Abstract
Starch granules from maize (Zea mays) contain a characteristic group of polypeptides that are tightly associated with the starch matrix (C. Mu-Forster, R. Huang, J.R. Powers, R.W. Harriman, M. Knight, G.W. Singletary, P.L. Keeling, B.P. Wasserman [1996] Plant Physiol 111: 821–829). Zeins comprise about 50% of the granule-associated proteins, and in this study their spatial distribution within the starch granule was determined. Proteolysis of starch granules at subgelatinization temperatures using the thermophilic protease thermolysin led to selective removal of the zeins, whereas granule-associated proteins of 32 kD or above, including the waxy protein, starch synthase I, and starch-branching enzyme IIb, remained refractory to proteolysis. Granule-associated proteins from maize are therefore composed of two distinct classes, the surface-localized zeins of 10 to 27 kD and the granule-intrinsic proteins of 32 kD or higher. The origin of surface-localized δ-zein was probed by comparing δ-zein levels of starch granules obtained from homogenized whole endosperm with granules isolated from amyloplasts. Starch granules from amyloplasts contained markedly lower levels of δ-zein relative to granules prepared from whole endosperm, thus indicating that δ-zein adheres to granule surfaces after disruption of the amyloplast envelope. Cross-linking experiments show that the zeins are deposited on the granule surface as aggregates. In contrast, the granule-intrinsic proteins are prone to covalent modification, but do not form intermolecular cross-links. We conclude that individual granule intrinsic proteins exist as monomers and are not deposited in the form of multimeric clusters within the starch matrix.
It has long been known that starch granules contain bound polypeptides, with protein levels of isolated starch granules from maize (Zea mays) ranging from 0.3 to 1.0% based upon measurement of N2 (May, 1987). A recent study by our laboratory demonstrates that isolated starch granules from maize contain several dozen strongly bound polypeptides (Mu-Forster et al., 1996). The granule-associated proteins include starch-biosynthetic enzymes such as the waxy protein, SSI, and SBEIIb. These polypeptides are not removed from intact starch granules by protease treatment or detergent washing; therefore, they are believed to bind to the starch and to become irreversibly entrapped within the starch matrix.
Based upon staining intensities of polypeptides extracted from the starch granule (Mu-Forster et al., 1996), approximately one-half of the granule-associated proteins in maize consist of low-molecular-mass polypeptides ranging between 10 and 27 kD. These bands fall within the size range displayed by the zein storage proteins, however, the spatial distribution of these polypeptides within the starch granule is unknown. Zeins have been defined as alcohol-soluble proteins that occur principally in protein bodies of maize endosperm and that may or may not require reduction before extraction (Wilson, 1991). The association of zeins with starch granules during endosperm development would not be expected because zein genes do not contain transit peptides that would target these proteins through the amyloplast envelope into the amyloplast stroma.
The objective of this study was to establish the topology of granule-associated zeins in starch granules from maize endosperm. To accomplish this, it was necessary to distinguish between surface-localized and internalized polypeptides. Our working hypothesis defines polypeptides localized at the starch granule surface as those that are susceptible to hydrolysis upon treatment of intact granules with exogenous proteases. Conversely, internal granule proteins are defined as those that (a) become susceptible to proteolysis only following thermal disruption of the starch matrix, and (b) resist extraction by 2% SDS at room temperatures (Denyer et al., 1993; Rahman et al., 1995; Mu-Forster et al., 1996).
In this study we were able to distinguish between surface-localized and internalized granule-associated polypeptides in starch granules from maize endosperm by use of the thermophilic protease thermolysin. Thermolysin is well suited for this purpose because it is highly active at starch-gelatinization temperatures, and has also been shown to effectively hydrolyze hydrophobic proteins located at the surfaces of chloroplasts and other subcellular organelles (Cline et al., 1984; Xu and Chitnis, 1995). Upon extended incubation of intact starch granules with thermolysin at subgelatinization temperatures, we found that zeins were selectively removed from the starch granule surface. All other granule-associated polypeptides remained inaccessible to proteolytic attack or to extraction by 2% SDS, unless the starch matrix was first disrupted by gelatinization. Our results distinguish between the surface-localized and granule-intrinsic proteins of maize endosperm, and establish that zeins are localized at the starch-granule surface. In addition, cross-linking experiments were conducted to determine nearest-neighbor relationships among zein subunits localized at the granule surface and granule intrinsic polypeptides localized within the starch matrix.
MATERIALS AND METHODS
Kernels of maize (Zea mays, inbred line B73) were collected from ears of greenhouse-grown plants at 18 to 21 DAP, frozen in liquid N2, and stored at −80°C. Industrial wet-milled starch inbred line W64 suspended in steeping solution was provided by Cerestar USA (Hammond, IN). Unless otherwise indicated, the steeping solution was removed by repeated aqueous washing, and washed granules were air dried. Where indicated, laboratory-isolated granules prepared from cv B73 as described by Mu-Forster et al. (1996) were utilized. Antibodies to SSI, SBEIIb, and waxy protein were previously described (Mu et al., 1994; Mu-Forster et al., 1996). A polyclonal antibody recognizing maize δ-zein (10 kD) was a generous gift from Dr. Joachim Messing (Rutgers University, New Brunswick, NJ). Thermolysin (protease type X from Bacillus thermoproteolyticus; EC 3.4.24.4) was obtained from Sigma.
