial imaging with PFC contrast agent and 19F MRI. A. Representative slices from each of three control animals (1, 2, 3) and each of three treated animals (4, 5, 6) are depicted on days 15, 22 and 29. Each column represents slices from a single, serially imaged animal over time. B. The degree of inflammation as assessed by quantification of the concentration of 19F per limb was depicted over time in vehicle (left) and prednisolone treated (right) animals. C. The average change in 19F signal from ti (day 15, the initial imaging session) to tn (day 22 or day 29) in untreated (open columns) and prednisolone treated (filled columns) at days 22 and day 29 are shown. *Statistically significant as determined by unpaired T-test (P =7.29 x10-6 at day 22 and P =1.65 x10-8 at day 29).
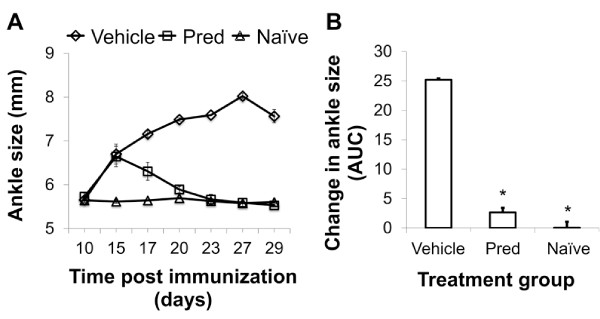
Figure 6 .
Clinical measurements of ankle size.A. Ankle swelling in 3 cohorts, naïve (Δ), diseased treated (□) and diseased vehicle control (◇) are plotted over time. B. Change in ankles size expressed as area under the curve (AUC). * P < 0.0001 as compared to vehicle control using Dunnett’s method.
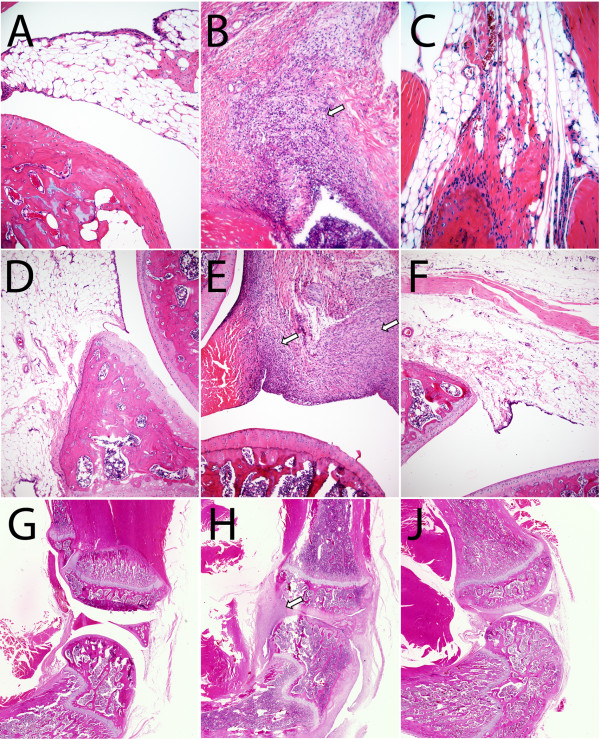
Figure 7 .
Representative hematoxylin- and eosin-stained histologic sections of rat knees and ankles on day 29. Each cohort was evaluated and examples are depicted of naïve controls (A, D, and G), vehicle-control treated CIA rats (B, E, and H) and prednisolone-treated CIA rats (C, F, and J). Images include photomicrographs of ankles (A, B, and C; 20X objective) and knees (D, E, and F; 10X objective) and knees taken with a slide scanner (G, H, and J). Inflammatory cell infiltration (arrows) is found in vehicle-control treated CIA rats.
