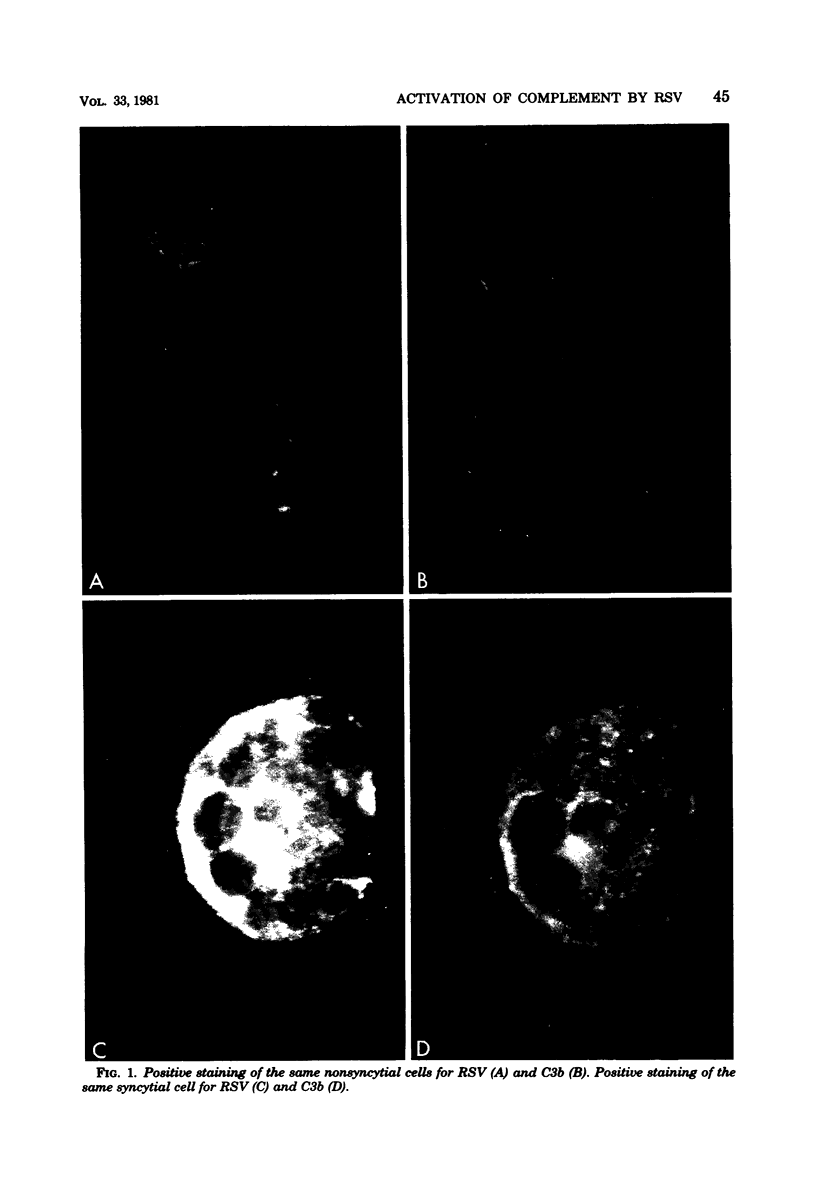
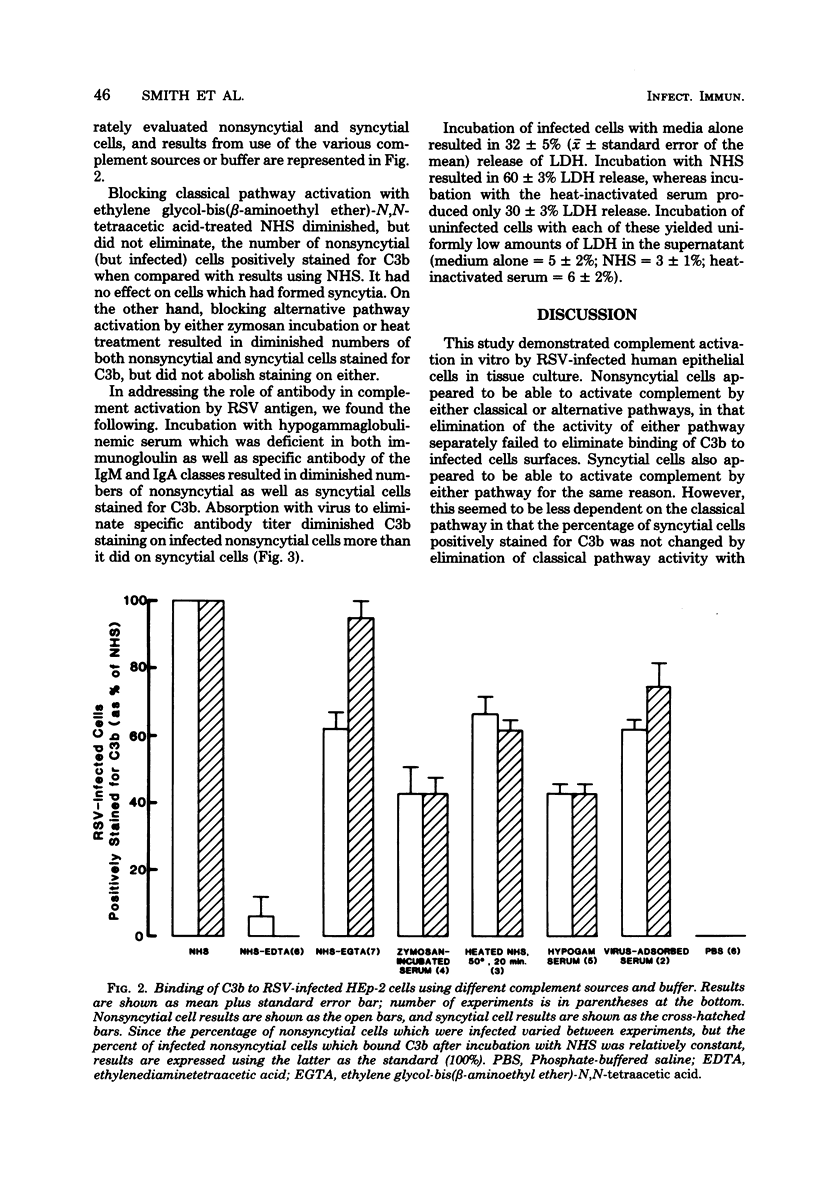
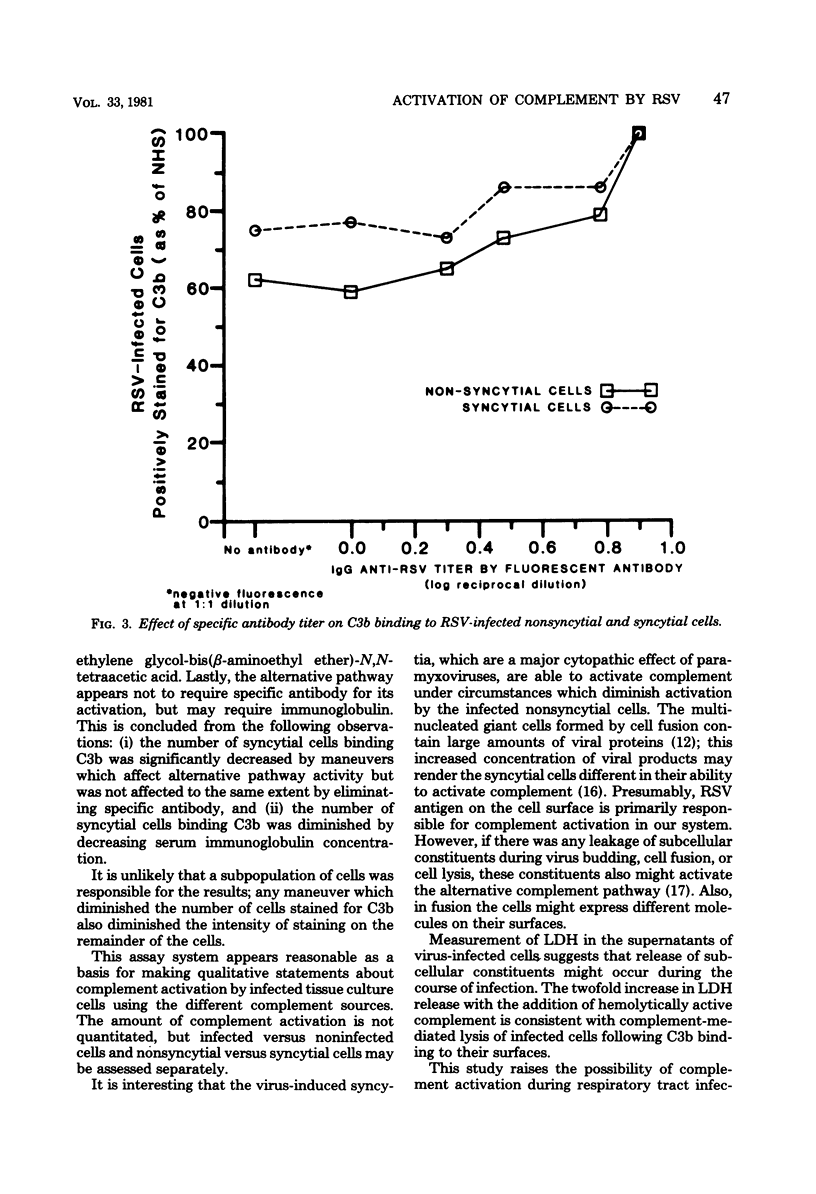
Abstract
The ability of respiratory syncytial virus (RSV)-infected HEp-2 cells in culture to activate complement was investigated. After incubation of cells with various complement sources and buffer, binding of C3b to surfaces of infected cells was demonstrated by immunofluorescence with a double-staining technique. Nonsyncytial and syncytial (i.e., fused, multinucleated) cells were separately enumerated. Also, lysis of RSV-infected cells was assessed by lactic dehydrogenase release. In this system only RSV-infected cells stained for C3b, and they did so only after incubation with functionally active complement. Blocking of classical pathway activation with ethylenediaminetetraacetic acid diminished the number of infected nonsyncytial cells positively stained for C3b, but had no effect on staining of syncytial cells. Blocking of alternative pathway activation with either zymosan incubation or heat treatment decreased the number of both syncytial and nonsyncytial cells stained for C3b. Decreasing immunoglobulin concentration of the serum used as the complement source also decreased numbers of both cell types stained for C3b. Eliminating specific anti-RSV antibody diminished numbers of both cell types stained for C3b, but staining was not eliminated. Lastly, incubation with functionally active complement markedly increased lactic dehydrogenase release from infected cells. This study demonstrated that RSV-infected nonsyncytial and syncytial cells are able to activate complement by both classical and alternative pathways. Activation