Starch Granule and Amyloplast Isolation
Starch granules were isolated by low-speed centrifugation as described (Mu et al., 1994; Mu-Forster et al., 1996). Amyloplasts were isolated from cv B73 endosperm harvested at 15 DAP as previously described (Denyer et al., 1996), with BSA omitted from the amyloplast isolation medium. Ten grams of endosperms was obtained by hand dissection, placed in a tilted Petri dish containing an amyloplast isolation medium consisting of buffer A (0.8 m sorbitol, 1 mm EDTA, 1 mm KCl, 2 mm MgCl2, 2 mm DTT, and 50 mm Hepes, pH 7.5), and incubated on ice for 30 min. A wide-bore pipette was used to slowly aspirate the cloudy liquid to a round-bottom centrifuge tube. Endosperms were re-immersed in buffer A and sliced in half with a razor blade. The resultant extract was transferred to a centrifuge tube using a pipette with its tip covered with a piece of cheesecloth to filter out large particles. A yellow amyloplast-enriched pellet was recovered by centrifugation at 36g for 10 min. The pellets were washed three times with buffer A and lysed in buffer B (10% glycerol, 10 mm EDTA, 1.25 mm DTT, and 50 mm Tris/HCl, pH 7.0) containing 0.3% Triton X-100. The clear, soluble fraction recovered by centrifugation (15,000g for 30 min) as amyloplast lysate was not further used in this study. SDS-PAGE was conducted, and no evidence was found to indicate that use of 0.3% Triton X-100 caused the release of any of the zeins into the lysis buffer. Amyloplast-derived granule-bound proteins were then extracted by boiling the pellets for 15 min in 200 μL of SDS-PAGE sample buffer (3% SDS, 5% β-mercaptoethanol, 10% glycerol, and 62.5 mm Tris/HCl, pH 6.9).
Protease Digestion of Starch Granules
Unless otherwise indicated, proteolytic digestion mixtures contained 50 mg (dry weight) of isolated starch granules, 100 μg of thermolysin, and 5 mm CaCl2 in a volume of 1 mL. Hydrolysis was conducted at 64°C, or as indicated, for defined intervals, and reactions were terminated by the addition of EDTA to 20 mm (Cline et al., 1984; Xu and Chitnis, 1995). Starch granules were recovered by centrifugation at 13,000g for 5 min. Residual thermolysin was removed by five successive washings with water. Proteins were extracted as described below. Controls contained buffer in place of thermolysin.
Protein Cross-Linking
Protein cross-linking was based upon a free radical coupling reaction using caffeic acid and potassium peroxymonosulfate (Oxone, Aldrich) (Gibson et al., 1994). Unless otherwise indicated, reaction mixtures contained 1 g (dry weight) of isolated starch granules in 1 mL of solution composed of caffeic acid at a concentration of 2.0 mm and potassium peroxymonosulfate at a concentration of 0.5 mm. Reactions were conducted at 55°C for 24 h and residual reagents were removed by five successive washings with water. Proteins were then extracted as described below. Controls contained water in place of the cross-linking reagents.
Protein Extraction and Analysis
Granule-associated proteins were recovered by extracting starch granules with SDS-PAGE sample buffer (20 μL of buffer per milligram dry weight of granule). Mixtures were then boiled for 15 min and cooled to room temperature, and annealed starch was removed by centrifugation at 13,000g for 15 min. Extracted proteins were analyzed by SDS-PAGE using 9 to 18% gradient gels (Porzio and Pearson, 1976) and were visualized by double-staining with Coomassie blue and silver (Integrated Separation Systems, Hyde Park, MA) or by immunoblotting (see below). Unless otherwise indicated, each lane was loaded with total protein extracted from 5 mg of isolated starch granules.
For immunoblotting, proteins were electrophoretically transferred from SDS gels to nitrocellulose membranes (Schleicher & Schuell) in 0.1% SDS, 100 mm Gly, and 10 mm Tris/HCl, pH 8.0 (Towbin et al., 1979; Harlow and Lane, 1988). The membranes were soaked for at least 1 h in TBS-T (0.15 m NaCl, 0.1% Tween 20, and 10 mm Tris/HCl, pH 7.4) containing 1% BSA to block nonspecific binding sites. The membranes were then washed with TBS-T once for 15 min and twice for 5 min. Antiserum (30 mL; 1:10,000 dilution) was then added and incubated for 1 h with gentle shaking. Following three more washes with TBS-T, blots were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (Bio-Rad) at 1:6,000 dilution for 1 h. Blots were then washed three times with TBS-T and were visualized using alkaline phosphatase (Fig. 3) or with enhanced chemiluminescence (Amersham) (Figs. 4–7).
Figure 3.
Immunoblot probed with the δ-zein antibody. Lane 1, Proteins extracted from 2.5 mg of starch isolated from purified amyloplasts of 15-DAP W64 maize. Lane 2, Proteins extracted from 2.5 mg of starch isolated from 15-DAP W64 maize endosperm. Lane 3, Protein extracted from thermolysin digested starch from 15-DAP W64 endosperm. The blot was developed colorimetrically using alkaline phosphatase.
Figure 4.
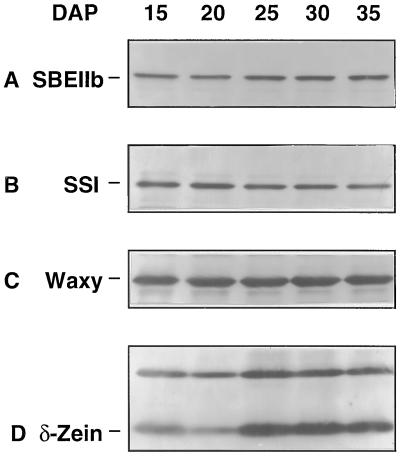
Immunoblots of proteins derived from starch granules were prepared using antibodies recognizing SBEIIb (A), SSI (B), the waxy protein (C), and δ-zein (D). cv B73 was harvested at 5-d intervals beginning at 15 DAP. Starch granules were recovered from the endosperm as described in Methods (Mu-Forster et al., 1996). Each lane contained the total protein extracted from 5 mg of isolated granules.
Figure 7.
Immunoblots were probed using antibodies recognizing SBEIIb (A), SSI (B), and the waxy protein (C). Lane arrangements and cross-linking reagent levels are identical to Figure 5.