Discussion
19F MRI with a PFC contrast agent is emerging as an effective approach to evaluate the onset of inflammation in both acute and chronic diseases [28-34]. Our results extend these findings to the detection and evaluation of CIA, a model with a quantifiable clinical surrogate of disease severity, enabling a direct comparison of disease activity with the 19F signal. Numerous studies have co-located the perfluorocarbon reagent within macrophage at the site of inflammation, allowing 19F MRI to image a general characteristic of inflammation at the cell-function level [33,37]. The detection of the 19F signal in diseased animals in this and other studies [28-34] and the lack of 19F signal in naïve animals indicates the specificity of this imaging approach. However, it was not clear whether the intensity of the signal could be used as an independent measure of disease activity. This study is the first to extend previous findings of the presence of inflammation to show the potential of 19F MRI to reveal the severity of inflammation. More importantly, serial 19F MRI monitoring could effectively be used to evaluate the persistence of inflammatory responses, progression of disease, and longitudinal study of the response to therapy. Limitations of the present study include the lack of methods to detect PFC within individual phagocytes at the site of inflammation histologically, and the relative insensitivity of MRI to detect very low amounts of 19F which might be present at sites of minimal but potentially relevant inflammation, leading to a false negative. We have recently developed a dual mode fluorescent version of the PFC contrast agent which will facilitate the evaluation of specific cells containing the contrast agent in future studies. The data reported here indicate the utility of PFC contrast agent with 19F MRI for monitoring the course of disease to assess the efficacy of a therapeutic.
Early in the disease process, a marked difference between individual animals was found, both in disease severity as well as in the accumulation of contrast agent. A linear relationship was observed between the amount of contrast agent at the site of inflammation and a clinical measurement of the severity of the experimental disease. In two subjects a high accumulation of contrast agent appeared in the tail (Figure 3B), with a lower level of 19F signal relative to ankle swelling measurement. Arthritis in the CIA model is typically restricted to the fore and hind limbs without axial involvement [35], and no clinical signs of disease were noted in the tails of any subjects. We surmised that signal in the tail could be a consequence of failure to completely deliver the contrast agent into the bloodstream, and the resulting misadministration enabled the local accumulation of the PFC emulsion at the site of injection, effectively reducing the amount systemically available to label circulating phagocytes. These results indicate that while the intensity of the 19F signal in the lesioned paws correlated with disease severity, care in administration of the contrast agent is necessary for the most reliable readout.
In the serial imaging studies, a difference in the pattern of 19F accumulation over time was found between the vehicle control and prednisolone treated cohorts. In the control cohort, 19F signal in the diseased limbs continued to accumulate upon repeated administration, whereas in the treated cohort, the signal remained stable over time, even after repeat administration of contrast agent. Histological and caliper measurements of ankle swelling point to continued infiltration of macrophage in the vehicle control cohort, consistent with 19F measurements. Histological endpoints and caliper measurements show fewer inflammatory cells and less swelling in the treated group, and a stable 19F signal. While 19F MRI did accurately reflect the abatement of macrophage infiltration to the site of inflammation (i.e., no increases in 19F were observed in treated animals), the persistence of signal after the departure of disease points to the need for future study and characterization of tissue clearance mechanisms and timescales of the 19F reagent. While a simple linear correlation between ankle swelling and 19F signals was not observed in the context of repeated administration of the contrast agent at days 22 and day 29 (data not shown), the 19F results nonetheless reflected the clinical responses, in which increases in 19F reflected disease progression and the inhibition of further 19F accumulation in animals undergoing successful therapy with a measureable clinical response. This data points to the utility of 19F imaging as a surrogate biomarker for evaluating therapeutic efficacy in RA.
While the CIA model in rats is largely restricted to the fore and hind limbs, and can be clinically assessed by measuring changes in ankle size, not all inflammatory diseases provide for a facile, rapid measurement of a response to a therapeutic drug [41]. Arthritis which affects the axial skeleton, such as ankylosing spondylitis or spondyloarthropathy, does not present simple external measurements for disease severity in preclinical models [42] and MRI is a standard clinical practice in the diagnosis of the disease [43]. In this case, the 19F MRI method of precisely measuring site-specific inflammation in vivo could enable an opportunity to facilitate study and treatment of disease, aiding the clinical development of therapeutics for ankylosing spondylitis and other inflammatory conditions [29,33,34].
As a preclinical tool, 19F MRI may have advantages over histological evaluation of tissues, given that a single, live animal may be imaged in less than one hour. In contrast, histology requires biopsy or necropsy of the particular tissues of interest, followed by fixation, preparation of frozen tissue blocks or paraffin embedding, slicing and mounting tissue sections, then staining and cover-slipping slides before the tissue is evaluated microscopically. 19F is taken up by macrophages in situ, and the signal intensity at sites of inflammation is directly related to the degree of cellular macrophage infiltration [29,31,33,34], providing a rapid means of assessing inflammatory infiltration. Further, MR methods provide more comprehensive information of the extent and location of inflammation compared with selected representative tissue sections evaluated by histology for phagocytic cells, although it may not replace detailed evaluation of cell subsets or subcellular biomarkers. MRI also allows longitudinal studies in the same animal over time, without biopsy or other invasive procedures, such as synovial aspiration [44]. Ultimately, this may speed the screening of inflammatory drugs against disease, particularly for those diseases where an external measurement on a live animal is unavailable. In the absence of imaging equipment, excised tissues may also be evaluated by 19F NMR spectrometers [28] for a more high throughput approach to quantitatively evaluate inflammatory lesions, with tissue potentially amenable to histology following NMR analysis.