of complement by syncytial cells appears to be less dependent on the classical pathway than is activation by nonsyncytial cells, and activation by syncytial cells may require immunoglobulin but not specific antibody. These experiments suggest the possibility of complement activation during respiratory tract infection by RSV. Implications of this are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brier A. M., Wohlenberg C., Rosenthal J., Mage M., Notkins A. L. Inhibition or enhancement of immunological injury of virus-infected cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3073–3077. doi: 10.1073/pnas.68.12.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N. R., Jensen F. C., Welsh R. M., Jr, Oldstone M. B. Lysis of RNA tumor viruses by human serum: direct antibody-independent triggering of the classical complement pathway. J Exp Med. 1976 Oct 1;144(4):970–984. doi: 10.1084/jem.144.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giclas P. C., Pinckard R. N., Olson M. S. In vitro activation of complement by isolated human heart subcellular membranes. J Immunol. 1979 Jan;122(1):146–151. [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. II. Mechanisms of release during phagocytosis, and adherence to nonphagocytosable surfaces. J Immunol. 1971 Dec;107(6):1547–1557. [PubMed] [Google Scholar]
- Hicks J. T., Ennis F. A., Kim E., Verbonitz M. The importance of an intact complement pathway in recovery from a primary viral infection: influenza in decomplemented and in C5-deficient mice. J Immunol. 1978 Oct;121(4):1437–1445. [PubMed] [Google Scholar]