N2 content of the starch was determined using a modified Kjeldahl method (American Association of Cereal Chemists, 1995). Protein content of starch granules was obtained by multiplying percent N2 content by a factor of 5.7 (Tkachuk, 1969).
RESULTS
Effect of Thermolysin on Starch Granule Protein Composition
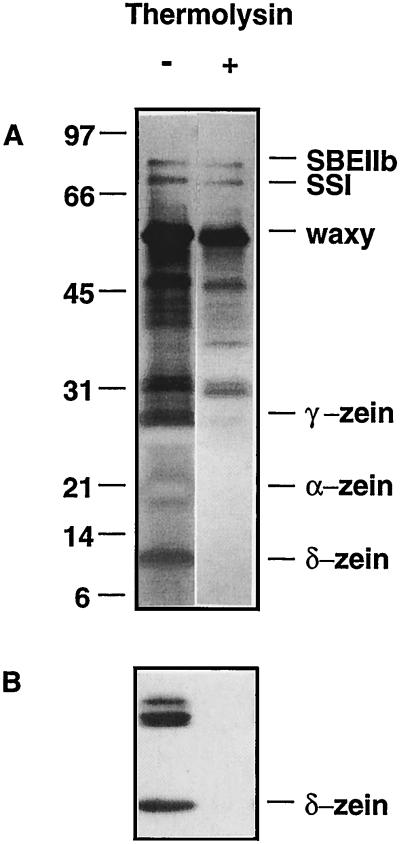
Isolated starch granules were subjected to thermolysin digestion at 64°C for 4 h. Granule-associated polypeptides were extracted and analyzed by SDS-PAGE (Fig. 1). Controls were subjected to identical heating and wash steps, however, thermolysin was omitted. Thermolysin digestion resulted in the selective removal of a group of low-molecular-mass proteins ranging between 10 and 27 kD. The 22- and 27-kD polypeptides, which were negatively stained with silver, corresponded to the positions of α- and γ-zein. In contrast, the prominent 60-kD granule-bound SSI (waxy protein), the 76-kD SSI, and the 85-kD SBEIIb remained intact following thermolysin digestion of intact granules under these conditions. However, when the starch matrix is disrupted by heating at 70° or higher, these polypeptides are readily proteolyzed by thermolysin, as would be consistent with an internal localization (Mu-Forster et al., 1996).
Figure 1.
Laboratory-isolated starch granules were incubated at 64°C for 4 h in the absence (−) or presence (+) of thermolysin as described in Methods. A, SDS-PAGE; B, immunoblot probed with antibodies recognizing the 10-kD δ-zein.
It is therefore clear that starch granules isolated from maize endosperm contain two distinct groups of granule-associated proteins. The low-molecular-mass proteins of 10 to 27 kD possessing the characteristic banding pattern of zeins (Larkins et al., 1984; Wilson, 1991) represent polypeptides located at or near the starch granule surface. However, the group that required granule disruption to expose internalized proteins to protease attack is comprised of proteins that are intrinsic to the starch granule. In maize all of the granule-associated polypeptides of 32 kD or higher fall within this group.
To establish that the low-molecular-mass proteins removed from the starch granule surface were in fact members of the zein family, immunoblotting was conducted using antibodies recognizing γ-zeins (16 or 27 kD) and δ-zeins (10 kD). Using enhanced chemiluminescence, the δ-zein antibody strongly recognized δ-zein, which runs at 10 kD, and a doublet of polypeptides at approximately 27 to 30 kD. Immunoblotting directly demonstrated that each of these polypeptides were completely digested by thermolysin (Fig. 1B). A similar digestion pattern was obtained with antibodies recognizing the γ-zeins (data not shown). These results clearly establish that digestion of intact starch granules with thermolysin results in the selective hydrolysis of zein proteins.
Analysis of residual N2 shows that thermolysin removed about 50% of total granule-associated protein (Table I). The protein content achieved after thermolysin digestion, based on N2, was generally reduced by 40 to 60%, depending upon the temperature of hydrolysis. The protein content of laboratory-isolated or commercial wet-milled corn starches generally ranges from 0.3 to 1% (Hoseney, 1994). These residual N2 levels provide a measurement of the matrix-embedded, intrinsically bound granule proteins that remain inaccessible to proteolytic digestion.
Table I.
Protein content of untreated and deproteinized maize starches
| Temperature | Protein
|
Reduction | |
|---|---|---|---|
| Control granules | Proteolyzed granules | ||
| °C | % dry wt | % | |
| 50 | 0.33 ± 0.03a | 0.14 ± 0.02 | 58 ± 4 |
| 64 | 0.24 ± 0.03 | 0.13 ± 0.02 | 46 ± 5 |
Starch granules were incubated with thermolysin at a concentration of 0.4 μg mg−1 in 5 mm CaCl2. Hydrolysis was conducted at 64 or 50°C for 4 h. The granules were washed five times with water to remove residual thermolysin and then air dried. Control starches were treated in parallel at each temperature with thermolysin omitted.
Values are the average of three measurements.