While the goal of this study was to evaluate the imaging potential, there were several incidental findings. The detection of high amounts of 19F in the proximity of the injection site suggests that tail vein injection was less successful then one might have predicted, and that inclusion of the contrast agent could enable one to quantify misadministration. It was also noted that administration of multiple large doses of the PFC contrast agent occurred in the absence of anaphylaxis or adverse clinical effects. While more extensive preclinical toxicological safety testing are necessary prior to drawing conclusions, the results here contribute to the accumulating data regarding the safety of systemic PFC administration for imaging and other applications [45-47].
Conclusions
Pairing a PFC contrast agent with 19F MRI in vivo enabled a highly specific indicator of disease activity that has a direct correlation with a clinical measurement (i.e., ankle swelling in the CIA model). Here, it is shown that 19F MRI with a PFC contrast agent is not only useful for identification of sites of inflammation in RA, but can serve as a quantitative indicator of disease activity, including detection of disease progression, or remission in response to a therapeutic when applied to longitudinal in vivo imaging studies. The ability to unambiguously discern the infiltrating inflammatory cells from other anatomical features is highly desirable for the ability to quantify and sensitively detect disease progression. As the 19F signal does not alter the ability to acquire typical anatomical 1H images, imaging of both inflammation and the unadulterated anatomical features are possible with this approach. This approach may facilitate the drug development process in evaluating the efficacy of novel therapeutic regimens for RA and other inflammatory-based diseases, and eventually enable image-guided interventions or inform the therapeutic decision process in the clinic.
Abbreviations
MRI: Magnetic resonance imaging; PFC: Perfluorocarbon; CIA: Collagen induced arthritis; RA: Rheumatoid arthritis; ROI: Region of interest; 19F: Fluorine-19; 1H: Hydrogen-1; AUC: area under the curve.
Competing interests
The authors, except ETA, are employees of Celsense, Inc. and receive salary and stock options. ETA serves as a paid consultant to Celsense and is a stock holder.
Authors’ contributions
AB contributed to the design of the studies, production of the contrast agent, and contributed to analysis of the MRI data, including rendering 3D images, and quantification. AB was involved in the interpretation of data and revision of manuscript. BMH contributed to the design and analysis of the studies, assessment of the contrast agent and also was involved in drafting and revising the manuscript. ETA assisted with design and aided in preparation of the manuscript. AKW and CFO were responsible for the conception and planning of the experiments, and CFO helped to revise the manuscript. AKW directed the study, designed the experiments, contributed to the analysis and interpretation of the data, and drafted the manuscript. All authors have read and approved the final version of this manuscript.
Supplementary Material
Table S1. Longitudinal analysis of 19F signal in CIA rats.
Contributor Information
Anthony Balducci, Email: abalducci@celsense.com.
Brooke M Helfer, Email: brooke@celsense.com.
Eric T Ahrens, Email: eta@cmu.com.
Charles F O’Hanlon, III, Email: charlie@celsense.com.
Amy K Wesa, Email: awesa@celsense.com.
Acknowledgements
We thank the expert support of the Covance Laboratories team in the implementation and execution of the studies including J. Wolos (contributed to study design) and M. Cockman, B. Oldham, M. Zhu, S. Chintalacharuvu (all contributed to data collection and analysis).