- Hirsch R. L., Griffin D. E., Winkelstein J. A. The effect of complement depletion on the course of Sindbis virus infection in mice. J Immunol. 1978 Oct;121(4):1276–1278. [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Joseph B. S., Cooper N. R., Oldstone M. B. Immunologic injury of cultured cells infected with measles virus. I. role of IfG antibody and the alternative complement pathway. J Exp Med. 1975 Apr 1;141(4):761–774. [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., McQuillin J., Gardner P. S. Cell-free and cell-bound antibody in nasal secretions from infants with respiratory syncytial virus infection. Infect Immun. 1979 Feb;23(2):276–281. doi: 10.1128/iai.23.2.276-281.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz D. C., Scheid A., Choppin P. W. Importance of antibodies to the fusion glycoprotein of paramyxoviruses in the prevention of spread of infection. J Exp Med. 1980 Feb 1;151(2):275–288. doi: 10.1084/jem.151.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills B. J., Cooper N. R. Antibody-independent neutralization of vesicular stomatitis virus by human complement. I. Complement requirements. J Immunol. 1978 Oct;121(4):1549–1557. [PubMed] [Google Scholar]
- Platts-Mills T. A., Ishizaka K. Activation of the alternate pathway of human complements by rabbit cells. J Immunol. 1974 Jul;113(1):348–358. [PubMed] [Google Scholar]
- Schiøtz P. O., Sørensen H., Høiby M. Activated complement in the sputum from patients with cystic fibrosis. Acta Pathol Microbiol Scand C. 1979 Feb;87C(1):1–5. [PubMed] [Google Scholar]
- Wohl M. E., Chernick V. State of the art: bronchiolitis. Am Rev Respir Dis. 1978 Oct;118(4):759–781. doi: 10.1164/arrd.1978.118.4.759. [DOI] [PubMed] [Google Scholar]