Effect of Ca2+
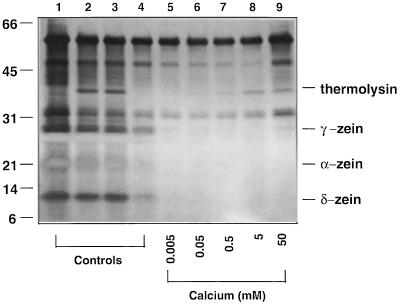
Thermolysin has an absolute requirement for Ca2+ (Feder et al., 1971; Tajima et al., 1976). Therefore, the effects of this divalent cation on starch granule surface deproteinization by thermolysin were investigated. Proteolysis would not be expected to occur in the absence of exogenous Ca2+, and this was the case since thermolysin failed to exert its proteolytic effect in the absence of Ca2+ (Fig. 2, lanes 2 and 3). When Ca2+ levels were increased to 0.5 mm or higher (Fig. 2, lanes 7–9), the zeins were removed in their entirety. It should be noted that thermolysin digestion of starch granules prepared by wet milling did not require the addition of exogenous Ca2+. Full-surface deproteinization occurred even in the absence of exogenous Ca2+ (data not shown). This could have been due to use of hard water during the commercial milling process, which could provide sufficient levels of Ca2+ to activate thermolysin. When wet-milled starch granules were washed with 20 mm EDTA to chelate divalent cations prior to incubation with thermolysin, reactions then became Ca2+ dependent, with the complete removal of surface polypeptides occurring at 0.5 mm Ca2+ (data not shown).
Figure 2.
Laboratory-isolated starch granules were incubated with thermolysin at the Ca2+ levels indicated (lanes 5–9). Lane 1 is a control with no additions. Lanes 2 through 9 each contained 0.1% thermolysin; however, in lane 2, starch granules were washed with 5 mm EDTA prior to the addition of thermolysin. In lane 3, starch granules were incubated with thermolysin in the presence of 5 mm EDTA. Lane 4 is a zero-time control containing thermolysin, but with both EDTA and Ca2+ omitted. Lanes 5 through 9 contained Ca2+ at the levels indicated with EDTA omitted. Proteins remaining associated with the starch granules were then extracted and analyzed by SDS-PAGE.
Origin of Granule-Associated δ-Zein
Since zein cDNAs do not contain plastid transit peptides (Pedersen et al., 1982, 1986; Liu and Rubinstein, 1992) and zeins are predominantly associated with protein bodies (Lending and Larkins, 1989), we hypothesized that the association of zeins with starch granules results from interactions of protein bodies with starch granules during kernel disruption. If this hypothesis is correct, we reasoned that starch granules isolated from amyloplasts should contain decreased levels of zeins relative to starches isolated from kernels or whole endosperm. To test this hypothesis, amyloplasts were purified from immature maize using a gentle mechanical-release method (Tetlow et al., 1996; Denyer et al., 1996), and starch granules were then isolated. As a control, starch granules were also isolated by grinding hand-dissected endosperm using a mortar and pestle followed by a series of aqueous washes. Proteins from the resultant granules were then extracted with hot SDS and separated by SDS-PAGE. Immunoblots demonstrated that starch granules from purified amyloplasts contained significantly less δ-zein relative to starch granule proteins isolated from the 15-DAP maize endosperm (Fig. 3, lanes 1 versus 2). This finding provides direct evidence that in undisrupted kernels, the bulk of the δ-zein is located outside of the amyloplast, indicating that the association of the zeins with the starch granules is likely to originate from protein bodies that are disrupted under the harsh conditions of kernel grinding and homogenization. We speculate that the residual amount of δ-zein associated with the amyloplast-derived starch may reflect a limited amount of envelope breakage during amyloplast isolation, despite precautions to minimize physical handling.
Association of δ-Zein with Starch Granules during Endosperm Development
The δ-zein content of starch granules was investigated over the course of endosperm development (Fig. 4). When normalized per unit weight of starch, levels of SBEIIb (Fig. 4A), SSI (Fig. 4B), and the waxy protein (Fig. 4C) remained constant over the course of kernel development (Mu-Forster et al., 1996). In contrast, δ-zein levels, which were relatively low at 15 and 20 DAP, exhibited a sharp increase between 20 and 25 DAP (Fig. 4D). δ-Zein levels remained constant between 25 and 35 DAP. This increase in δ-zein content could be a consequence of amyloplast envelope breakage during the developmental process, providing a means for protein bodies to interact with exposed granule surfaces as the process of starch deposition and grain filling proceeds.
Differential Cross-Linking Patterns of Surface-Bound δ-Zein and Granule-Intrinsic Proteins
Patterns of starch granule-associated proteins after cross-linking were determined following free-radical-based cross-linking conducted in the presence of the phenolic compound caffeic acid, which has been shown to cross-link isolated zeins (Gibson et al., 1994). This method is based upon the intercalation of the monophenolic compound caffeic acid with proteins (Loomis and Bataile, 1966; McManus et al., 1981; Mason and Wasserman, 1987), followed by in situ cross-linking initiated by the addition of potassium peroxymonosulfate (Gibson et al., 1994). Both reagents are small enough to diffuse within the pores and amorphous regions of the starch granule (Appelqvist and Debet, 1997). Following the noncovalent complexation of caffeic acid with granule-associated proteins, the potassium peroxymonosulfate induces a reaction that results in the formation of covalent complexes between adjacent species. As with bifunctional cross-linking agents, intermolecular complexes are formed between neighboring polypeptides. Alternatively, if a single polypeptide is internally cross-linked, intramolecular cross-links such as hairpin loops would result. In contrast to bifunctional reagents that may or may not bind to form noncovalent complexes with protein, the apparent affinity of the granule-associated proteins for phenolics such as caffeic acid is believed to provide a driving force for diffusion and equilibration of the phenolic compound within the starch granule.