References
- Silman AJ, Hochberg MC. Epidemiology of the rheumatic diseases. 2. Oxford University Press, Oxford; New York; 2001. [Google Scholar]
- Firestein GS, Panayi GS, Wollheim FA. Rheumatoid arthritis. 2. Oxford University Press, Oxford; New York; 2006. [Google Scholar]
- Wunder A, Straub RH, Gay S, Funk J, Muller-Ladner U. Molecular imaging: novel tools in visualizing rheumatoid arthritis. Rheumatology (Oxford) 2005;44:1341–1349. doi: 10.1093/rheumatology/keh709. [DOI] [PubMed] [Google Scholar]
- Sugimoto H, Takeda A, Hyodoh K. Early-stage rheumatoid arthritis: prospective study of the effectiveness of MR imaging for diagnosis. Radiology. 2000;216:569–575. doi: 10.1148/radiology.216.2.r00au20569. [DOI] [PubMed] [Google Scholar]
- Woodburn J, Hennessy K, Steultjens MPM, McInnes IB, Turner DE. Looking through the 'window of opportunity': is there a new paradigm of podiatry care on the horizon in early rheumatoid arthritis. Journal of Foot and Ankle Research. 2010;3:10. doi: 10.1186/1757-1146-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cush JJ. Early rheumatoid arthritis – is there a window of opportunity? The Journal of Rheumatology. 2007;80:1–7. [PubMed] [Google Scholar]
- Huizinga TWJ, Landewe RBM. Early aggressive therapy in rheumatoid arthritis: a 'window of opportunity'? Nat Clin Pract Rheum. 2005;1:2–3. doi: 10.1038/ncprheum0015. [DOI] [PubMed] [Google Scholar]
- McQueen FM, Stergaard M. Established rheumatoid arthritis – new imaging modalities. Best Practice & Research Clinical Rheumatology. 2007;21:841–856. doi: 10.1016/j.berh.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Strouse PJ, Londy F, DiPietro MA, Teo ELHJ, Chrisp CE, Doi K. MRI evaluation of infectious and non-infectious synovitis: preliminary studies in a rabbit model. Pediatr Radiol. 1999;29:367–371. doi: 10.1007/s002470050609. [DOI] [PubMed] [Google Scholar]
- Biswal S, Resnick DL, Hoffman JM, Gambhir SS. Molecular imaging: integration of molecular imaging into the musculoskeletal imaging practice. Radiology. 2007;244:651–671. doi: 10.1148/radiol.2443060295. [DOI] [PubMed] [Google Scholar]
- Chan K-WA, Felson D, Yood R, Walker A. The lag time between onset of symptoms and diagnosis of rheumatoid arthritis. Arthritis Rheum. 1994;37:814–820. doi: 10.1002/art.1780370606. [DOI] [PubMed] [Google Scholar]
- Gandjbakhch F, Conaghan PG, Ejbjerg B, Haavardsholm EA, Foltz V, Brown AK. Moller Dohn U, Lassere M, Freeston J, Boyesen P, et al: Synovitis and osteitis are very frequent in rheumatoid arthritis clinical remission: results from an MRI study of 294 patients in clinical remission or low disease activity state. J Rheumatol. 2011;38:2039–2044. doi: 10.3899/jrheum.110421. [DOI] [PubMed] [Google Scholar]
- Beckers C, Ribbens C, Andre B, Marcelis S, Kaye O, Mathy L, Kaiser MJ, Hustinx R, Foidart J, Malaise MG. Assessment of disease activity in rheumatoid arthritis with (18)F-FDG PET. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2004;45:956–964. [PubMed] [Google Scholar]
- Walther M, Harms H, Krenn V, Radke S, Faehndrich TP, Gohlke F. Correlation of power Doppler sonography with vascularity of the synovial tissue of the knee joint in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2001;44:331–338. doi: 10.1002/1529-0131(200102)44:2<331::AID-ANR50>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Hansch A, Frey O, Hilger I, Sauner D, Haas M, Schmidt D, Kurrat C, Gajda M, Malich A, Brauer R. Kaiser WA. Diagnosis of arthritis using near-infrared fluorochrome Cy5.5. Invest Radiol. 2004;39:626–632. doi: 10.1097/01.rli.0000139008.04288.fd. [DOI] [PubMed] [Google Scholar]
- Weber U, Østergaard M, Lambert R, Maksymowych W. The impact of MRI on the clinical management of inflammatory arthritides. Skeletal Radiol. 2011;40:1153–1173. doi: 10.1007/s00256-011-1204-5. [DOI] [PubMed] [Google Scholar]
- Beckmann N, Cannet C, Babin AL, Ble FX, Zurbruegg S, Kneuer R, Dousset V. In vivo visualization of macrophage infiltration and activity in inflammation using magnetic resonance imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:272–298. doi: 10.1002/wnan.16. [DOI] [PubMed] [Google Scholar]
- König H, Sieper J, Wolf KJ. Rheumatoid arthritis: evaluation of hypervascular and fibrous pannus with dynamic MR imaging enhanced with Gd-DTPA. Radiology. 1990;176:473–477. doi: 10.1148/radiology.176.2.2367663. [DOI] [PubMed] [Google Scholar]
- Tamai K, Yamato M, Yamaguchi T, Ohno W. Dynamic magnetic resonance imaging for the evaluation of synovitis in patients with rheumatoid arthritis. Arthritis Rheum. 1994;37:1151–1157. doi: 10.1002/art.1780370807. [DOI] [PubMed] [Google Scholar]
- van der Leij C, van de Sande MGH, Lavini C, Tak PP, Maas M. Rheumatoid Synovial Inflammation: Pixel-by-Pixel Dynamic Contrast-enhanced MR Imaging Time-Intensity Curve Shape Analysis—A Feasibility Study1. Radiology. 2009;253:234–240. doi: 10.1148/radiol.2531081722. [DOI] [PubMed] [Google Scholar]
- Gaffney K, Cookson J, Blades S, Coumbe A, Blake D. Quantitative assessment of the rheumatoid synovial microvascular bed by gadolinium-DTPA enhanced magnetic resonance imaging. Ann Rheum Dis. 1998;57:152–157. doi: 10.1136/ard.57.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard M, Stoltenberg M, Henriksen O, Lorenzen I. Quantitative assessment of synovial inflammation by dynamic gadolinium-enhanced magnetic resonance imaging. A study of the effect of intra-articular methylprednisolone on the rate of early synovial enhancement. Br J Rheumatol. 1996;35:50–59. doi: 10.1093/rheumatology/35.1.50. [DOI] [PubMed] [Google Scholar]
- Denis MC, Mahmood U, Benoist C, Mathis D, Weissleder R. Imaging inflammation of the pancreatic islets in type 1 diabetes. PNAS. 2004;101:12634–12639. doi: 10.1073/pnas.0404307101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann N, Falk R, Zurbrugg S, Dawson J, Engelhardt P. Macrophage infiltration into the rat knee detected by MRI in a model of antigen-induced arthritis. Magn Reson Med. 2003;49:1047–1055. doi: 10.1002/mrm.10480. [DOI] [PubMed] [Google Scholar]
- Lutz AM, Seemayer C, Corot C, Gay RE, Goepfert K, Michel BA, Marincek B, Gay S, Weishaupt D. Detection of Synovial Macrophages in an Experimental Rabbit Model of Antigen-induced Arthritis: Ultrasmall Superparamagnetic Iron Oxide-enhanced MR Imaging. Radiology. 2004;233:149–157. doi: 10.1148/radiol.2331031402. [DOI] [PubMed] [Google Scholar]
- Dardzinski BJ, Schmithorst VJ, Holland SK, Boivin GP, Imagawa T, Watanabe S, Lewis JM, Hirsch R. MR imaging of murine arthritis using ultrasmall superparamagnetic iron oxide particles. Magn Reson Imaging. 2001;19:1209–1216. doi: 10.1016/S0730-725X(01)00448-9. [DOI] [PubMed] [Google Scholar]
- Simon GH, von Vopelius-Feldt J, Fu Y, Schlegel J, Pinotek G, Wendland MF, Chen M-H, Daldrup-Link HE. Ultrasmall Supraparamagnetic Iron Oxide-Enhanced Magnetic Resonance Imaging of Antigen-Induced Arthritis: A Comparative Study Between SHU 555 C, Ferumoxtran-10, and Ferumoxytol. Invest Radiol. 2006;41:45–51. doi: 10.1097/01.rli.0000191367.61306.83. [DOI] [PubMed] [Google Scholar]
- Ahrens ET, Young WB, Xu H, Pusateri LK. Rapid quantification of inflammation in tissue samples using perfluorocarbon emulsion and fluorine-19 nuclear magnetic resonance. Biotechniques. 2011;50:229–234. doi: 10.2144/000113652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchens TK, Ye Q, Eytan DF, Janjic JM, Ahrens ET, Ho C. 19F MRI detection of acute allograft rejection with in vivo perfluorocarbon labeling of immune cells. Magn Reson Med. 2011;65:1144–1153. doi: 10.1002/mrm.22702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flogel U, Su S, Kreideweiss I, Ding Z, Galbarz L, Fu J, Jacoby C, Witzke O, Schrader J. Noninvasive detection of graft rejection by in vivo (19) F MRI in the early stage. Am J Transplant. 2011;11:235–244. doi: 10.1111/j.1600-6143.2010.03372.x. [DOI] [PubMed] [Google Scholar]
- Kadayakkara DK, Ranganathan S, Young WB, Ahrens ET. Assaying macrophage activity in a murine model of inflammatory bowel disease using fluorine-19 MRI. 2012. [DOI] [PMC free article] [PubMed]