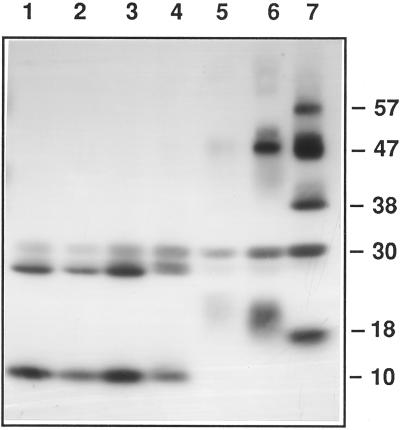
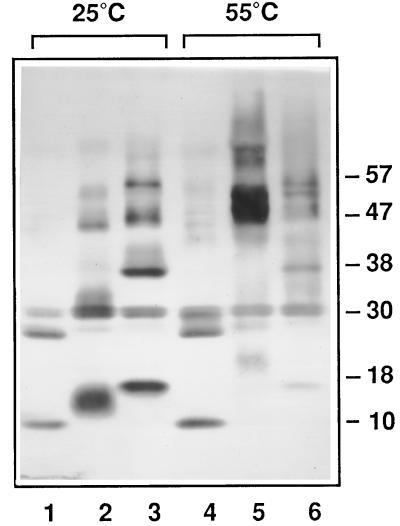
Since zein monomers are highly prone to associate, we would expect zein subunits to form cross-linked species that do not revert to monomers in the presence of reducing agent. Following extraction of proteins from starch granules and immunoblot analysis, extensive intermolecular cross-linking was observed, as followed by the formation of DTT-resistant complexes. Using the δ-zein antibody, the 10- and 27-kD immunoreactive species sharply declined at a caffeic acid level of 1 mm and at a potassium peroxymonosulfate level of 0.25 mm (Fig. 5, lane 5). The decline of the 10- and 27-kD immunoreactive species was accompanied by formation of cross-linked species with apparent masses of 18, 30, 38, 47, and 57 kD. With further increases in cross-linking agent concentration (Figs. 5 and 6), or temperature (Fig. 6), this marked shift toward multimer formation continued. High temperatures could disrupt hydrogen bonds within zein clusters, causing them to become more accessible to cross-linking reagents. We were unable to detect cross-link formation between δ-zein and internal granule-associated proteins such as the waxy protein, SSI, or SBEIIb.
Figure 5.
Immunoblots were probed using antibodies recognizing δ-zein. Lane 1 is a control with no cross-linking reagents added. In lanes 2 through 7, 1-g samples of starch granules were incubated with cross-linking reagents as follows: Caffeic acid levels, 0.02, 0.1, 0.2, 1, 2, and 10 mm, respectively; and potassium peroxymonosulfate levels, 0.005, 0.025, 0.05, 0.25, 0.5, and 2.5 mm, respectively. Each lane contained the total protein extracted from 5 mg of isolated granules.
Figure 6.
Immunoblots were probed using antibodies recognizing δ-zein as described in Figure 5. Reaction temperatures: Lanes 1 through 3, 25°C; lanes 4 through 6, 55°C. Lanes 1 and 4 are controls incubated in the absence of cross-linking reagents. Concentrations of caffeic acid: lanes 2 and 5, 2 mm; lanes 3 and 6, 10 mm. Concentrations of potassium peroxymonosulfate: lanes 2 and 5, 0.5 mm; lanes 3 and 6, 2.5 mm.
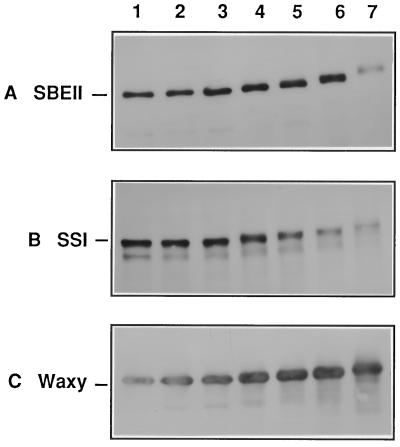
Using this approach, we then sought to determine whether internal granule proteins were also subject to cross-linking under these conditions. This information is important because it provides a means to establish whether granule-intrinsic proteins are deposited as individual monomers or as clusters within the granule. Polypeptide profiles from SDS extracts of cross-linked starch granules were therefore further analyzed with antibodies to SBEIIb (Fig. 7A), SSI (Fig. 7B), and the waxy protein (Fig. 7C). Our results show that cross-linking profiles of the granule intrinsic proteins differed markedly from the zeins in that (a) individual monomeric subunits did not disappear, and (b) intermolecularly cross-linked species containing the waxy protein, SSI, or SBEIIb were not generated with increased concentrations of cross-linking reagents. From these observations we conclude that the individual granule-intrinsic proteins are not situated in close enough proximity to enable intermolecular cross-linking.
The results of this cross-linking study show that granule-associated δ-zein was highly subject to the formation of intermolecular cross-linked species, whereas the granule-intrinsic proteins did not form covalently linked multimeric species under the same conditions. This leads us to conclude that the surface zeins occur in the form of aggregates and that the starch granule-intrinsic proteins are independently distributed within the starch granule matrix. However, similar cross-linking profiles would have also been generated if the cross-linking agents had been unable to penetrate the starch granule matrix. This latter possibility was readily excluded because the upward shifts in electrophoretic mobility observed in Figure 6 provided strong evidence that the reagents were able to penetrate within the granule matrix. Moreover, the starch granule contains amorphous interior channels positioned roughly in the radial direction (Fannon et al., 1992; Gallant et al., 1997), which are permeable to solutes of a Mr of 1000 or less (Appelqvist and Debet, 1997).
Figure 7, A through C, shows that SBEIIb, SSI, and the waxy protein each exhibited a gradual but steady increase in mass as levels of the cross-linking agents were increased. The introduction of covalent bonds within these proteins could arise by two means. Intramolecular hairpin loops, such as the kind induced by classical bifunctional reagents, are known to impede the migration of proteins in denaturing gels (Griffith, 1972; McIntosh, 1992). Alternatively, the increased apparent mass could be due to simple modification of the proteins by incorporation of caffeic acid groups. These results provide strong evidence to indicate that the individual granule intrinsic proteins are separately entrapped within the starch granule matrix and do not exist as multimeric clusters. To our knowledge, this result provides the first experimental evidence to verify the schematic model of independently distributed starch-granule proteins, as depicted in a recent review (Martin and Smith, 1995).