- Hertlein T, Sturm V, Kircher S, Basse-Lusebrink T, Haddad D, Ohlsen K, Jakob P. Visualization of Abscess Formation in a Murine Thigh Infection Model of Staphylococcus aureus by F-Magnetic Resonance Imaging (MRI) PLoS One. 2011;6:e18246. doi: 10.1371/journal.pone.0018246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner B, Behm P, Jacoby C, Burghoff S, French BA, Schrader J, Flogel U. Early assessment of pulmonary inflammation by 19F MRI in vivo. Circ Cardiovasc Imaging. 2010;3:202–210. doi: 10.1161/CIRCIMAGING.109.902312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flogel U, Ding Z, Hardung H, Jander S, Reichmann G, Jacoby C, Schubert R, Schrader J. In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation. 2008;118:140–148. doi: 10.1161/CIRCULATIONAHA.107.737890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977;146:857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou J, Bull CM, Li L, Qian HR, Wei T, Luo S, Perkins D, Solenberg PJ, Tan SL, Chen XY. et al. Identification of blood biomarkers of rheumatoid arthritis by transcript profiling of peripheral blood mononuclear cells from the rat collagen-induced arthritis model. Arthritis Res Ther. 2006;8:R28. doi: 10.1186/ar1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise G. Basse-Luesebrink TC, Wessig C, Jakob PM, Stoll G: In vivo imaging of inflammation in the peripheral nervous system by (19)F MRI. Exp Neurol. 2011;229:494–501. doi: 10.1016/j.expneurol.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Gudbjartsson H, Patz S. The Rician distribution of noisy MRI data. Magn Reson Med. 1995;34:910–914. doi: 10.1002/mrm.1910340618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas M, Turner MS, Janjic JM, Morel PA, Laidlaw DH, Ahrens ET. In Vivo Cytometry of Antigen-Specific T Cells Using F-19 MRI. Magn Reson Med. 2009;62:747–753. doi: 10.1002/mrm.22063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas M, Morel PA, Ernst LA, Laidlaw DH, Ahrens ET. Fluorine-19 MRI for visualization and quantification of cell migration in a diabetes model. Magn Reson Med. 2007;58:725–734. doi: 10.1002/mrm.21352. [DOI] [PubMed] [Google Scholar]
- Bolon B, Stolina M, King C, Middleton S, Gasser J, Zack D, Feige U. Rodent preclinical models for developing novel antiarthritic molecules: comparative biology and preferred methods for evaluating efficacy. J Biomed Biotechnol. 2011;2011:569068. doi: 10.1155/2011/569068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardos T, Szabo Z, Czipri M, Vermes C, Tunyogi-Csapo M, Urban RM, Mikecz K, Glant TT. A longitudinal study on an autoimmune murine model of ankylosing spondylitis. Ann Rheum Dis. 2005;64:981–987. doi: 10.1136/ard.2004.029710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo-Ortega H, McGonagle D, Bennett AN. Magnetic resonance imaging in spondyloarthritis. Curr Opin Rheumatol. 2010;22:381–387. doi: 10.1097/BOR.0b013e328339381e. [DOI] [PubMed] [Google Scholar]
- Barton NJ, Stevens DA, Hughes JP, Rossi AG, Chessell IP, Reeve AJ, McQueen DS. Demonstration of a novel technique to quantitatively assess inflammatory mediators and cells in rat knee joints. J Inflamm (Lond) 2007;4:13. doi: 10.1186/1476-9255-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riess JG. Fluorous micro- and nanophases with a biomedical perspective. Tetrahedron. 2002;58:4113–4131. doi: 10.1016/S0040-4020(02)00262-4. [DOI] [Google Scholar]
- Krafft MP, Riess JG. Perfluorocarbons: Life sciences and biomedical uses dedicated to the memory of Professor Guy Ourisson, a true RENAISSANCE man. Journal of Polymer Science, Part A: Polymer Chemistry. 2007;45:1185–1198. doi: 10.1002/pola.21937. [DOI] [Google Scholar]
- Cohn CS, Cushing MM. Oxygen therapeutics: perfluorocarbons and blood substitute safety. Crit Care Clin. 2009;25:399–414. doi: 10.1016/j.ccc.2008.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Longitudinal analysis of 19F signal in CIA rats.