DISCUSSION
This study demonstrates that starch-granule-associated proteins in maize may be divided into two categories based upon the susceptibility of individual proteins to proteolytic attack. One group consists of internalized proteins intrinsically associated with the starch granule matrix. The granule-intrinsic proteins become accessible to protease digestion only after the starch granules are gelatinized (Mu-Forster et al., 1996). A second group consists of protease-accessible proteins located at the starch granule surface. These polypeptides are virtually all zeins and constitute approximately 50% of total granule-associated N2 content. δ-Zein readily forms multimeric species when subjected to cross-linking (Figs. 5 and 6). In contrast, the trend toward decreased electrophoretic mobility observed with the granule-intrinsic proteins is indicative of either covalent modification or intramolecular cross-link formation (Fig. 7). The inability to form intermolecular cross-links within the granule-intrinsic proteins clearly demonstrates that individual proteins embedded within the starch matrix do not exist in clustered form.
The finding that individual granule intrinsic proteins are separately entrapped within the starch granule matrix and do not exist as multimeric clusters is consistent with existing models of starch granule structure. In a starch cross-linking study (Kasemsuwan and Jane, 1994), phosphorus oxychloride was used for in situ cross-linking of starch granules. 31P-NMR spectroscopy was used to differentiate between phosphomonoesters and diesters. In conjunction, gel-filtration chromatography was used to demonstrate that no cross-links were formed among amylose, and that amylose only formed cross-links with amylopectin. The study concluded that amylose molecules are randomly interspersed within the amylopectin matrix. Analogous to amylose, the current results demonstrate that starch granule proteins do not appear to exist in the form of bundles or clusters.
A comparison of maize with other species shows that some granule-intrinsic polypeptides are common to a range of plants. For example, the 60-kD waxy protein is found in starch granules from virtually all monocots and many dicots. Wheat (Rahman et al., 1995; Takaoka et al., 1997) and maize (Mu-Forster et al., 1996) contain the 76-kD SSI, the 85-kD SBEIIb, and a 32-kD polypeptide of unknown identity (Rayas-Duarte et al., 1995), each of which is embedded within the starch matrix. In pea, the granule-bound SSII and SBE are also intrinsic proteins (Denyer et al., 1993). Prior to this study, the granule surface-bound proteins of maize were not known. The surface-bound proteins of maize starch granules are distinct from the surface proteins of wheat. Starch granules from wheat contain a surface-localized 15-kD polypeptide referred to as puroindoline or friabilin, which is extractable with 1% SDS or 50% isopropanol at room temperature (Greenwell and Schofield, 1986; Schofield and Greenwell, 1987; Morrison et al., 1992; Jolly et al., 1993; Morris et al., 1994). This study provides direct evidence that polypeptides of this class are not found at the surface of starch granules from maize. Since granule-surface proteins arise in part from processes that disrupt amyloplast envelope integrity, and the nature of storage proteins varies greatly, granule-surface proteins may show large interspecies variations.
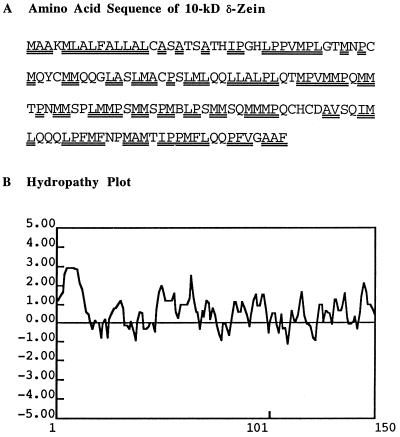
Thermolysin has long been used as an analytical tool for biochemical studies of membrane topology because it effectively hydrolyzes peripheral membrane proteins located at phospholipid/aqueous interfaces (Cline et al., 1984; Xu and Chitnis, 1995). To our knowledge, this is the first utilization of thermolysin at a protein-carbohydrate/aqueous interface. Thermolysin is ideally suited for hydrolysis of zeins because it cleaves at domains containing bulky hydrophobic or aromatic residues such as Ile, Leu, Val, Ala, Met, and Phe. Figure 8A shows the location of numerous thermolysin cleavage sites in the polypeptide chain of δ-zein, and a corresponding hydropathy plot (Fig. 8B).
Figure 8.
A, Amino acid sequence. Double underlines denote thermolysin recognition sites, which are generally rich in Leu and Met (Kirihara et al., 1988). B, Hydropathy plot (Kyte and Doolittle, 1982).
Zeins are formally defined as alcohol-soluble storage proteins that accumulate as disulfide-/hydrophobically linked aggregates in protein bodies localized within the endosperm, and that may comprise up to 60% of total endosperm protein (Wilson, 1987). Genetic and biochemical studies strongly rule out the possibility that zeins are amyloplast components. Our data showing that the zein content of starch granules recovered from isolated amyloplasts is markedly reduced relative to starch granules from homogenized whole endosperm further supports this conclusion (Fig. 3).
The temporal association of δ-zein with the starch granule appears to be independent of the onset or duration of δ-zein biosynthesis. In an expression study, transcripts for the 10-kD δ-zein were observed at 12 DAP and peaked at 15 to 18 DAP (Kirihara et al., 1988). However, granule-associated zeins peaked at approximately 25 DAP (Fig. 4). It is unlikely that this rise could be due to a sudden increase in the rate of zein synthesis. More likely, increases in granule-associated zeins could be indicative of amyloplast envelope breakage during the later stages of starch deposition. However, the extent to which amyloplast envelope rupture may occur during kernel development and the factors that influence envelope integrity are unknown.
The subunit composition of the granule-associated zeins generally reflects the zein composition of the endosperm-localized protein bodies. The α- and the γ-zeins are believed to constitute more than 90% of the total zein content, and these two zeins are also the predominant species associated with the starch granule (Esen, 1987; Wilson, 1991). Consistent with their levels within the protein body, the δ- and β-zeins are present in lesser amounts. Although the various zeins appear to be differentially distributed within endosperm-localized protein bodies (Lending and Larkins, 1989; Geetha et al., 1991), sufficient information does not exist at this time to conclude whether the subunit composition of the granule-bound zeins closely parallels the distribution of zein subunits at the surface of maize protein bodies. Because zein subunits assemble into multimeric networks via disulfide-linkages and various types of noncovalent interactions, one would expect that zeins are deposited on the starch granule surface in the form of multimeric clusters, and this was confirmed by the chemical cross-linking experiments (Figs. 5 and 7).
In summary, this study provides a direct demonstration of the interaction of a distinct class of polypeptides, consisting in large part of zeins, with the starch granule surface. Moreover, granule-intrinsic proteins appear to be randomly interspersed within the starch matrix. These surface proteins appear to be sequestered from the starch, whereas the amyloplast envelope remains intact but adheres to the starch granule upon physical disruption of endosperm tissue and subsequent rupture of the envelope. An effective enzymatic process for the removal of surface proteins provides a means to further characterize the molecular properties of the starch granule surface. Moreover, since the granule-intrinsic proteins are unaffected by thermolysin treatment, surface protein removal could greatly simplify the purification and characterization of polypepeptides located within the granule interior.
ACKNOWLEDGMENTS
We thank Dr. Joachim Messing, Ms. Helen Mu, and Mr. Justin Belles of Rutgers University; Dr. Jeff Habben and Dr. Brian Larkins of the University of Arizona; Dr. Peter L. Keeling of ExSeed Genetics; Dr. Robert Friedman of Cerestar USA; and Dr. Harold Corke of the University of Hong Kong for their generous assistance.
Abbreviations:
- DAP
days after pollination
- SBEIIb
starch-branching enzyme IIb
- SSI
starch synthase I
Footnotes
This research was supported in part by the U.S. Department of Agriculture National Research Initiative (grant nos. 91-37304-6579 and 95-02531), ExSeed Genetics, the Center for Advanced Food Technology, and the New Jersey Agricultural Experiment Station with State and Hatch Act Funds.
LITERATURE CITED
- American Association of Cereal Chemists (1995) Crude protein–improved Kjeldahl method, copper catalyst modification, Method 46–11A. In Approved Methods of the AACC, Ed 9. The Association, St. Paul, MN
- Appelqvist IAM, Debet MRM. Starch-biopolymer interactions: a review. Food Rev Int. 1997;13:163–224. [Google Scholar]
- Cline K, Werner-Washburne M, Andrews J, Keegstra K. Thermolysin is a suitable protease for probing the surface of intact pea chloroplasts. Plant Physiol. 1984;75:675–678. doi: 10.1104/pp.75.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer K, Dunlap F, Thorbjornsen T, Keeling P, Smith AM. The major form of ADP-glucose pyrophosphorylase in maize (Zea mays L.) endosperm is extra-plastidial. Plant Physiol. 1996;112:779–785. doi: 10.1104/pp.112.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer K, Sidebottom C, Hylton CM, Smith AM. Soluble isoforms of starch synthase and starch-branching enzyme also occur within starch granules in developing pea embryos. Plant J. 1993;4:191–198. doi: 10.1046/j.1365-313x.1993.04010191.x. [DOI] [PubMed] [Google Scholar]
- Esen A. A proposed nomenclature for the alcohol-soluble proteins (zein) of maize (Zea mays L.) J Cereal Sci. 1987;5:117–128. [Google Scholar]
- Fannon JE, Hauber RJ, BeMiller JN. Surface pores of starch granules. Cereal Chem. 1992;69:284–288. [Google Scholar]
- Feder J, Garrett LR, Wildi BS. Studies on the role of calcium in thermolysin. Biochemistry. 1971;10:4552–4555. doi: 10.1021/bi00800a032. [DOI] [PubMed] [Google Scholar]
- Gallant DJ, Bouchet B, Baldwin PM. Microscopy of starch: evidence of a new level of granule organization. Carbohydr Polym. 1997;32:177–191. [Google Scholar]
- Geetha KB, Lending CR, Lopes MA, Wallace JC, Larkins BA. Opaque-2 Modifiers increase γ-zein synthesis and alter its spatial distribution in maize endosperm. Plant Cell. 1991;3:1207–1219. doi: 10.1105/tpc.3.11.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson SM, Strauss G, Wasserman BP, inventors. Heat stable fat substitute compositions and process. US patent 5,374,441. 1994 December 20.
- Greenwell P, Schofield JD. A starch granule protein associated with endosperm softness in wheat. Cereal Chem. 1986;63:379–380. [Google Scholar]
- Griffith IP. The effects of cross-links on the mobility of proteins in dodecyl sulphate-polyacrylamide gels. Biochem J. 1972;126:553–560. doi: 10.1042/bj1260553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies, A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- Hoseney RC (1994) Principles of Cereal Science and Technology, Ed. 2. American Association of Cereal Chemists, St. Paul, MN
- Jolly CJ, Rahman S, Kortt AA, Higgens TJV. Characterisation of the wheat Mr 15,000 “grain-softness protein” and analysis of the relationship between its accumulation in the whole seed and grain softness. Theor Appl Genet. 1993;86:589–597. doi: 10.1007/BF00838714. [DOI] [PubMed] [Google Scholar]
- Kasemsuwan T, Jane J. Location of amylose in normal starch granules. II. Locations of phosphodiester cross-linking revealed by phosphorus-31 nuclear magnetic resonance. Cereal Chem. 1994;71:282–287. [Google Scholar]
- Kirihara JA, Hunsperger JP, Mahoney WC, Messing JW. Differential expression of a gene for a methionine-rich storage protein in maize. Mol Gen Genet. 1988;211:477–484. doi: 10.1007/BF00425704. [DOI] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Larkins BA, Pedersen M, Marks D, Wilson DR. The zein proteins of maize endosperm. Trends Biochem Sci. 1984;9:306–308. [Google Scholar]
- Lending CR, Larkins BA. Changes in the zein composition of protein bodies during maize endosperm development. Plant Cell. 1989;1:1011–1023. doi: 10.1105/tpc.1.10.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CN, Rubinstein I. Molecular characterization of two types of 22 kilodalton α-zein genes in a gene cluster in maize. Mol Gen Genet. 1992;234:244–253. doi: 10.1007/BF00283845. [DOI] [PubMed] [Google Scholar]
- Loomis WD, Bataile J. Plant phenolic compounds and the isolation of plant enzymes. Phytochemistry. 1966;5:423–438. [Google Scholar]
- Martin C, Smith AM. Starch biosynthesis. Plant Cell. 1995;7:971–985. doi: 10.1105/tpc.7.7.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason TL, Wasserman BP. Inactivation of red beet root β-glucan synthase by native and oxidized phenolic compounds. Phytochemistry. 1987;26:2197–2202. [Google Scholar]
- May JB (1987) Wet milling: process and products. In SA Watson, PE Ramstad, eds, Corn: Chemistry and Technology. American Association of Cereal Chemists, St. Paul, MN, pp 377–397
- McIntosh DB. Glutaraldehyde cross-links Lys-492 and Arg-678 at the active site of sarcoplasmic reticulum Ca2+ ATPase. J Biol Chem. 1992;267:22328–22335. [PubMed] [Google Scholar]
- McManus JP, Davis KG, Lilley TH, Haslam E. The association of proteins with polyphenols. J Chem Soc. 1981;24:309–311. [Google Scholar]
- Morris CF, Greenblatt GA, Bettge AD, Malkawi HI. Isolation and characterization of multiple forms of friabilin. J Cereal Sci. 1994;21:167–174. [Google Scholar]
- Morrison WR, Greenwell P, Law CN, Sulaiman BD. Occurrence of friabilin, a low molecular weight protein associated with grain softness, on starch granules isolated from wheats and related species. J Cereal Sci. 1992;15:143–149. [Google Scholar]
- Mu C, Harn C, Ko YT, Singletary GW, Keeling PL, Wasserman BP. Association of a 76 kDa polypeptide with soluble starch synthase I activity in maize (cv 73) endosperm. Plant J. 1994;6:151–159. [Google Scholar]
- Mu-Forster C, Huang R, Powers JR, Harriman RW, Knight M, Singletary GW, Keeling PL, Wasserman BP. Physical association of starch biosynthetic enzymes with starch granules of maize endosperm. Granule-associated forms of starch synthase I and starch branching enzyme II. Plant Physiol. 1996;111:821–829. doi: 10.1104/pp.111.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen K, Argos P, Naravana SVL, Larkins BA. Sequence analysis and characterization of a maize gene encoding a high-sulfur zein protein of Mr 15,000. J Biol Chem. 1986;261:6279–6284. [PubMed] [Google Scholar]
- Pedersen K, Devereux J, Wilson DR, Sheldon E, Larkins BA. Cloning and sequence analysis reveal structural variation among related zein genes in maize. Cell. 1982;29:1015–1026. doi: 10.1016/0092-8674(82)90465-2. [DOI] [PubMed] [Google Scholar]
- Porzio MA, Pearson AM. Improved resolution of myofibrillar proteins with sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1976;490:27–34. doi: 10.1016/0005-2795(77)90102-7. [DOI] [PubMed] [Google Scholar]
- Rahman S, Kosar Hashemi B, Samuel MS, Hill A, Abbott DC, Skeritt JH, Preiss J, Appels R, Morell MK. The major proteins of wheat endosperm starch granules. Aust J Plant Physiol. 1995;22:793–803. [Google Scholar]
- Rayas-Duarte P, Robinson SF, Freeman TP. In situ location of a starch granule protein in durum wheat endosperm by immunocytochemistry. Cereal Chem. 1995;72:269–274. [Google Scholar]
- Schofield JD, Greenwell P. Wheat starch granule proteins and their technological significance. In: Morton ID, editor. Cereals in a European Context. London: VCH; 1987. pp. 407–420. [Google Scholar]
- Tajima M, Urabe I, Yutani K, Odada H. Role of calcium ions in the thermostability of thermolysin and Bacillus subtilis var. amylosacchariticus neutral protease. Eur J Biochem. 1976;64:243–247. doi: 10.1111/j.1432-1033.1976.tb10293.x. [DOI] [PubMed] [Google Scholar]
- Takaoka M, Watanabe S, Sassa H, Yamamori M, Nakamura T, Sasakuma T, Hirano H. Structural characterization of high molecular weight starch granule-bound proteins in wheat (Triticum aestivum L) J Agric Food Chem. 1997;45:2929–2934. [Google Scholar]
- Tetlow IJ, Bowsher CG, Emes MJ. Reconstitution of the hexose phosphate translocator from the envelope membranes of wheat endosperm amyloplasts. Biochem J. 1996;319:717–723. doi: 10.1042/bj3190717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachuk R. Nitrogen-to-protein conversion factors for cereals and oil seed meals. Cereal Chem. 1969;46:419–423. [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CM (1987) Proteins of the kernel. In SA Watson, PE Ramstad, eds, Corn Chemistry and Technology. American Association of Cereal Chemists, St. Paul, MN, pp 273–310
- Wilson CM. Multiple zeins from maize endosperms characterized by reversed-phase high performance liquid chromatography. Plant Physiol. 1991;95:777–786. doi: 10.1104/pp.95.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Chitnis PR. Organization of photosystem I polypeptides. Plant Physiol. 1995;108:1067–1075. doi: 10.1104/pp.108.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]